Effects of L-Carnitine Intake on Exercise-Induced Muscle Damage and Oxidative Stress: A Narrative Scoping Review
Abstract
1. Introduction
1.1. Muscle Damage and Exercise
1.2. L-Carnitine
2. Objective
3. Materials and Methods
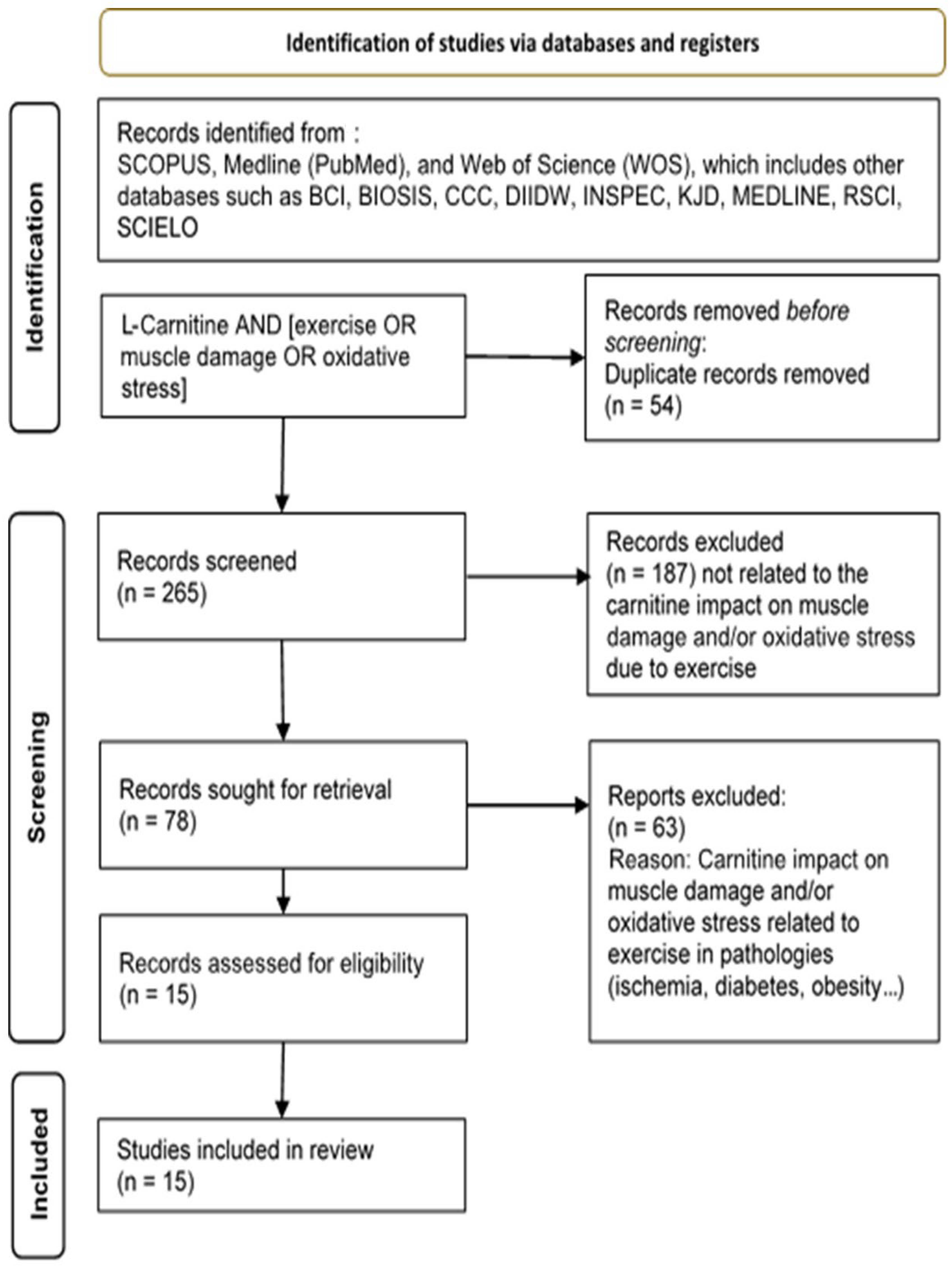
Study Analysis and Search Strategy
4. Results
5. Discussion
5.1. Oxidative Stress in Different Types of Exercise
5.2. Oxidative Stress and L-Carnitine Supplementation
5.3. Oxidative Stress, Muscle Damage and L-Carnitine Supplementation
5.4. L-Carnitine Supplementation and Recovery from Exercise
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef] [PubMed]
- Montesano, A.; Senesi, P.; Luzi, L.; Benedini, S.; Terruzzi, I. Potential Therapeutic Role of L-Carnitine in Skeletal Muscle Oxidative Stress and Atrophy Conditions. Oxid. Med. Cell. Longev. 2015, 2015, 646171. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L. Modulation of skeletal muscle antioxidant defense by exercise: Role of redox signaling. Free Radic. Biol. Med. 2008, 44, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Córdova-Martínez, A.; Caballero-García, A.; Bello, H.J.; Perez-Valdecantos, D.; Roche, E. Effects of Eccentric vs. Concentric Sports on Blood Muscular Damage Markers in Male Professional Players. Biology 2022, 11, 343. [Google Scholar] [CrossRef]
- Owens, D.J.; Twist, C.; Cobley, J.N.; Howatson, G.; Close, G.L. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur. J. Sport Sci. 2019, 19, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Manfredi, T.J.; Ding, W.; Fiatarone, M.A.; Evans, W.J.; Cannon, J.G. Acute phase response in exercise. III. Neutrophil and IL-1 beta accumulation in skeletal muscle. Am. J. Physiol. 1993, 265, R166–R172. [Google Scholar] [CrossRef]
- Malech, H.L.; Gallin, J.I. Neutrophils in Human Diseases. N. Engl. J. Med. 1987, 317, 687–694. [Google Scholar] [CrossRef]
- Fatouros, I.; Jamurtas, A. Insights into the molecular etiology of exercise-induced inflammation: Opportunities for optimizing performance. J. Inflamm. Res. 2016, 9, 175–186. [Google Scholar] [CrossRef]
- Córdova, A.; Mielgo-Ayuso, J.; Fernandez-Lazaro, C.; Caballero-García, A.; Roche, E.; Fernández-Lázaro, D. Effect of Iron Supplementation on the Modulation of Iron Metabolism, Muscle Damage Biomarkers and Cortisol in Professional Cyclists. Nutrients 2019, 11, 500. [Google Scholar] [CrossRef]
- Córdova, A.; Mielgo-Ayuso, J.; Roche, E.; Caballero-García, A.; Fernandez-Lázaro, D. Impact of Magnesium Supplementation in Muscle Damage of Professional Cyclists Competing in a Stage Race. Nutrients 2019, 11, 1927. [Google Scholar] [CrossRef]
- Carrera-Quintanar, L.; Funes, L.; Herranz-López, M.; Martínez-Peinado, P.; Pascual-García, S.; Sempere, J.M.; Boix-Castejón, M.; Córdova, A.; Pons, A.; Micol, V.; et al. Antioxidant Supplementation Modulates Neutrophil Inflammatory Response to Exercise-Induced Stress. Antioxidants 2020, 9, 1242. [Google Scholar] [CrossRef] [PubMed]
- Drobnic, F.; Riera, J.; Appendino, G.; Togni, S.; Franceschi, F.; Valle, X.; Pons, A.; Tur, J. Reduction of delayed onset muscle soreness by a novel curcumin delivery system (Meriva®): A randomised, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2014, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Tominaga, T.; Ruhee, R.T.; Ma, S. Characterization and Modulation of Systemic Inflammatory Response to Exhaustive Exercise in Relation to Oxidative Stress. Antioxidants 2020, 9, 401. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef]
- Salisbury, D.; Bronas, U. Reactive Oxygen and Nitrogen Species: Impact on Endothelial Dysfunction. Nurs. Res. 2015, 64, 53–66. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Talalay, P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol. Nutr. Food Res. 2008, 52, S128–S138. [Google Scholar] [CrossRef]
- Braakhuis, A.J.; Hopkins, W.G. Impact of Dietary Antioxidants on Sport Performance: A Review. Sports Med. 2015, 45, 939–955. [Google Scholar] [CrossRef]
- Bieber, L.L. Carnitine. Annu. Rev. Biochem. 1988, 57, 261–283. [Google Scholar] [CrossRef]
- Strijbis, K.; Vaz, F.M.; Distel, B. Enzymology of the carnitine biosynthesis pathway. IUBMB Life 2010, 62, 357–362. [Google Scholar] [CrossRef]
- Carter, A.L.; Abney, T.O.; Lapp, D.F. Biosynthesis and metabolism of carnitine. J. Child Neurol. 1995, 10, S3–S7. [Google Scholar] [CrossRef]
- D’Antona, G.; Nabavi, S.M.; Micheletti, P.; Di Lorenzo, A.; Aquilani, R.; Nisoli, E.; Rondanelli, M.; Daglia, M. Creatine, L-Carnitine, and ω 3 Polyunsaturated Fatty Acid Supplementation from Healthy to Diseased Skeletal Muscle. BioMed Res. Int. 2014, 2014, 613890. [Google Scholar] [CrossRef]
