β-Hydroxy-β-methyl Butyrate Regulates the Lipid Metabolism, Mitochondrial Function, and Fat Browning of Adipocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Cell Proliferation Analysis
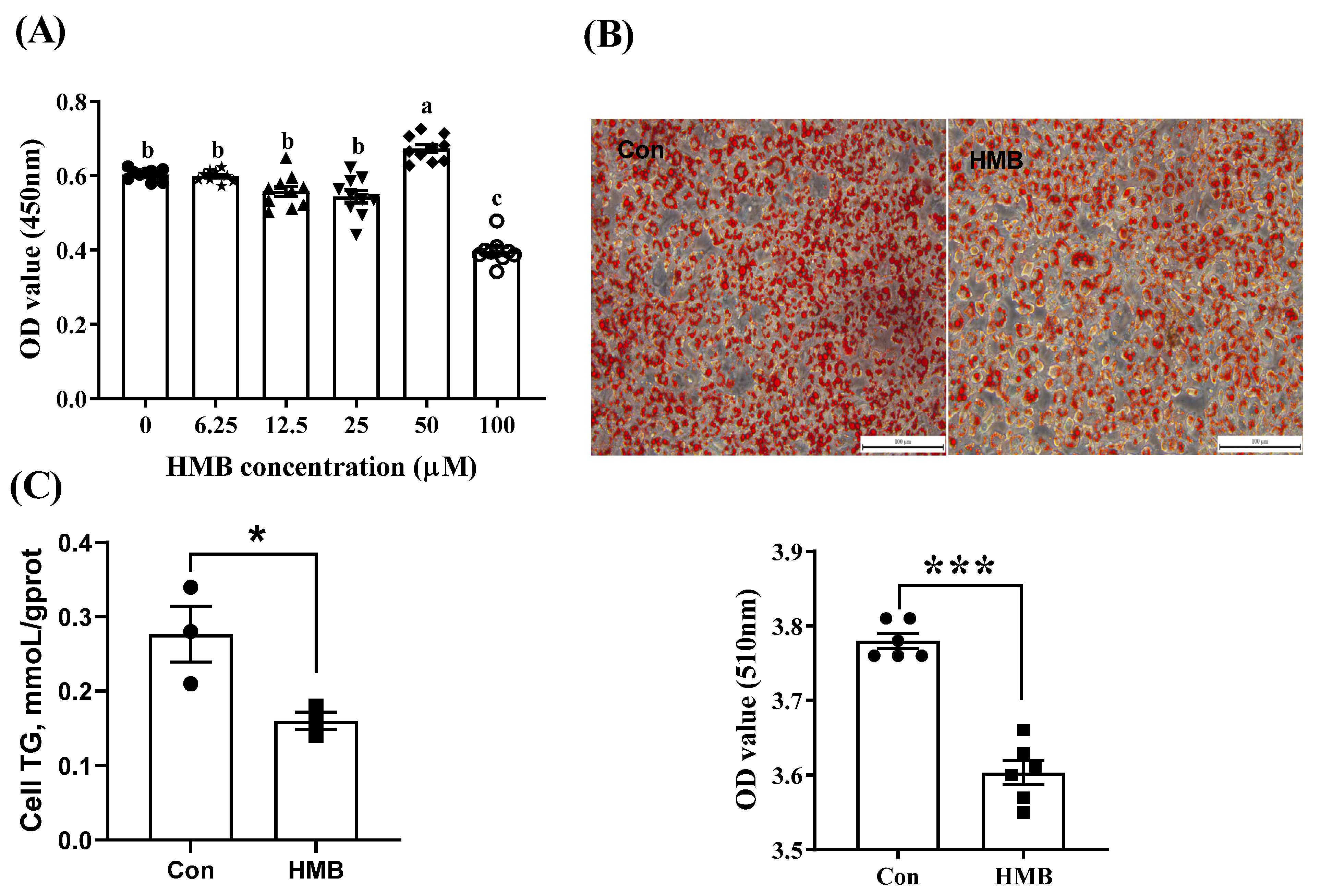
2.4. Oil Red O Staining
2.5. The Determination of TG Levels
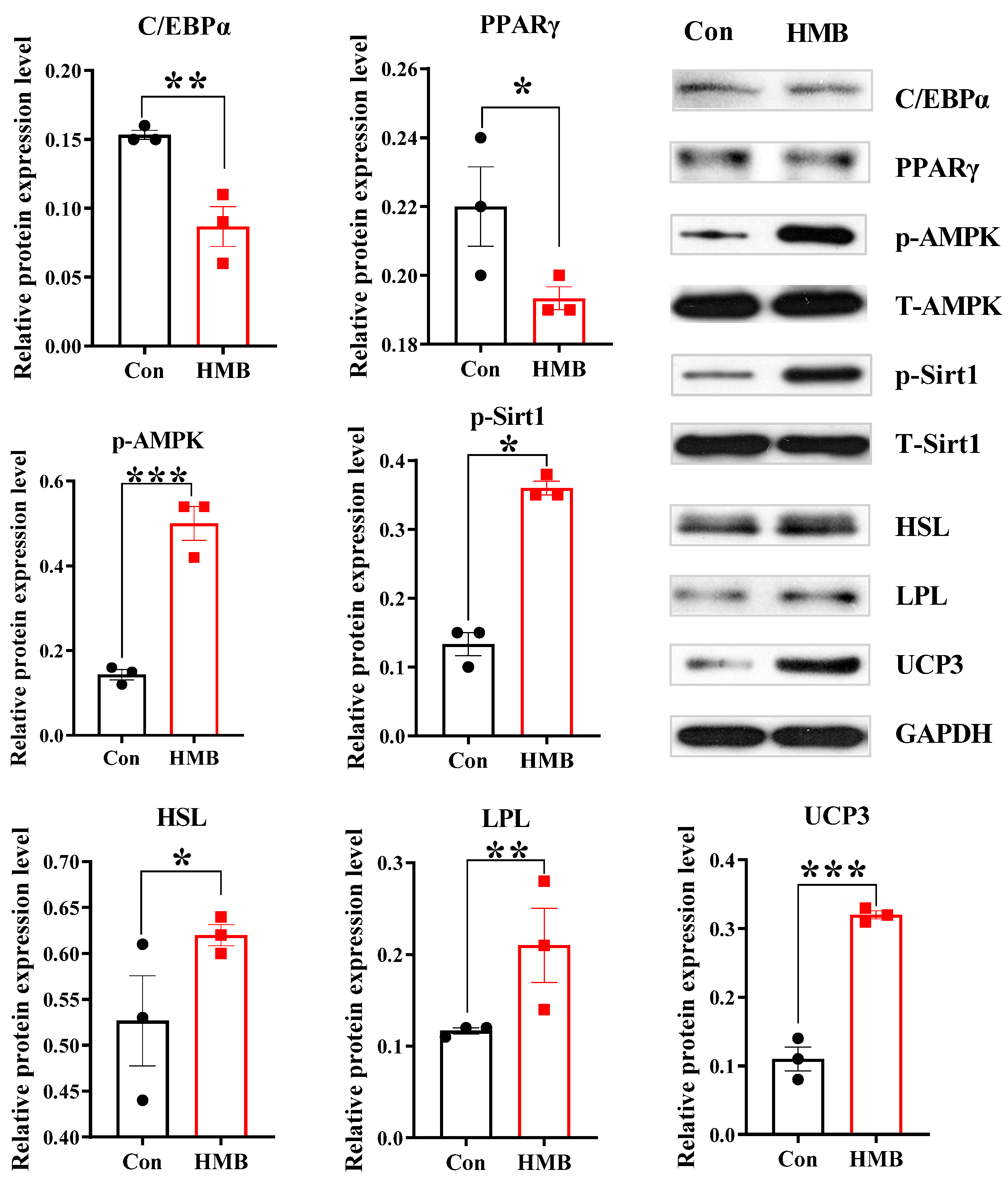
2.6. Western Blotting Analysis
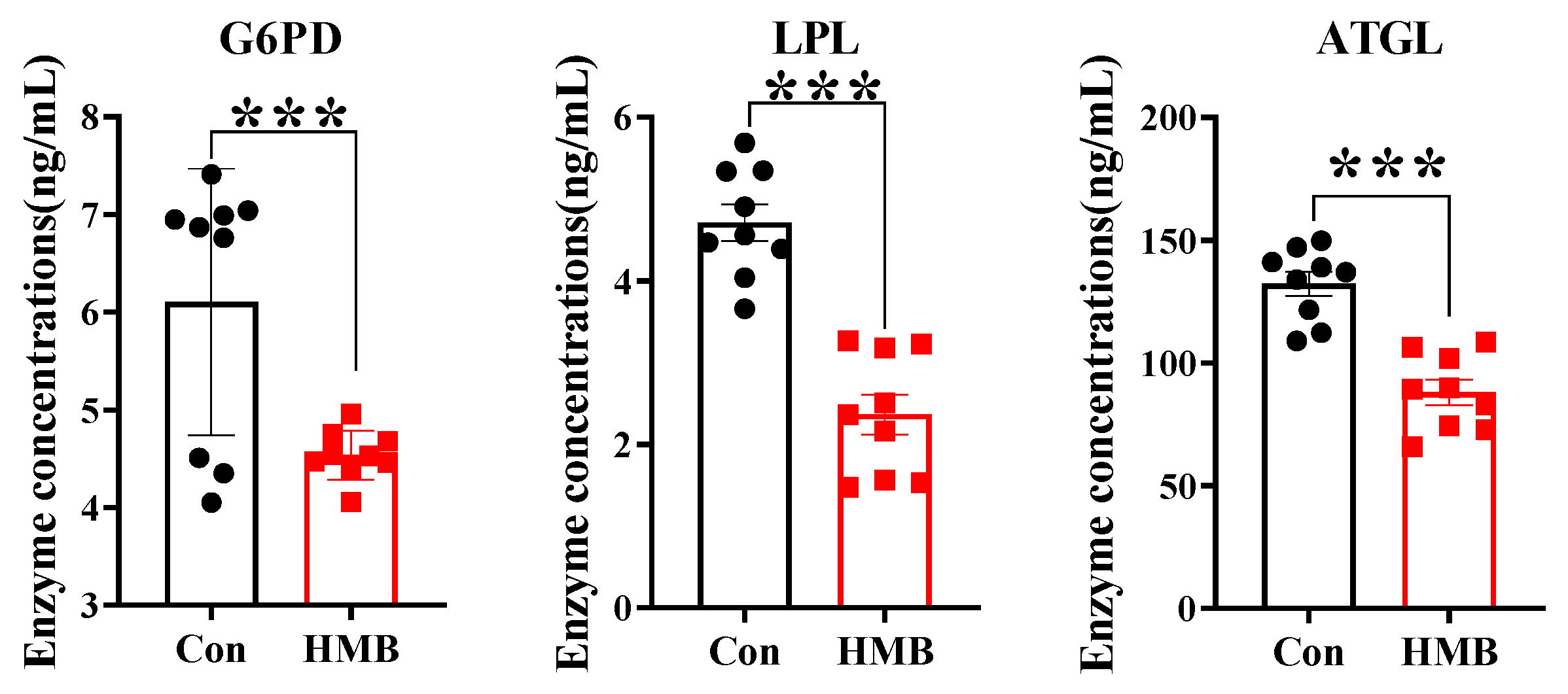
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Fatty Acid Composition Analysis
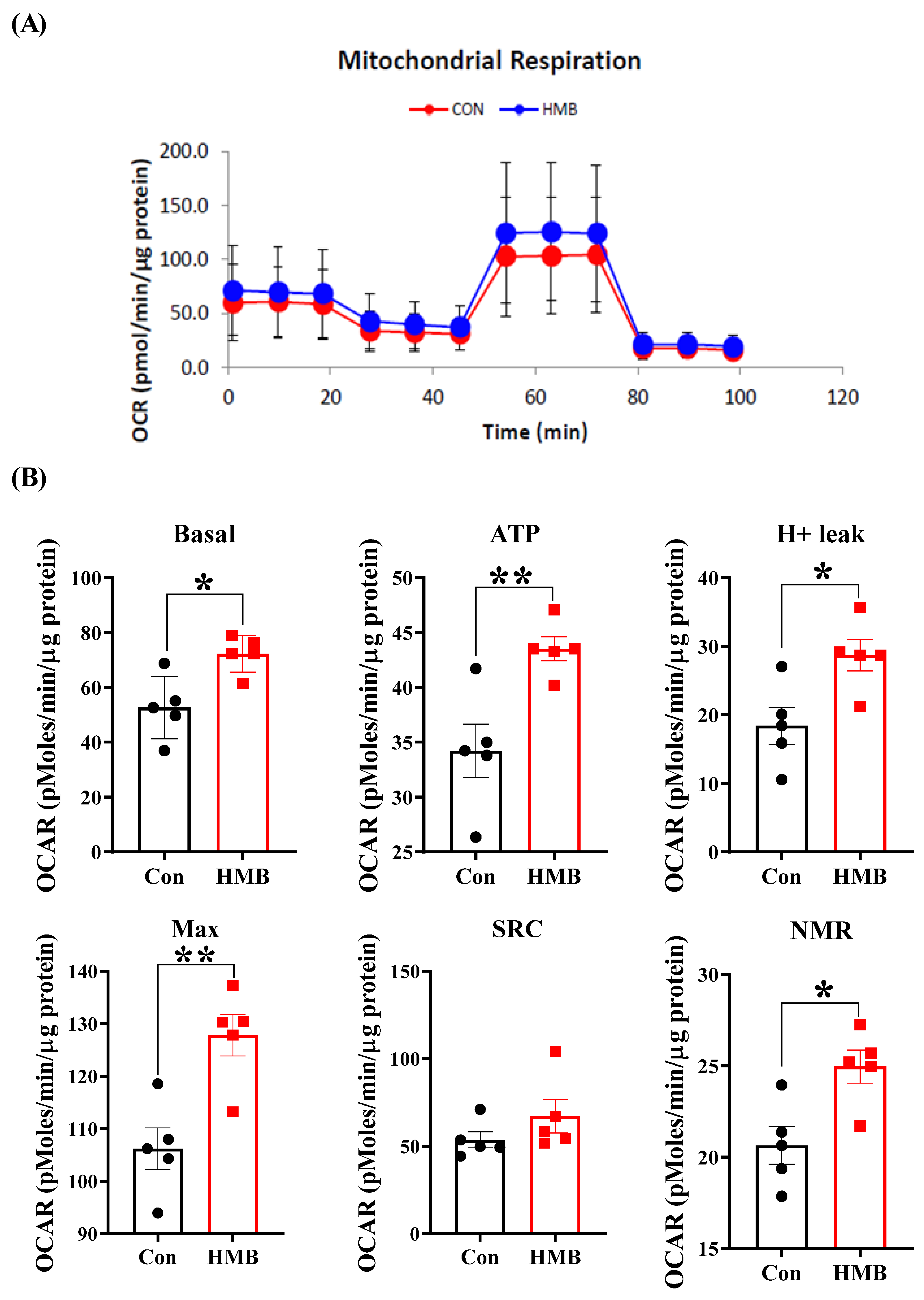
2.9. Seahorse Metabolic Assay
2.10. Statistical Analysis
3. Results
3.1. HMB Suppressed Lipid Accumulation in 3T3-L1 Adipocytes
3.2. HMB Altered the Concentrations of Lipid Metabolism-Related Enzymes and Fatty Acid Composition in 3T3-L1 Adipocytes
3.3. HMB Improved the Mitochondrial Respiratory Function of 3T3-L1 Adipocytes
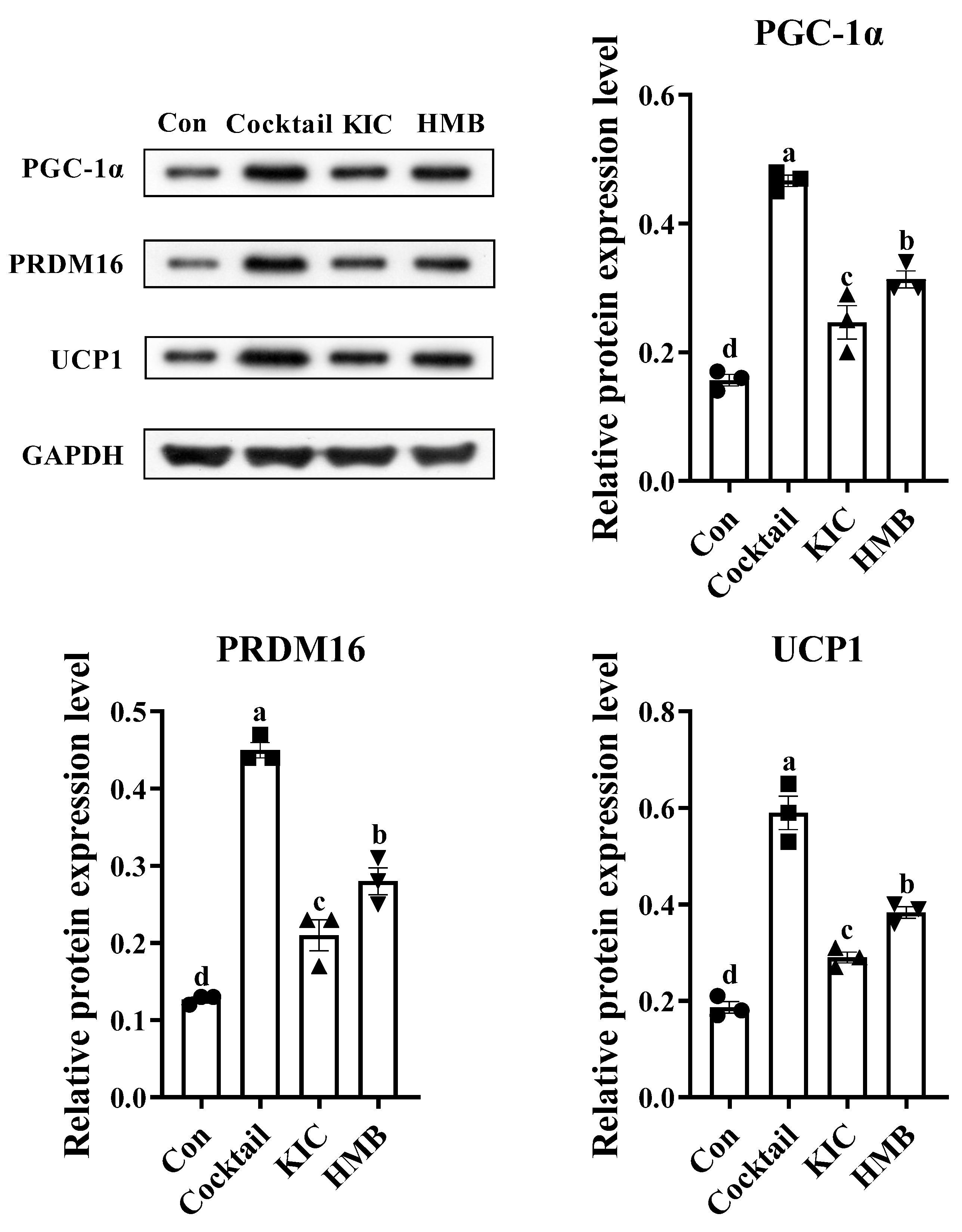
3.4. HMB Promoted the Browning of 3T3-L1 Adipocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, J.M.; Grant, S.C.; Lee, S.-R.; Masad, I.S.; Park, Y.-M.; Henning, P.C.; Stout, J.R.; Loenneke, J.P.; Arjmandi, B.H.; Panton, L.B.; et al. Beta-hydroxy-beta-methyl-butyrate blunts negative age-related changes in body composition, functionality and myofiber dimensions in rats. J. Int. Soc. Sport. Nutr. 2012, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-S.; Henning, P.C.; Grant, S.; Lee, W.J.; Lee, S.-R.; Arjmandi, B.H.; Kim, J.-S. HMB attenuates muscle loss during sustained energy deficit induced by calorie restriction and endurance exercise. Metabolism 2013, 62, 1718–1729. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zheng, C.; Song, B.; Guo, Q.; Zhong, Y.; Zhang, S.; Zhang, L.; Duan, G.; Li, F.; Duan, Y. HMB Improves Lipid Metabolism of Bama Xiang Mini-Pigs via Modulating the Bacteroidetes-Acetic Acid-AMPKα Axis. Front. Microbiol. 