Human Milk Oligosaccharide Profile across Lactation Stages in Israeli Women—A Prospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Human Milk Samples
2.2.1. Chemical Analysis and OS Composition
2.2.2. LC-MS Analysis
2.2.3. GC-MS Analysis
2.3. Statistical Analysis
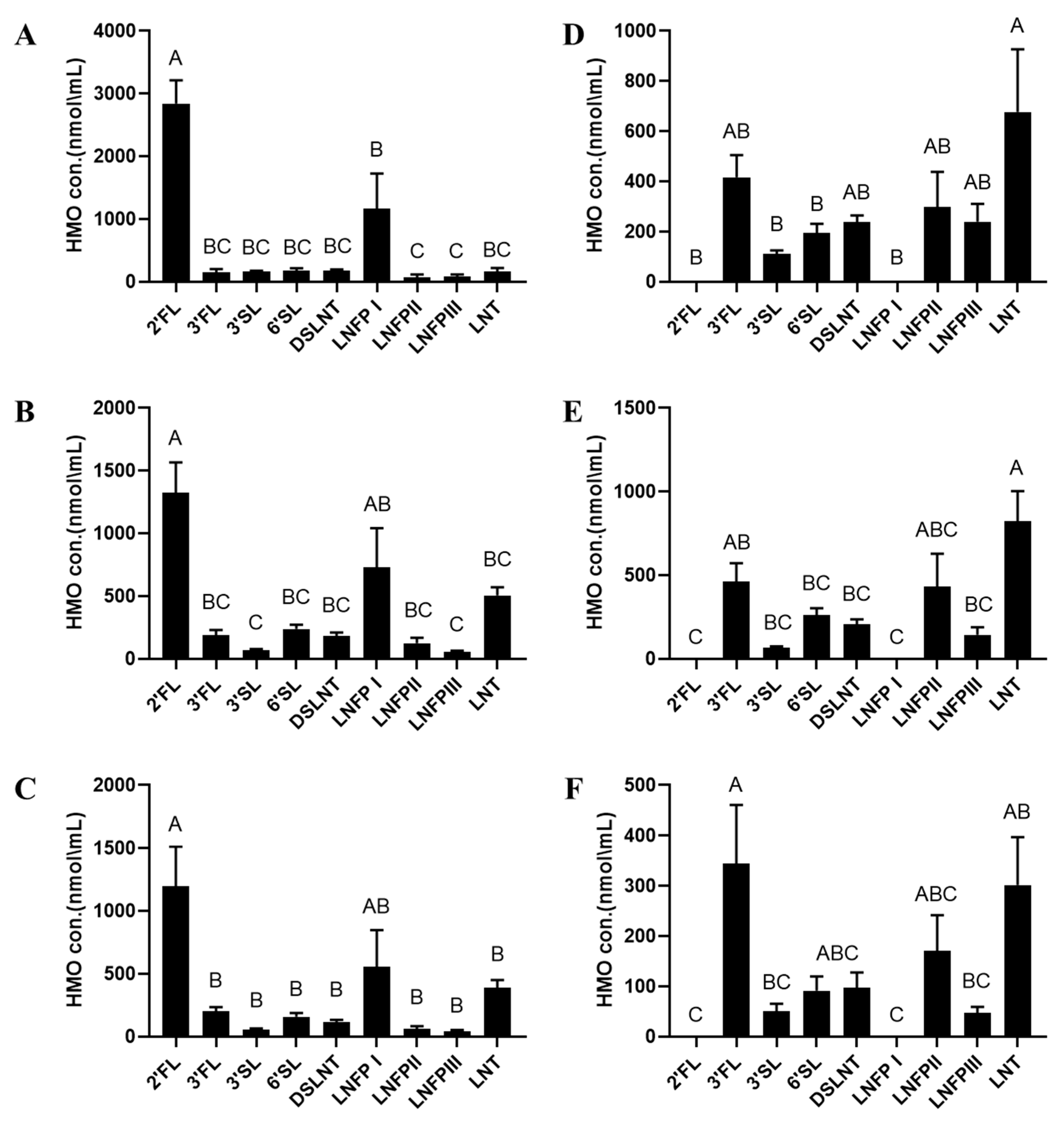
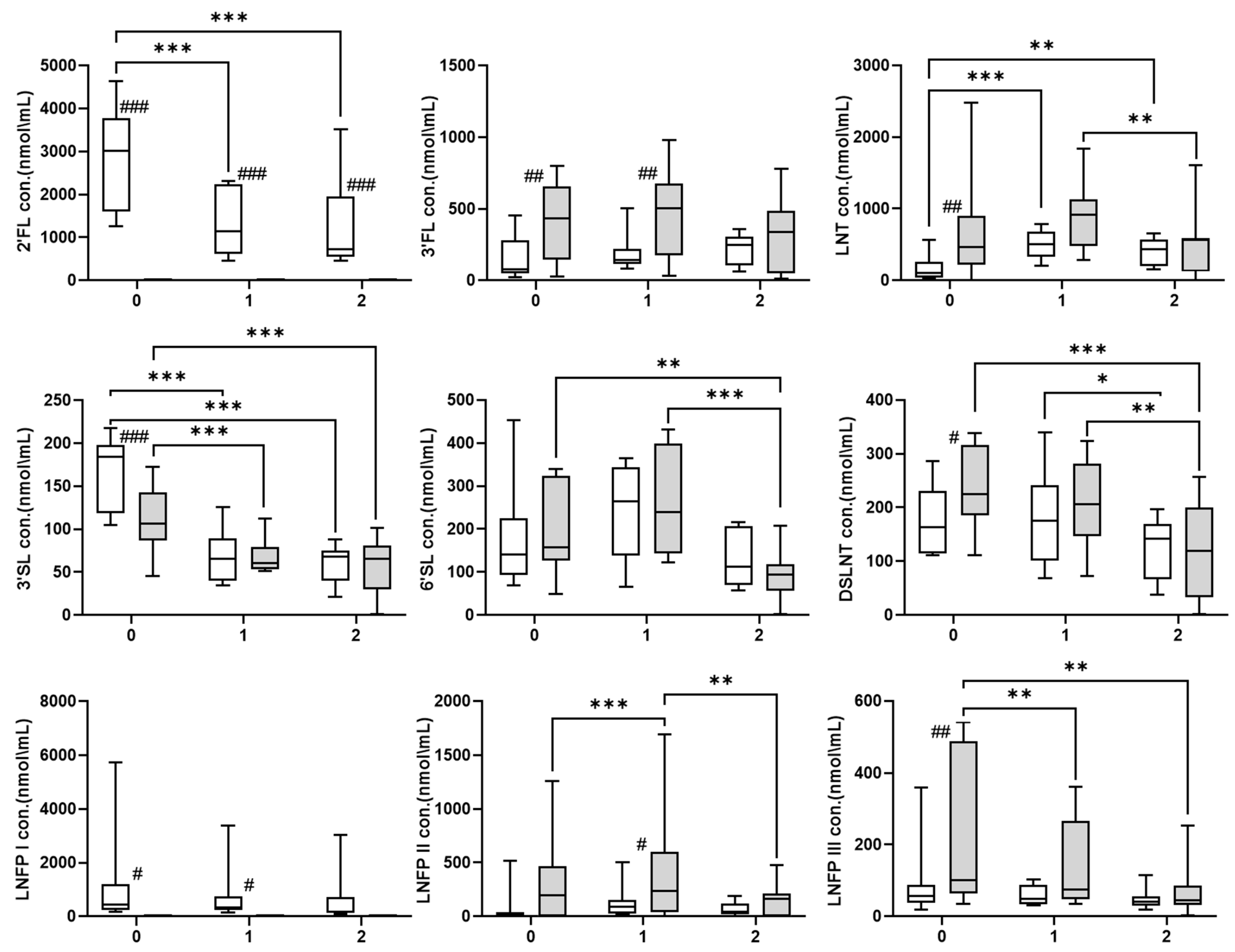
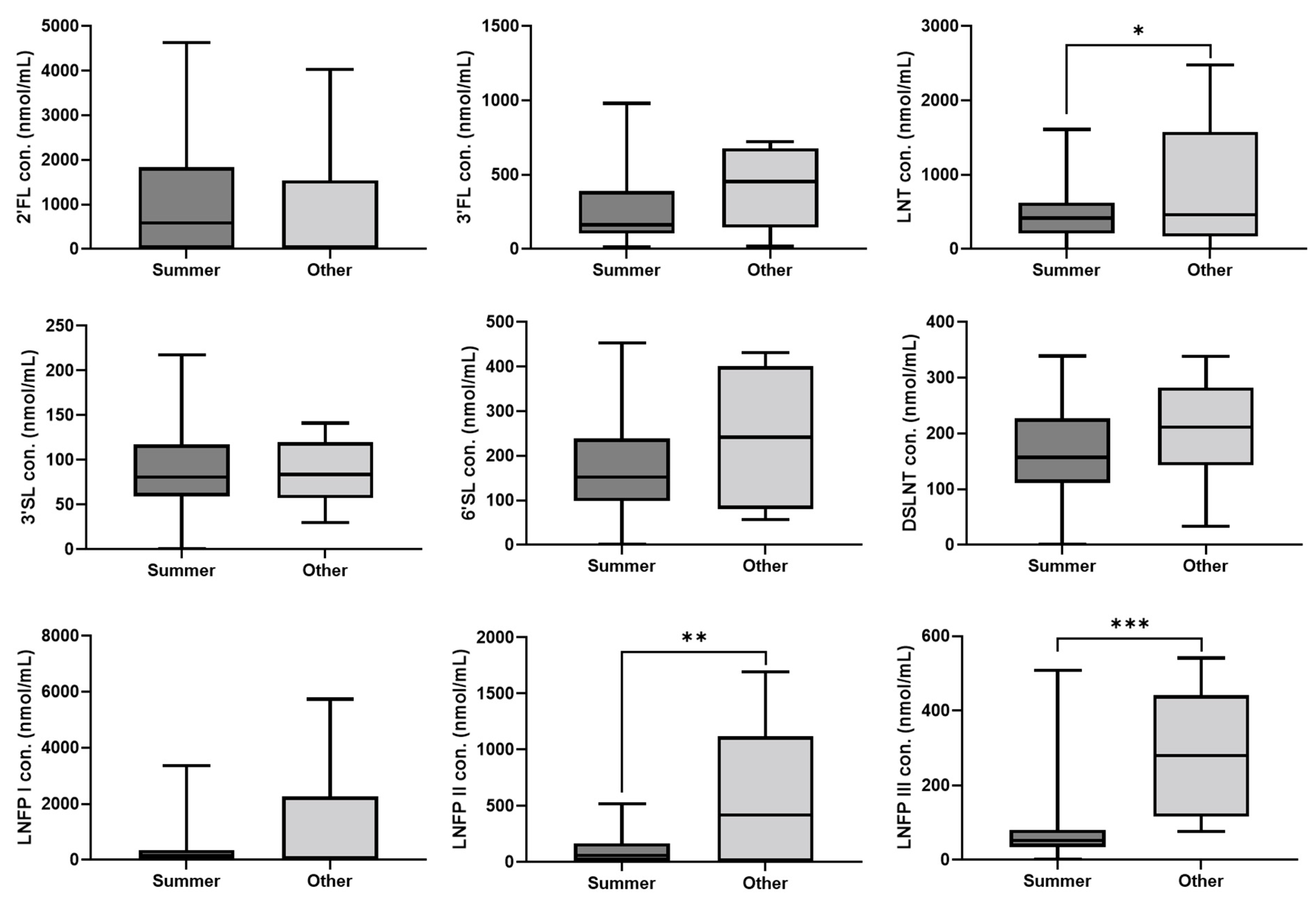
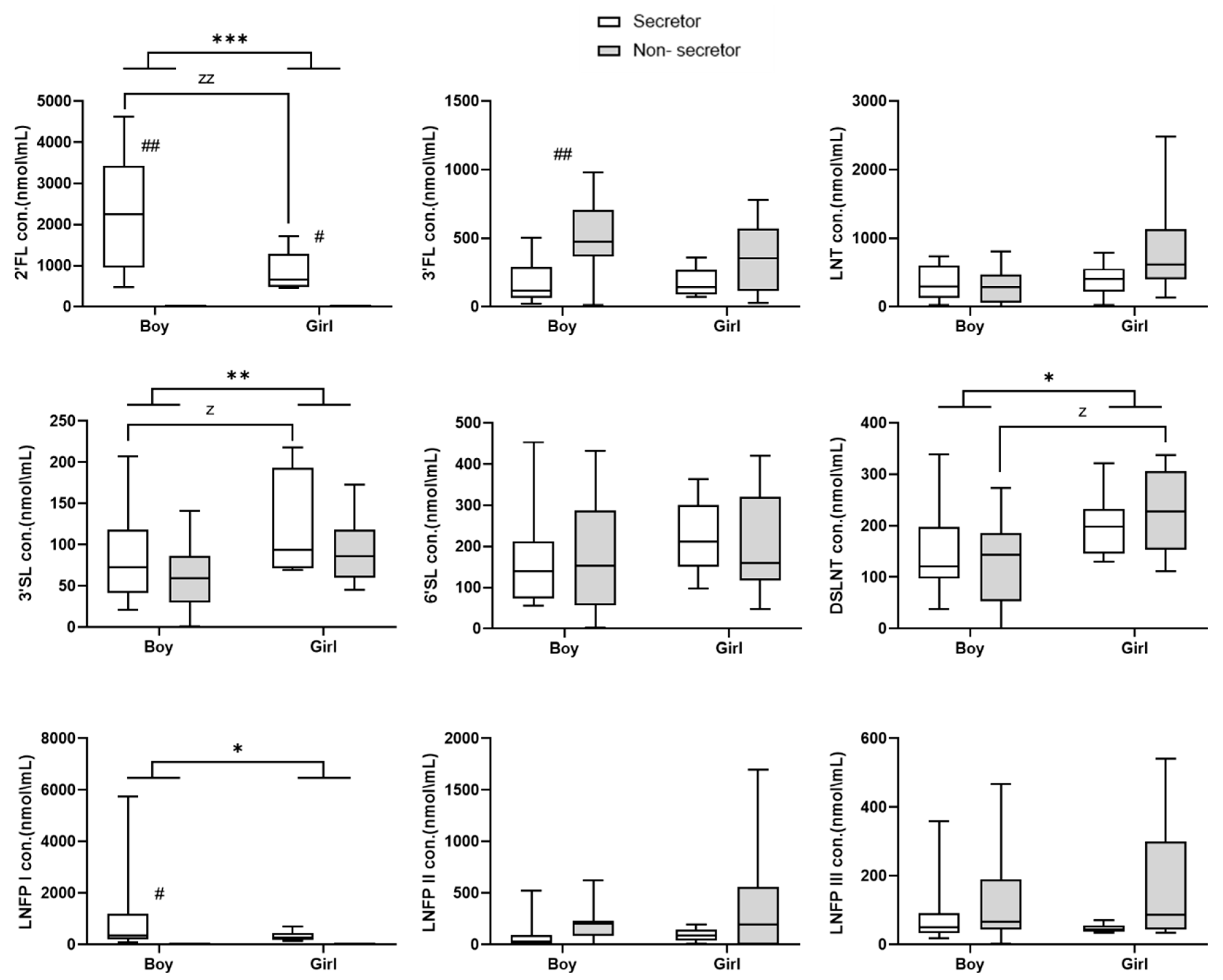
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Discussion Paper—The Extension of the 2025 Maternal, Infant and Young Child Nutrition Targets to 2030; WHO: Geneva, Switzerland, 2017; pp. 1–12. [Google Scholar]
- Gidrewicz, D.A.; Fenton, T.R. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014, 14, 216. [Google Scholar] [CrossRef]
- Wu, R.Y.; Li, B.; Koike, Y.; Määttänen, P.; Miyake, H.; Cadete, M.; Johnson-Henry, K.C.; Botts, S.R.; Lee, C.; Abrahamsson, T.R.; et al. Human Milk Oligosaccharides Increase Mucin Expression in Experimental Necrotizing Enterocolitis. Mol. Nutr. Food Res. 2019, 63, e1800658. [Google Scholar] [CrossRef]