- Rebouche, C.J. Carnitine function and requirements during the life cycle. FASEB J. 1992, 6, 3379–3386. [Google Scholar] [CrossRef]
- Karlic, H.; Lohninger, A. Supplementation of L-carnitine in athletes: Does it make sense? Nutrition 2004, 20, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Dodson, W.; Sachan, D. Choline supplementation reduces urinary carnitine excretion in humans. Am. J. Clin. Nutr. 1996, 63, 904–910. [Google Scholar] [CrossRef]
- Schmidt-Sommerfeld, E.; Werner, D.; Penn, D. Carnitine plasma concentrations in 353 metabolically healthy children. Eur. J. Pediatr. 1988, 147, 356–360. [Google Scholar] [CrossRef]
- Valkner, K.J.; Bieber, L.L. Short-chain acylcarnitines of human blood and urine. Biochem. Med. 1982, 28, 197–203. [Google Scholar] [CrossRef]
- Reuter, S.E.; Evans, A.M. Carnitine and Acylcarnitines: Pharmacokinetic, Pharmacological and Clinical Aspects. Clin. Pharmacokinet. 2012, 51, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Steiber, A. Carnitine: A nutritional, biosynthetic, and functional perspective. Mol. Aspects Med. 2004, 25, 455–473. [Google Scholar] [CrossRef] [PubMed]
- Vaz, F.M.; Wanders, R.J.A. Carnitine biosynthesis in mammals. Biochem. J. 2002, 361, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Bremer, J. Carnitine–metabolism and functions. Physiol. Rev. 1983, 63, 1420–1480. [Google Scholar] [CrossRef]
- Fielding, R.; Riede, L.; Lugo, J.; Bellamine, A. L-Carnitine Supplementation in Recovery after Exercise. Nutrients 2018, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Traina, G. The neurobiology of acetyl-L-carnitine. Front. Biosci. 2016, 21, 1314–1329. [Google Scholar] [CrossRef]
- Vescovo, G.; Ravara, B.; Gobbo, V.; Sandri, M.; Angelini, A.; Della Barbera, M.; Dona, M.; Peluso, G.; Calvani, M.; Mosconi, L.; et al. L-Carnitine: A potential treatment for blocking apoptosis and preventing skeletal muscle myopathy in heart failure. Am. J. Physiol.-Cell Physiol. 2002, 283, C802–C810. [Google Scholar] [CrossRef] [PubMed]
- Spiering, B.A.; Kraemer, W.J.; Vingren, J.L.; Hatfield, D.L.; Fragala, M.S.; Ho, J.Y.; Maresh, C.M.; Anderson, J.M.; Volek, J.S. Responses of criterion variables to different supplemental doses of L-carnitine L-tartrate. J. Strength Cond. Res. 2007, 21, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Li, J.; Chen, Y.; Lu, Z.; Lyu, W. L-carnitine’s role in KAATSU training- induced neuromuscular fatigue. BioMed Pharmacother. 2020, 125, 109899. [Google Scholar] [CrossRef]
- Saaiq, M.; Ashraf, B. Modifying ‘Pico’ Question into ‘Picos’ Model for More Robust and Reproducible Presentation of the Methodology Employed in A Scientific Study. World J. Plast Surg. 2017, 6, 390–392. [Google Scholar]
- Harbour, R.; Miller, J. A new system for grading recommendations in evidence based guidelines. BMJ 2001, 323, 334–336. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Hutton, B.; Catalá-López, F.; Moher, D. La extensión de la declaración PRISMA para revisiones sistemáticas que incorporan metaanálisis en red: PRISMA-NMA. Med. Clín. 2016, 147, 262–266. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. Int. J. Evid. Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, S.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Arenas, J.; Huertas, R.; Campos, Y.; Díaz, A.E.; Villalón, J.M.; Vilas, E. Effects of L-carnitine on the pyruvate dehydrogenase complex and carnitine palmitoyl transferase activities in muscle of endurance athletes. FEBS Lett. 1994, 341, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Colombani, P.; Wenk, C.; Kunz, I.; Krähenbühl, S.; Kuhnt, M.; Arnold, M.; Frey-Rindova, P.; Frey, W.; Langhans, W. The effects of L-carnitine supplementation on physical performance and energy metabolism of endurance-trained athletes: A double-blind crossover field study. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 73, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Giamberardino, M.; Dragani, L.; Valente, R.; Di Lisa, F.; Saggin, R.; Vecchiet, L. Effects of Prolonged L-Carnitine Administration on Delayed Muscle Pain and CK Release After Eccentric Effort. Int. J. Sports Med. 1996, 17, 320–324. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Volek, J.S.; French, D.N.; Rubin, M.R.; Sharman, M.J.; Gómez, A.L.; Ratamess, N.A.; Newton, R.U.; Jemiolo, B.; Craig, B.W.; et al. The Effects of L-Carnitine L-Tartrate Supplementation on Hormonal Responses to Resistance Exercise and Recovery. J. Strength Cond. Res. 2003, 17, 455–462. [Google Scholar]
- Naclerio, F.; Larumbe-Zabala, E.; Cooper, R.; Jimenez, A.; Goss-Sampson, M. Effect of a carbohydrate-protein multi-ingredient supplement on intermittent sprint performance and muscle damage in recreational athletes. Appl. Physiol. Nutr. Metab. 2014, 39, 1151–1158. [Google Scholar] [CrossRef]