2021, 12, 736997. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhang, L.; Li, F.; Guo, Q.; Long, C.; Yin, Y.; Kong, X.; Peng, M.; Wang, W. β-Hydroxy-β-methylbutyrate modulates lipid metabolism in adipose tissues of growing pigs. Food Funct. 2018, 9, 4836–4846. [Google Scholar] [CrossRef]
- Duan, Y.; Zhong, Y.; Xiao, H.; Zheng, C.; Song, B.; Wang, W.; Guo, Q.; Li, Y.; Han, H.; Gao, J.; et al. Gut microbiota mediates the protective effects of dietary β-hydroxy-β-methylbutyrate (HMB) against obesity induced by high-fat diets. FASEB J. 2019, 33, 10019–10033. [Google Scholar] [CrossRef]
- Cedikova, M.; Kripnerová, M.; Dvorakova, J.; Pitule, P.; Grundmanova, M.; Babuska, V.; Mullerova, D.; Kuncova, J. Mitochondria in White, Brown, and Beige Adipocytes. Stem Cells Int. 2016, 2016, 6067349. [Google Scholar] [CrossRef]
- De Pauw, A.; Tejerina, S.; Raes, M.; Keijer, J.; Arnould, T. Mitochondrial (Dys)function in Adipocyte (De)differentiation and Systemic Metabolic Alterations. Am. J. Pathol. 2009, 175, 927–939. [Google Scholar] [CrossRef]
- White, C.R.; Kearney, M.R. Determinants of inter-specific variation in basal metabolic rate. J. Comp. Physiol. B 2013, 183, 1–26. [Google Scholar] [CrossRef]
- Heinonen, S.; Buzkova, J.; Muniandy, M.; Kaksonen, R.; Ollikainen, M.; Ismail, K.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Vuolteenaho, K.; et al. Impaired Mitochondrial Biogenesis in Adipose Tissue in Acquired Obesity. Diabetes 2015, 64, 3135–3145. [Google Scholar] [CrossRef]
- Fischer, B.; Schöttl, T.; Schempp, C.; Fromme, T.; Hauner, H.; Klingenspor, M.; Skurk, T. Inverse relationship between body mass index and mitochondrial oxidative phosphorylation capacity in human subcutaneous adipocytes. Am. J. Physiol.-Endocrinol. Metab. 2015, 309, E380–E387. [Google Scholar] [CrossRef]