- Triantis, V.; Bode, L.; van Neerven, R.J. Immunological Effects of Human Milk Oligosaccharides. Front. Pediatr. 2018, 6, 190. [Google Scholar] [CrossRef] [PubMed]
- Ashline, D.J.; Yu, Y.; Lasanajak, Y.; Song, X.; Hu, L.; Ramani, S.; Prasad, V.; Estes, M.K.; Cummings, R.D.; Smith, D.F.; et al. Structural Characterization by Multistage Mass Spectrometry (MSn) of Human Milk Glycans Recognized by Human Rotaviruses. Mol. Cell. Proteom. 2014, 13, 2961–2974. [Google Scholar] [CrossRef]
- Remoroza, C.A.; Mak, T.D.; De Leoz, M.L.A.; Mirokhin, Y.A.; Stein, S.E. Creating a Mass Spectral Reference Library for Oligosaccharides in Human Milk. Anal. Chem. 2018, 90, 8977–8988. [Google Scholar] [CrossRef]
- Urashima, T.; Hirabayashi, J.; Sato, S.; Kobata, A. Human Milk Oligosaccharides as Essential Tools for Basic and Application Studies on Galectins. Trends Glycosci. Glycotechnol. 2018, 30, SE51–SE65. [Google Scholar] [CrossRef]