- Naclerio, F.; Larumbe-Zabala, E.; Cooper, R.; Allgrove, J.; Earnest, C.P. A Multi-Ingredient Containing Carbohydrate, Proteins L-Glutamine and L-Carnitine Attenuates Fatigue Perception with No Effect on Performance, Muscle Damage or Immunity in Soccer Players. PLoS ONE 2015, 10, e0125188. [Google Scholar] [CrossRef] [PubMed]
- Spiering, B.A.; Kraemer, W.J.; Hatfield, D.L.; Vingren, J.L.; Fragala, M.S.; Ho, J.Y.; Thomas, G.A.; Häkkinen, K.; Volek, J.S. Effects of L-Carnitine L-Tartrate Supplementation on Muscle Oxygenation Responses to Resistance Exercise. J. Strength Cond. Res. 2008, 22, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Kraemer, W.J.; Rubin, M.R.; Gómez, A.L.; Ratamess, N.A.; Gaynor, P. L-Carnitine L-tartrate supplementation favorably affects markers of recovery from exercise stress. Am. J. Physiol.-Endocrinol. Metab. 2002, 282, E474–E482. [Google Scholar] [CrossRef] [PubMed]
- Atalay Guzel, N.; Erikoglu Orer, G.; Sezen Bircan, F.; Coskun Cevher, S. Effects of acute L-carnitine supplementation on nitric oxide production and oxidative stress after exhaustive exercise in young soccer players. J. Sports Med. Phys. Fit. 2015, 55, 9–15. [Google Scholar]
- Bloomer, R.J.; Smith, W.A. Oxidative Stress in Response to Aerobic and Anaerobic Power Testing: Influence of Exercise Training and Carnitine Supplementation. Res. Sports Med. 2009, 17, 1–16. [Google Scholar] [CrossRef]
- Cao, Y.; Qu, H.-J.; Li, P.; Wang, C.-B.; Wang, L.-X.; Han, Z.-W. Single Dose Administration of L-Carnitine Improves Antioxidant Activities in Healthy Subjects. Tohoku J. Exp. Med. 2011, 224, 209–213. [Google Scholar] [CrossRef]
- Parandak, K.; Arazi, H.; Khoshkhahesh, F.; Nakhostin-Roohi, B. The effect of two-week L-carnitine supplementation on exercise -induced oxidative stress and muscle damage. Asian J. Sports Med. 2014, 5, 123–128. [Google Scholar]
- Ho, J.Y.; Kraemer, W.J.; Volek, J.S.; Fragala, M.S.; Thomas, G.A.; Dunn-Lewis, C.; Coday, M.; Häkinnen, K.; Maresh, C.M. L-Carnitine l-tartrate supplementation favorably affects biochemical markers of recovery from physical exertion in middle-aged men and women. Metabolism 2010, 59, 1190–1199. [Google Scholar] [CrossRef]
- Stefan, M.; Sharp, M.; Gheith, R.; Lowery, R.; Ottinger, C.; Wilson, J.; Durkee, S.; Bellamine, A. L-Carnitine Tartrate Supplementation for 5 Weeks Improves Exercise Recovery in Men and Women: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 3432. [Google Scholar] [CrossRef]
- Harris, R.C.; Foster, C.V.; Hultman, E. Acetylcarnitine formation during intense muscular contraction in humans. J. Appl. Physiol. 1987, 63, 440–442. [Google Scholar] [CrossRef]
- Hiatt, W.R.; Regensteiner, J.G.; Wolfel, E.E.; Ruff, L.; Brass, E.P. Carnitine and acylcarnitine metabolism during exercise in humans. Dependence on skeletal muscle metabolic state. J. Clin. Investig. 1989, 84, 1167–1173. [Google Scholar] [CrossRef]
- Sahlin, K. Muscle carnitine metabolism during incremental dynamic exercise in humans. Acta Physiol. Scand. 1990, 138, 259–262. [Google Scholar] [CrossRef]
- Bremmer, J. Pyruvate Dehydrogenase, Substrate Specificity and Product Inhibition. Eur. J. Biochem. 1969, 8, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Carlin, J.I.; Harris, R.C.; Cederblad, G.; Constantin-Teodosiu, D.; Snow, D.H.; Hultman, E. Association between muscle acetyl-CoA and acetylcarnitine levels in the exercising horse. J. Appl. Physiol. 1990, 69, 42–45. [Google Scholar] [CrossRef]
- Friolet, R.; Hoppeler, H.; Krähenbühl, S. Relationship between the coenzyme A and the carnitine pools in human skeletal muscle at rest and after exhaustive exercise under normoxic and acutely hypoxic conditions. J. Clin. Investig. 1994, 94, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Brass, E.P.; Scarrow, A.M.; Ruff, L.J.; Masterson, K.A.; Van Lunteren, E. Carnitine delays rat skeletal muscle fatigue in vitro. J. Appl. Physiol. 1993, 75, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Wächter, S.; Vogt, M.; Kreis, R.; Boesch, C.; Bigler, P.; Hoppeler, H.; Krähenbühl, S. Long-term administration of l-carnitine to humans: Effect on skeletal muscle carnitine content and physical performance. Clin. Chim. Acta 2002, 318, 51–61. [Google Scholar] [CrossRef]
- Spagnoli, L.G.; Palmieri, G.; Mauriello, A.; Vacha, G.M.; D’Iddio, S.; Giorcelli, G.; Corsi, M. Morphometric Evidence of the Trophic Effect of L-Carnitine on Human Skeletal Muscle. Nephron 1990, 55, 16–23. [Google Scholar] [CrossRef]