- Yin, X.; Lanza, I.R.; Swain, J.M.; Sarr, M.G.; Nair, K.S.; Jensen, M.D. Adipocyte Mitochondrial Function Is Reduced in Human Obesity Independent of Fat Cell Size. J. Clin. Endocrinol. Metab. 2014, 99, E209–E216. [Google Scholar] [CrossRef]
- Schöttl, T.; Kappler, L.; Fromme, T.; Klingenspor, M. Limited OXPHOS capacity in white adipocytes is a hallmark of obesity in laboratory mice irrespective of the glucose tolerance status. Mol. Metab. 2015, 4, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, M.; GuhaThakurta, I.; Behera, P.; Ranjan, K.R.; Khanna, M.; Mukhopadhyay, S.; Chakrabarti, S. Mitochondrial bioenergetics is not impaired in nonobese subjects with type 2 diabetes mellitus. Metabolism 2011, 60, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Choo, H.-J.; Kim, J.-H.; Kwon, O.-B.; Lee, C.S.; Mun, J.Y.; Han, S.S.; Yoon, Y.-S.; Yoon, G.; Choi, K.-M.; Ko, Y.-G. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia 2006, 49, 784–791. [Google Scholar] [CrossRef]
- Ratner, C.; Madsen, A.N.; Kristensen, L.V.; Skov, L.J.; Pedersen, K.S.; Mortensen, O.H.; Knudsen, G.M.; Raun, K.; Holst, B. Impaired oxidative capacity due to decreased CPT1b levels as a contributing factor to fat accumulation in obesity. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2015, 308, R973–R982. [Google Scholar] [CrossRef]
- Giralt, M.; Villarroya, F. White, Brown, Beige/Brite: Different Adipose Cells for Different Functions? Endocrinology 2013, 154, 2992–3000. [Google Scholar] [CrossRef] [PubMed]
- Park, A.; Kim, W.K.; Bae, K.-H. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J. Stem Cells 2014, 6, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef]