- Urashima, T.; Katayama, T.; Fukuda, K.; Hirabayashi, J. Human Milk Oligosaccharides and Innate Immunity. In Comprehensive Glycoscience, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 389–439. [Google Scholar] [CrossRef]
- Thurl, S.; Munzert, M.; Boehm, G.; Matthews, C.; Stahl, B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr. Rev. 2017, 75, 920–933. [Google Scholar] [CrossRef]
- Conze, D.B.; Kruger, C.L.; Symonds, J.M.; Lodder, R.; Schönknecht, Y.B.; Ho, M.; Derya, S.M.; Parkot, J.; Parschat, K. Weighted analysis of 2′-fucosyllactose, 3-fucosyllactose, lacto-N-tetraose, 3′-sialyllactose, and 6′-sialyllactose concentrations in human milk. Food Chem. Toxicol. 2022, 163, 112877. [Google Scholar] [CrossRef]
- Sánchez, C.; Fente, C.; Regal, P.; Lamas, A.; Lorenzo, M.P. Human Milk Oligosaccharides (HMOs) and Infant Microbiota: A Scoping Review. Foods 2021, 10, 1429. [Google Scholar] [CrossRef]
- Elwakiel, M.; Hageman, J.A.; Wang, W.; Szeto, I.; van Goudoever, J.B.; Hettinga, K.A.; Schols, H.A. Human Milk Oligosaccharides in Colostrum and Mature Milk of Chinese Mothers: Lewis Positive Secretor Subgroups. J. Agric. Food Chem. 2018, 66, 7036–7043. [Google Scholar] [CrossRef]