- Huertas, R.; Campos, Y.; Díaz, E.; Esteban, J.; Vechietti, L.; Montanari, G.; D’Iddio, S.; Corsi, M.; Arenas, J. Respiratory chain enzymes in muscle of endurance athletes: Effect of L-carnitine. Biochem. Biophys. Res. Commun. 1992, 188, 102–107. [Google Scholar] [CrossRef]
- Arenas, J.; Ricoy, J.R.; Encinas, A.R.; Pola, P.; D’Iddio, S.; Zeviani, M.; Didonato, S.; Corsi, M. Carnitine in muscle, serum, and urine of nonprofessional athletes: Effects of physical exercise, training, and L-carnitine administration. Muscle Nerve 1991, 14, 598–604. [Google Scholar] [CrossRef]
- Pekala, J.; Patkowska-Sokola, B.; Bodkowski, R.; Jamroz, D.; Nowakowski, P.; Lochynski, S.; Librowski, T. L-Carnitine–Metabolic Functions and Meaning in Humans Life. Curr. Drug Metab. 2011, 12, 667–678. [Google Scholar] [CrossRef]
- Gohil, K.; Viguie, C.; Stanley, W.C.; Brooks, G.A.; Packer, L. Blood glutathione oxidation during human exercise. J. Appl. Physiol. 1988, 64, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.C.; Silva, A.N.; de Oliveira, M.R. Oxidants, Antioxidants, and the Beneficial Roles of Exercise-Induced Production of Reactive Species. Oxid. Med. Cell. Longev. 2012, 2012, 756132. [Google Scholar] [CrossRef] [PubMed]
- Leaf, D.A.; Kleinman, M.T.; Hamilton, M.; Barstow, T.J. The effect of exercise intensity on lipid peroxidation. Med. Sci. Sports Exerc. 1997, 29, 1036–1039. [Google Scholar] [CrossRef]
- Michailidis, Y.; Jamurtas, A.Z.; Nikolaidis, M.G.; Fatouros, I.G.; Koutedakis, Y.; Papassotiriou, I.; Kouretas, D. Sampling Time is Crucial for Measurement of Aerobic Exercise-Induced Oxidative Stress. Med. Sci. Sports Exerc. 2007, 39, 1107–1113. [Google Scholar] [CrossRef]
- Niess, A.; Hartmann, A.; Grünert-Fuchs, M.; Poch, B.; Speit, G. DNA Damage After Exhaustive Treadmill Running in Trained and Untrained Men. Int. J. Sports Med. 1996, 17, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Sakellariou, G.K.; Jackson, M.J.; Vasilaki, A. Redefining the major contributors to superoxide production in contracting skeletal muscle. The role of NAD(P)H oxidases. Free Radic. Res. 2014, 48, 12–29. [Google Scholar] [CrossRef]
- Radak, Z.; Zhao, Z.; Koltai, E.; Ohno, H.; Atalay, M. Oxygen Consumption and Usage During Physical Exercise: The Balance Between Oxidative Stress and ROS-Dependent Adaptive Signaling. Antioxid. Redox Signal. 2013, 18, 1208–1246. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Li, J.; Liu, Z.; Chuang, C.C.; Yang, W.; Zuo, L. Redox Mechanism of Reactive Oxygen Species in Exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Involvement of neutrophils in exercise-induced muscle damage and its modulation. Gen. Intern. Med. Clin. Innov. 2018, 3, 1–8. [Google Scholar] [CrossRef]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive Oxygen Species: Impact on Skeletal Muscle. In Comprehensive Physiology, 1st ed.; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2011; pp. 941–969. [Google Scholar]
- Knez, W.L.; Coombes, J.S.; Jenkins, D.G. Ultra-Endurance Exercise and Oxidative Damage: Implications for Cardiovascular Health. Sports Med. 2006, 36, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Goldfarb, A.H.; McKenzie, M.J. Oxidative Stress Response to Aerobic Exercise: Comparison of Antioxidant Supplements. Med. Sci. Sports Exerc. 2006, 38, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Marzatico, F.; Pansarasa, O.; Bertorelli, L.; Somenzini, L.; Della Valle, G. Blood free radical antioxidant enzymes and lipid peroxides following long-distance and lactacidemic performances in highly trained aerobic and sprint athletes. J. Sports Med. Phys. Fit. 1997, 37, 235–239. [Google Scholar]
- Groussard, C.; Rannou-Bekono, F.; Machefer, G.; Chevanne, M.; Vincent, S.; Sergent, O.; Cillard, J.; Gratas-Delamarche, A. Changes in blood lipid peroxidation markers and antioxidants after a single sprint anaerobic exercise. Eur. J. Appl. Physiol. 2003, 89, 14–20. [Google Scholar] [CrossRef]
- Groussard, C.; Machefer, G.; Rannou, F.; Faure, H.; Zouhal, H.; Sergent, O.; Chevanne, M.; Cillard, J.; Gratas-Delamarche, A. Physical Fitness and Plasma Non-Enzymatic Antioxidant Status at Rest and After a Wingate Test. Can. J. Appl. Physiol. 2003, 28, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.S.; Bailey, D.M.; Hullin, D.; Young, I.; Davies, B. Metabolic implications of resistive force selection for oxidative stress and markers of muscle damage during 30s of high-intensity exercise. Eur. J. Appl. Physiol 2004, 92, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.; Trabelsi, K.; Boukhris, O.; Glenn, J.; Bott, N.; Masmoudi, L.; Hakim, A.; Chtourou, H.; Driss, T.; Hoekelmann, A.; et al. Effects of Aerobic-, Anaerobic- and Combined-Based Exercises on Plasma Oxidative Stress Biomarkers in Healthy Untrained Young Adults. Int. J. Environ. Res. Public Health 2020, 17, 2601. [Google Scholar] [CrossRef] [PubMed]