- Bargut, T.C.L.; Souza-Mello, V.; Aguila, M.B.; Mandarim-De-Lacerda, C.A. Browning of white adipose tissue: Lessons from experimental models. Horm. Mol. Biol. Clin. Investig. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Kajimura, S.; Yang, W.; Chin, S.; Rohas, L.M.; Uldry, M.; Tavernier, G.; Langin, D.; Spiegelman, B.M. Transcriptional Control of Brown Fat Determination by PRDM16. Cell Metab. 2007, 6, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, S.; Seale, P.; Tomaru, T.; Erdjument-Bromage, H.; Cooper, M.P.; Ruas, J.L.; Chin, S.; Tempst, P.; Lazar, M.A.; Spiegelman, B.M. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes Dev. 2008, 22, 1397–1409. [Google Scholar] [CrossRef]
- Ohno, H.; Shinoda, K.; Spiegelman, B.M.; Kajimura, S. PPARγ agonists Induce a White-to-Brown Fat Conversion through Stabilization of PRDM16 Protein. Cell Metab. 2012, 15, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A Cold-Inducible Coactivator of Nuclear Receptors Linked to Adaptive Thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef]
- Asano, H.; Kanamori, Y.; Higurashi, S.; Nara, T.; Kato, K.; Matsui, T.; Funaba, M. Induction of Beige-like Adipocytes in 3T3-L1 Cells. J. Vet.-Med. Sci. 2014, 76, 57–64. [Google Scholar] [CrossRef]
- Li, Y.; Yang, P.; Chang, Q.; Wang, J.; Liu, J.; Lv, Y.; Wang, T.T.Y.; Gao, B.; Zhang, Y.; Yu, L.L. Inhibitory Effect of Piceatannol on TNF-α-Mediated Inflammation and Insulin Resistance in 3T3-L1 Adipocytes. J. Agric. Food Chem. 2017, 65, 4634–4641. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Duan, Y.; Zheng, C.; Yu, J.; Li, F.; Guo, Q.; Yin, Y. Long-Term Protein Restriction Modulates Lipid Metabolism in White Adipose Tissues and Alters Colonic Microbiota of Shaziling Pigs. Animals 2022, 12, 2944. [Google Scholar] [CrossRef]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Rho, H.K.; Kim, K.H.; Choe, S.S.; Lee, Y.S.; Kim, J.B. Overexpression of Glucose-6-Phosphate Dehydrogenase Is Associated with Lipid Dysregulation and Insulin Resistance in Obesity. Mol. Cell. Biol. 2005, 25, 5146–5157. [Google Scholar] [CrossRef]
- Park, Y.J.; Choe, S.S.; Sohn, J.H.; Kim, J.B. The role of glucose-6-phosphate dehydrogenase in adipose tissue inflammation in obesity. Adipocyte 2017, 6, 147–153. [Google Scholar] [CrossRef]
- Wang, H.; Eckel, R.H. Lipoprotein lipase: From gene to obesity. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E271–E288. [Google Scholar] [CrossRef]
- Patel, R.; Santoro, A.; Hofer, P.; Tan, D.; Oberer, M.; Nelson, A.T.; Konduri, S.; Siegel, D.; Zechner, R.; Saghatelian, A.; et al. ATGL is a biosynthetic enzyme for fatty acid esters of hydroxy fatty acids. Nature 2022, 606, 968–975. [Google Scholar] [CrossRef]
- Sun, X.; Zemel, M.B. Leucine and Calcium Regulate Fat Metabolism and Energy Partitioning in Murine Adipocytes and Muscle Cells. Lipids 2007, 42, 297–305. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Gerhart-Hines, Z.; Rodgers, J.T.; Bare, O.; Lerin, C.; Kim, S.-H.; Mostoslavsky, R.; Alt, F.W.; Wu, Z.; Puigserver, P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α. EMBO J. 2007, 26, 1913–1923. [Google Scholar] [CrossRef]
- Picard, F.; Kurtev, M.; Chung, N.; Topark-Ngarm, A.; Senawong, T.; De Oliveira, R.M.; Leid, M.; McBurney, M.W.; Guarente, L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature 2004, 429, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Recazens, E.; Mouisel, E.; Langin, D. Hormone-sensitive lipase: Sixty years later. Prog. Lipid Res. 2021, 82, 101084. [Google Scholar] [CrossRef] [PubMed]