- El Abed, K.; Ammar, A.; Boukhris, O.; Trabelsi, K.; Masmoudi, L.; Bailey, S.J.; Hakim, A.; Bragazzi, N.L. Independent and Combined Effects of All-Out Sprint and Low-Intensity Continuous Exercise on Plasma Oxidative Stress Biomarkers in Trained Judokas. Front. Physiol. 2019, 10, 842. [Google Scholar] [CrossRef]
- Parker, L.; McGuckin, T.A.; Leicht, A.S. Influence of exercise intensity on systemic oxidative stress and antioxidant capacity. Clin. Physiol. Funct. Imaging 2014, 34, 377–383. [Google Scholar] [CrossRef]
- Child, R.; Brown, S.; Day, S.; Donnelly, A.; Roper, H.; Saxton, J. Changes in indices of antioxidant status, lipid peroxidation and inflammation in human skeletal muscle after eccentric muscle actions. Clin. Sci. 1999, 96, 105–115. [Google Scholar] [CrossRef]
- Radák, Z.; Pucsok, J.; Mecseki, S.; Csont, T.; Ferdinandy, P. Muscle soreness-induced reduction in force generation is accompanied by increased nitric oxide content and DNA damage in human skeletal muscle. Free Radic. Biol. Med. 1999, 26, 1059–1063. [Google Scholar] [CrossRef]
- Sunemi, S.; Silva, F.; Antonio, E.; Tucci, P.; Serra, A. Photobiomodulation: Newly Discovered Actions in Resistance Exercise. React. Oxyg. Species 2019, 7, 148–153. [Google Scholar] [CrossRef]
- Bouviere, J.; Fortunato, R.S.; Dupuy, C.; Werneck-de-Castro, J.P.; Carvalho, D.P.; Louzada, R.A. Exercise-Stimulated ROS Sensitive Signaling Pathways in Skeletal Muscle. Antioxidants 2021, 10, 537. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Radak, Z.; Ji, L.L. Exercise-induced oxidative stress: Past, present and future: Exercise-induced oxidative stress. J. Physiol. 2016, 594, 5081–5092. [Google Scholar] [CrossRef] [PubMed]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Kita, K.; Kato, S.; Yaman, M.A.; Okumura, J.; Yokota, H. Dietary L-carnitine increases plasma insulin-like growth factor-I concentration in chicks fed a diet with adequate dietary protein level. Br. Poult. Sci. 2002, 43, 117–121. [Google Scholar] [CrossRef]
- Keller, J.; Ringseis, R.; Eder, K. Supplemental carnitine affects the microRNA expression profile in skeletal muscle of obese Zucker rats. BMC Genom. 2014, 15, 512. [Google Scholar] [CrossRef]
- Glass, D.J. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat. Cell Biol. 2003, 5, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Couturier, A.; Haferkamp, M.; Most, E.; Eder, K. Supplementation of carnitine leads to an activation of the IGF-1/PI3K/Akt signalling pathway and down regulates the E3 ligase MuRF1 in skeletal muscle of rats. Nutr. Metab. 2013, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Antioxidant and antiradical activities of l-carnitine. Life Sci. 2006, 78, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Finaud, J.; Lac, G.; Filaire, E. Oxidative Stress: Relationship with Exercise and Training. Sports Med. 2006, 36, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Twist, C.; Eston, R. Neuromuscular Function After Exercise-Induced Muscle Damage: Theoretical and Applied Implications. Sports Med. 2004, 34, 49–69. [Google Scholar] [CrossRef]
- Leeuwenburgh, C.; Hansen, P.A.; Holloszy, J.O.; Heinecke, J.W. Hydroxyl radical generation during exercise increases mitochondrial protein oxidation and levels of urinary dityrosine. Free Radic. Biol. Med. 1999, 27, 186–192. [Google Scholar] [CrossRef]
- Di Meo, S.; Venditti, P. Mitochondria in Exercise-Induced Oxidative Stress. Neurosignals 2001, 10, 125–140. [Google Scholar] [CrossRef]
- Reid, M.B. Invited Review: Redox modulation of skeletal muscle contraction: What we know and what we don’t. J. Appl. Physiol. 2001, 90, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.H.; Reid, M.B.; Allen, D.G.; Westerblad, H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J. Physiol. 1998, 509, 565–575. [Google Scholar] [CrossRef]
- Cooper, C.E.; Vollaard, N.B.; Choueiri, T.; Wilson, M.T. Exercise, free radicals and oxidative stress. Biochem. Soc. Trans. 2002, 30, 280–285. [Google Scholar] [CrossRef]
- Jackson, M.J.; Farrell, S.O. Free radicals and muscle damage. Br. Med. Bull. 1993, 49, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Tiidus, P.M. Radical species in inflammation and overtraining. Can. J. Physiol. Pharmacol. 1998, 76, 533–538. [Google Scholar] [CrossRef]
- McArdle, A.; Pattwell, D.; Vasilaki, A.; Griffiths, R.D.; Jackson, M.J. Contractile activity-induced oxidative stress: Cellular origin and adaptive responses. Am. J. Physiol.-Cell Physiol. 2001, 280, C621–C627. [Google Scholar] [CrossRef]
- Peake, J.; Nosaka, K.; Suzuki, K. Characterization of inflammatory responses to eccentric exercise in humans. Exerc. Immunol. Rev. 2005, 11, 64–85. [Google Scholar] [PubMed]