- Pohl, E.E.; Rupprecht, A.; Macher, G.; Hilse, K.E. Important Trends in UCP3 Investigation. Front. Physiol. 2019, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Hernandez-Morante, J.J.; Lujan, J.; Tebar, F.J.; Zamora, S. Relationship between fat cell size and number and fatty acid composition in adipose tissue from different fat depots in overweight/obese humans. Int. J. Obes. 2006, 30, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-Y.; Wang, J.; Lee, Y.; Lee, J.; Kwon, K.-S.; Bae, E.J.; Park, B.-H. Enhanced M2 macrophage polarization in high n-3 polyunsaturated fatty acid transgenic mice fed a high-fat diet. Mol. Nutr. Food Res. 2016, 60, 2481–2492. [Google Scholar] [CrossRef]
- Li, J.; Li, F.R.; Wei, D.; Jia, W.; Kang, J.X.; Stefanovic-Racic, M.; Dai, Y.; Zhao, A.Z. Endogenous ω-3 Polyunsaturated Fatty Acid Production Confers Resistance to Obesity, Dyslipidemia, and Diabetes in Mice. Mol. Endocrinol. 2014, 28, 1316–1328. [Google Scholar] [CrossRef]
- van der Bliek, A.M.; Sedensky, M.M.; Morgan, P.G. Cell Biology of the Mitochondrion. Genetics 2017, 207, 843–871. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zeng, L.; Deng, J.; Duan, Y.; Li, F. β-hydroxy-β-methylbutyrate (HMB) improves mitochondrial function in myocytes through pathways involving PPARβ/δ and CDK4. Nutrition 2019, 60, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Stancliffe, R.A. Role of Beta-Hydroxy-Beta-Methylbutyrate (HMB) in Leucine Stimulation of Mitochondrial Biogenesis and Fatty Acid Oxidation. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 2012. [Google Scholar]
- Sun, X.; Zemel, M.B. Leucine modulation of mitochondrial mass and oxygen consumption in skeletal muscle cells and adipocytes. Nutr. Metab. 2009, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Finck, B.N.; Kelly, D.P. PGC-1 coactivators: Inducible regulators of energy metabolism in health and disease. J. Clin. Investig. 2006, 116, 615–622. [Google Scholar] [CrossRef]