- Caputo, F.; Vegliante, R.; Ghibelli, L. Redox modulation of the DNA damage response. Biochem. Pharmacol. 2012, 84, 1292–1306. [Google Scholar] [CrossRef] [PubMed]
- Chilelli, N.; Ragazzi, E.; Valentini, R.; Cosma, C.; Ferraresso, S.; Lapolla, A.; Sartore, G. Curcumin and Boswellia serrata Modulate the Glyco-Oxidative Status and Lipo-Oxidation in Master Athletes. Nutrients 2016, 8, 745. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Cánoves, P.; Scheele, C.; Pedersen, B.K.; Serrano, A.L. Interleukin-6 myokine signaling in skeletal muscle: A double-edged sword? FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef]
- Cordova, A.; Monserrat, J.; Villa, G.; Reyes, E.; Soto, M.A.M. Effects of AM3 (Inmunoferon®) on increased serum concentrations of interleukin-6 and tumour necrosis factor receptors I and II in cyclists. J. Sports Sci. 2006, 24, 565–573. [Google Scholar] [CrossRef]
- Córdova, A.; Sureda, A.; Pons, A.; Alvarez-Mon, M. Modulation of TNF-α, TNF-α receptors and IL-6 after treatment with AM3 in professional cyclists. J. Sports Med. Phys. Fit. 2015, 55, 345–351. [Google Scholar]
- Sureda, A.; Batle, J.M.; Capó, X.; Martorell, M.; Córdova, A.; Tur, J.A.; Pons, A. Scuba diving induces nitric oxide synthesis and the expression of inflammatory and regulatory genes of the immune response in neutrophils. Physiol. Genom. 2014, 46, 647–654. [Google Scholar] [CrossRef]
- Reid, M.B.; Haack, K.E.; Franchek, K.M.; Valberg, P.A.; Kobzik, L.; West, M.S. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J. Appl. Physiol. 1992, 73, 1797–1804. [Google Scholar] [CrossRef]
- Miles, M.P.; Andring, J.M.; Pearson, S.D.; Gordon, L.K.; Kasper, C.; Depner, C.M.; Kidd, J.R. Diurnal variation, response to eccentric exercise, and association of inflammatory mediators with muscle damage variables. J. Appl. Physiol. 2008, 10, 451–458. [Google Scholar] [CrossRef]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Zielinski, M.R.; Groschwitz, C.M.; Brown, A.S.; Gangemi, J.D.; Ghaffar, A.; Mayer, E.P. Curcumin effects on inflammation and performance recovery following eccentric exercise-induced muscle damage. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2007, 292, R2168–R2173. [Google Scholar] [CrossRef]
- Johnson, M.L.; Robinson, M.M.; Nair, K.S. Skeletal muscle aging and the mitochondrion. Trends Endocrinol. Metab. 2013, 24, 247–256. [Google Scholar] [CrossRef]
- Tidball, J.G. Mechanisms of Muscle Injury, Repair, and Regeneration. In Comprehensive Physiology, 1st ed.; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2011; pp. 2029–2062. [Google Scholar]
- Suzuki, K.; Ohno, H.; Oh-ishi, S.; Kizaki, T.; Ookawara, T.; Fujii, J.; Radák, Z.; Taniguchi, N. Superoxide dismutases in exercise and disease. In Handbook of Oxidants and Antioxidants in Exercise; Sen, C.K., Packer, L., Hänninen, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 243–295. [Google Scholar]
- Alessio, H.M.; Goldfarb, A.H. Lipid peroxidation and scavenger enzymes during exercise: Adaptive response to training. J. Appl. Physiol. 1988, 64, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.K.; Powers, S.K.; Stewart, D.J.; Demirel, H.A.; Shanely, R.A.; Naito, H. Short-term exercise training improves diaphragm antioxidant capacity and endurance. Eur. J. Appl. Physiol. 2000, 81, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Salo, D.C.; Donovan, C.M.; Davies, K.J.A. HSP70 and other possible heat shock or oxidative stress proteins are induced in skeletal muscle, heart, and liver during exercise. Free Radic. Biol. Med. 1991, 11, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Ray, K.; Singh, V.; Vats, P.; Singh, S.; Singh, S. L-carnitine supplementation attenuates intermittent hypoxia-induced oxidative stress and delays muscle fatigue in rats. Exp. Physiol. 2008, 93, 1139–1146. [Google Scholar] [CrossRef]
- Yu, J.; Ye, J.; Liu, X.; Han, Y.; Wang, C. Protective effect of L-carnitine against H2O2-induced neurotoxicity in neuroblastoma (SH-SY5Y) cells. Neurol. Res. 2011, 33, 708–716. [Google Scholar] [CrossRef]
- Huang, A.; Owen, K. Role of Supplementary L-Carnitine in Exercise and Exercise Recovery. In Medicine and Sport Science; Lamprecht, M., Ed.; S. Karger AG: Basel, Switzerland, 2012; pp. 135–142. [Google Scholar]
- Ringseis, R.; Keller, J.; Eder, K. Mechanisms underlying the anti-wasting effect of l-carnitine supplementation under pathologic conditions: Evidence from experimental and clinical studies. Eur. J. Nutr. 2013, 52, 1421–1442. [Google Scholar] [CrossRef] [PubMed]