- Cao, W.; Daniel, K.W.; Robidoux, J.; Puigserver, P.; Medvedev, A.V.; Bai, X.; Floering, L.M.; Spiegelman, B.M.; Collins, S. p38 Mitogen-Activated Protein Kinase Is the Central Regulator of Cyclic AMP-Dependent Transcription of the Brown Fat Uncoupling Protein 1 Gene. Mol. Cell. Biol. 2004, 24, 3057–3067. [Google Scholar] [CrossRef]
- Duan, Y.; Li, F.; Li, Y.; Tang, Y.; Kong, X.; Feng, Z.; Anthony, T.G.; Watford, M.; Hou, Y.; Wu, G.; et al. The role of leucine and its metabolites in protein and energy metabolism. Amino Acids 2016, 48, 41–51. [Google Scholar] [CrossRef]
- Duan, Y.; Zhong, Y.; Song, B.; Zheng, C.; Xu, K.; Kong, X.; Li, F. Suppression of protein degradation by leucine requires its conversion to β-hydroxy-β-methyl butyrate in C2C12 myotubes. Aging 2019, 11, 11922–11936. [Google Scholar] [CrossRef]
- Duan, Y.; Li, F.; Guo, Q.; Wang, W.; Zhang, L.; Wen, C.; Chen, X.; Yin, Y. β-Hydroxy-β-methyl Butyrate Is More Potent Than Leucine in Inhibiting Starvation-Induced Protein Degradation in C2C12 Myotubes. J. Agric. Food Chem. 2017, 66, 170–176. [Google Scholar] [CrossRef]
- Kaisanlahti, A.; Glumoff, T. Browning of white fat: Agents and implications for beige adipose tissue to type 2 diabetes. J. Physiol. Biochem. 2019, 75, 1–10. [Google Scholar] [CrossRef]
- Chevalier, C.; Stojanović, O.; Colin, D.J.; Suarez-Zamorano, N.; Tarallo, V.; Veyrat-Durebex, C.; Rigo, D.; Fabbiano, S.; Stevanović, A.; Hagemann, S.; et al. Gut Microbiota Orchestrates Energy Homeostasis during Cold. Cell 2015, 163, 1360–1374. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Tomiya, S.; Takegaki, J.; Kouzaki, K.; Tsutaki, A.; Nakazato, K. Apple polyphenols induce browning of white adipose tissue. J. Nutr. Biochem. 2020, 77, 108299. [Google Scholar] [CrossRef] [PubMed]
- Hammarstedt, A.; Gogg, S.; Hedjazifar, S.; Nerstedt, A.; Smith, U. Impaired Adipogenesis and Dysfunctional Adipose Tissue in Human Hypertrophic Obesity. Physiol. Rev. 2018, 98, 1911–1941. [Google Scholar] [CrossRef] [PubMed]