- Barnett, C.; Costill, D.L.; Vukovich, M.D.; Cole, K.J.; Goodpaster, B.H.; Trappe, S.W.; Fink, W.J. Effect of L-Carnitine Supplementation on Muscle and Blood Camitine Content and Lactate Accumulation during High-Intensity Sprint Cycling. Int. J. Sport Nutr. 1994, 4, 280–288. [Google Scholar] [CrossRef]
- Vukovich, M.D.; Costill, D.L.; Fink, W.J. Carnitine supplementation: Effect on muscle carnitine and glycogen content during exercise. Med. Sci. Sports Exerc. 1994, 26, 1122–1129. [Google Scholar] [CrossRef]
- Wall, B.T.; Stephens, F.B.; Constantin-Teodosiu, D.; Marimuthu, K.; Macdonald, I.A.; Greenhaff, P.L. Chronic oral ingestion of L-carnitine and carbohydrate increases muscle carnitine content and alters muscle fuel metabolism during exercise in humans: Muscle carnitine loading and fuel utilisation. J. Physiol. 2011, 589, 963–973. [Google Scholar] [CrossRef]
- Iyer, R.N.; Khan, A.A.; Gupta, A.; Vajifdar, B.U.; Lokhandwala, Y.Y. L-carnitine moderately improves the exercise tolerance in chronic stable angina. J. Assoc. Physicians India 2000, 48, 1050–1052. [Google Scholar]
- Swart, I.; Rossouw, J.; Loots, J.M.; Kruger, M.C. The effect of L-carnitine supplementation on plasma carnitine levels and various performance parameters of male marathon athletes. Nutr. Res. 1997, 17, 405–414. [Google Scholar] [CrossRef]
- Vecchiet, L.; Di Lisa, F.; Pieralisi, G.; Ripari, P.; Menabò, R.; Giamberardino, M.A.; Siliprandi, N. Influence of L-carnitine administration on maximal physical exercise. Eur. J. Appl. Physiol. 1990, 61, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Krähenbuhl, S. L-Carnitine and physical performance. In Proceedings of the Symposium on L-Carnitine, a ‘Vitamin-Like Substance’, Zermatt, Switzerland, 28 April–1 May 2000. [Google Scholar]
- Dubelaar, M.L.; Lucas, C.M.B.H.; Hülsmann, W.C. The Effect of L-Carnitine on Force Development of the Latissimus Dorsi Muscle in Dogs. J. Card. Surg. 1991, 6, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Córdova, A.; Caballero-García, A.; Noriega-González, D.; Bello, H.J.; Pons, A.; Roche, E. Nitric-Oxide-Inducing Factors on Vitamin D Changes in Older People Susceptible to Suffer from Sarcopenia. Int. J. Environ. Res. Public Health 2022, 19, 5938. [Google Scholar] [CrossRef] [PubMed]

| Reference | Molecule/s | Daily Dosage | Route | Days | Placebo/Control | n | Type of Subjects | Age (Years) | Tests | Impact on Resolution | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arenas J et al., (1994) | [47] | L-carnitine | 2 g | Orally | 28 | P and C | 8P/8S/ 22 C | High level male athletes | 28 ± 7 | Histology (muscle biopsies) | ⊕ ↑ pyruvate dehydrogenase, ⊕ ↑ in the activities of complexes I, III and IV of the respiratory chain. |
| Colombani P et al., (1996) | [48] | L-carnitine | 4 g (2 + 2) | Orally | 1 | P | 10 | High level male athletes | 36 ± 3 | Blood analysis after marathon race | ↔ marathon running time, ↔ plasma concentrations of carbohydrate metabolites; ↔ fat metabolites, ↔ hormones (insulin, glucagon, cortisol), ↔ enzyme activities (CK). |
| Giamberardino MA et al., (1996) | [49] | L-carnitine | 3 g | Orally | 21 | P | 6 | Healthy males | 26 ± 4 | Blood analysis after eccentric effort, VAS | ⊕ ↓ pain, ↓ tenderness and ↓ CK release. |
| Kraemer WJ et al., (2003) | [50] | L-carnitine + L-tartrate | 2 g | Orally | 21 | P | 10 | Resistance-trained males | 26 ± 2 | Blood analysis after resistance effort, MRI | ⊕ ↓ exercise-induced muscle tissue damage, ↑ IGFBP-3. |
| Naclerio F et al., (2014) | [51] | L-carnitine + L-tartrate + MI | 3 g | Orally | 1 | P | 16 | Amateur soccer male players | 24 ± 4 | Blood analysis after intermittent repeated sprint test, RPE | ⊕ perception of fatigue, ↓ myoglobin, ↔ intermittent performance, ↔ inflammatory or immune function. |
| Naclerio F et al., (2015) | [52] | L-carnitine + L-tartrate + MI | 3 g | Orally | 1 | P | 10 | Team sport male players | 25 ± 4 | Blood analysis after intermittent repeated sprint test, RPE | ⊕ ↓ myoglobin, ↓ CK, ↔ perception of fatigue, ↔ sprint performance, ↔ inflammatory or immune function. |
| Spiering BA et al., (2007) | [37] | L-carnitine + L-tartrate | 1 or 2 g | Orally | 21 | P | 8 | Resistance-trained male | 22 ± 3 | Blood analysis after resistance effort. | ⊕ ↓ hypoxanthine, xanthine oxidase, myoglobin, and perceived muscle soreness. |
| Spiering BA et al., (2008) | [53] | L-carnitine + L-tartrate | 2 g | Orally | 23 | P | 9 | Resistance-trained male | 25 ± 6 | Blood analysis after resistance effort. | ⊕ ↓ muscle oxygenation during upper arm occlusion and following each set of resistance exercise. |
| Volek JS et al., (2002) | [54] | L-carnitine tartrate | 2 g | Orally | 21 | P | 10 | Resistance-trained male | 24 ± 2 | Blood analysis after resistance effort, MRI | ⊕ ↓ markers of purine catabolism (hypoxanthine, xanthine oxidase, and serum uric acid) and ↓ circulating muscle proteins (myoglobin, fatty acid-binding protein, and creatine kinase). ↓ muscle disruption from MRI scans. |
| Atalay Guzel N et al., (2014) | [55] | L-carnitine | 3 or 4 g or P | Orally | 1 | P | 13 | Healthy males | 17–19 | Maximal exercise test | ⊕ ↑ GSH and NO, ↓ TBARs |
| Bloomer RJ et al., (2009) | [56] | Propionyl L-carnitine | 1 or 3 g or P | Orally | 56 | P | 32 | Healthy males and females | 27 ± 2, P 26 ± 2, 1 g 27 ± 2, 3 g | Aerobic–anaerobic exercise testing | Both aerobic and anaerobic power testing increase oxidative stress to a similar extent. ⊕ ↓ MDA, but little impact on exercise-induced oxidative stress biomarkers. |
| Cao Y et al., (2011) | [57] | L-carnitine | 2 g | Orally | 1 | U | 12 | Healthy males and females | 28 ± 5 | Blood analysis | ⊕ ↑ SOD, ↑ GSH-Px, ↑ catalase and ↑ TAC following the first 3,5 h post-administration. |
| Parandak K et al., (2014) | [58] | L-carnitine | 2 g | Orally | 14 | P | 21 | Healthy males | 22 ± 1 | Blood analysis after endurance exercise | ⊕ ↑ TAC, ↓ MDA-TBARS, CK, and LDH 24 h after exercise. |
| Ho JY et al., (2010) | [59] | L-carnitine | 2 g | Orally | 24 | P | 18 | Healthy males and females | 45 ± 5, m 52 ± 5, f | Blood analysis after resistance effort | ⊕ ↓ biochemical markers of purine metabolism, ↓ MDA, ↓ muscle tissue disruption (myoglobin, CK), ↓ muscle soreness. |
| Stefan M et al., (2021) | [60] | L-carnitine tartrate | 2 g | Orally | 35 | P | 73 | Healthy males and females | 39 ± 1, m 41 ± 2, f | Blood salivary analysis, soreness scale | ⊕ ↑ SOD, ↓ perceived recovery and soreness, ↓ CK. |
| Reference | Limitations |
|---|---|
| Arenas et al. [47] Colombani et al. [48] Giamberardino et al. [49] Parandak et al. [58] | Small sample size conducted in endurance athletes, limiting the extension to other populations. |
| Spiering et al. [37] Kraemer et al. [50] Spiering et al. [53] Volek et al. [54] | Small sample size conducted in resistance male athletes, limiting the extension to other populations. |
| Nacleiro et al. [51] Nacleiro et al. [52] Atalay Guzel et al. [55] | Small sample size conducted in intervallic athletes, limiting the extension to other populations. |
| Cao et al. [57] | Small sample size conducted in healthy individuals, no representative of a broader population. |
| Ho et al. [59] | Small sample size conducted in middle-aged individuals, no representative of a broader population. |
| Cao et al. [57] Parandak et al. [58] Ho et al. [59] | No control group. |
| Arenas et al. [47] Colombani et al. [48] Kraemer et al. [50] Nacleiro et al. [51] Bloomer et al. [56] | The study does not investigate the role of L-carnitine supplementation on postexercise recovery. |
| Spiering et al. [37] Arenas et al. [47] Colombani et al. [48] Kraemer et al. [50] Nacleiro et al. [51] | The study does not investigate the role of L-carnitine supplementation on postexercise oxidative stress. |
| Colombani et al. [48] | The study was conducted in a field setting, limiting the control of other variables. |
| Giamberardino et al. [49] Stefan et al. [60] | Only data from CK release, but no data from other markers of postexercise muscle damage or oxidative stress were presented. |
| Atalay Guzel et al. [55] Bloomer et al. [56] | The study did not provide data on muscle damage. |
| Cao et al. [57] | Short duration and single dose administration. This limits the possibility to draw long-term conclusions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caballero-García, A.; Noriega-González, D.C.; Roche, E.; Drobnic, F.; Córdova, A. Effects of L-Carnitine Intake on Exercise-Induced Muscle Damage and Oxidative Stress: A Narrative Scoping Review. Nutrients 2023, 15, 2587. https://doi.org/10.3390/nu15112587
Caballero-García A, Noriega-González DC, Roche E, Drobnic F, Córdova A. Effects of L-Carnitine Intake on Exercise-Induced Muscle Damage and Oxidative Stress: A Narrative Scoping Review. Nutrients. 2023; 15(11):2587. https://doi.org/10.3390/nu15112587
Chicago/Turabian StyleCaballero-García, Alberto, David C. Noriega-González, Enrique Roche, Franchek Drobnic, and Alfredo Córdova. 2023. "Effects of L-Carnitine Intake on Exercise-Induced Muscle Damage and Oxidative Stress: A Narrative Scoping Review" Nutrients 15, no. 11: 2587. https://doi.org/10.3390/nu15112587
APA StyleCaballero-García, A., Noriega-González, D. C., Roche, E., Drobnic, F., & Córdova, A. (2023). Effects of L-Carnitine Intake on Exercise-Induced Muscle Damage and Oxidative Stress: A Narrative Scoping Review. Nutrients, 15(11), 2587. https://doi.org/10.3390/nu15112587