| Item | Control | HMB | SEM | p-Value |
|---|---|---|---|---|
| C10:0 | 0.61 b | 0.90 a | 0.00076 | <0.05 |
| C14:0 | 3.71 a | 2.68 b | 0.00254 | <0.05 |
| C15:0 | 8.12 a | 5.45 b | 0.00655 | <0.05 |
| C16:0 | 31.71 | 35.24 | 0.01101 | 0.11 |
| C16:1 | 21.38 a | 12.60 b | 0.02117 | <0.01 |
| C17:0 | 2.41 a | 2.06 b | 0.00090 | <0.05 |
| C18:0 | 12.31 b | 21.28 a | 0.02119 | <0.01 |
| C18:1n9c | 13.06 | 11.96 | 0.00739 | 0.55 |
| C22:1n9 | 2.53 | 4.10 | 0.00502 | 0.12 |
| C20:4n6 | 1.56 b | 2.15 a | 0.00132 | <0.001 |
| C22:6n3 | 0.68 b | 0.93 a | 0.00057 | <0.001 |
| ∑n6 PUFAs | 1.56 b | 2.15 a | 0.00132 | <0.001 |
| ∑n3 PUFAs | 0.68 b | 0.93 a | 0.00057 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, G.; Zheng, C.; Yu, J.; Zhang, P.; Wan, M.; Zheng, J.; Duan, Y. β-Hydroxy-β-methyl Butyrate Regulates the Lipid Metabolism, Mitochondrial Function, and Fat Browning of Adipocytes. Nutrients 2023, 15, 2550. https://doi.org/10.3390/nu15112550
Duan G, Zheng C, Yu J, Zhang P, Wan M, Zheng J, Duan Y. β-Hydroxy-β-methyl Butyrate Regulates the Lipid Metabolism, Mitochondrial Function, and Fat Browning of Adipocytes. Nutrients. 2023; 15(11):2550. https://doi.org/10.3390/nu15112550
Chicago/Turabian StyleDuan, Geyan, Changbing Zheng, Jiayi Yu, Peiwen Zhang, Mengliao Wan, Jie Zheng, and Yehui Duan. 2023. "β-Hydroxy-β-methyl Butyrate Regulates the Lipid Metabolism, Mitochondrial Function, and Fat Browning of Adipocytes" Nutrients 15, no. 11: 2550. https://doi.org/10.3390/nu15112550
APA StyleDuan, G., Zheng, C., Yu, J., Zhang, P., Wan, M., Zheng, J., & Duan, Y. (2023). β-Hydroxy-β-methyl Butyrate Regulates the Lipid Metabolism, Mitochondrial Function, and Fat Browning of Adipocytes. Nutrients, 15(11), 2550. https://doi.org/10.3390/nu15112550