- Totten, S.M.; Zivkovic, A.M.; Wu, S.; Ngyuen, U.; Freeman, S.L.; Ruhaak, L.R.; Darboe, M.K.; German, J.B.; Prentice, A.M.; Lebrilla, C.B. Comprehensive Profiles of Human Milk Oligosaccharides Yield Highly Sensitive and Specific Markers for Determining Secretor Status in Lactating Mothers. J. Proteome Res. 2012, 11, 6124–6133. [Google Scholar] [CrossRef] [PubMed]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.L.; Alves, R.; Figueiredo, A.; Alves-Santos, N.; Freitas-Costa, N.; Batalha, M.; Yonemitsu, C.; Manivong, N.; Furst, A.; Bode, L.; et al. Human Milk Oligosaccharide Profile Variation Throughout Postpartum in Healthy Women in a Brazilian Cohort. Nutrients 2020, 12, 790. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Robertson, B.; Atakora, F.; Becker, A.B.; Subbarao, P.; Moraes, T.J.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; et al. Human Milk Oligosaccharide Concentrations Are Associated with Multiple Fixed and Modifiable Maternal Characteristics, Environmental Factors, and Feeding Practices. J. Nutr. 2018, 148, 1733–1742. [Google Scholar] [CrossRef]
- Korpela, K.; Salonen, A.; Hickman, B.; Kunz, C.; Sprenger, N.; Kukkonen, K.; Savilahti, E.; Kuitunen, M.; de Vos, W.M. Fucosylated oligosaccharides in mother’s milk alleviate the effects of caesarean birth on infant gut microbiota. Sci. Rep. 2018, 8, 13757. [Google Scholar] [CrossRef] [PubMed]
- Samuel, T.M.; Binia, A.; De Castro, C.A.; Thakkar, S.K.; Billeaud, C.; Agosti, M.; Al-Jashi, I.; Costeira, M.J.; Marchini, G.; Martínez-Costa, C.; et al. Impact of maternal characteristics on human milk oligosaccharide composition over the first 4 months of lactation in a cohort of healthy European mothers. Sci. Rep. 2019, 9, 11767. [Google Scholar] [CrossRef] [PubMed]
- Lahdenperä, M.; Galante, L.; Gonzales-Inca, C.; Vahtera, J.; Pentti, J.; Rautava, S.; Käyhkö, N.; Yonemitsu, C.; Gupta, J.; Bode, L.; et al. Residential green environments are associated with human milk oligosaccharide diversity and composition. Sci. Rep. 2023, 13, 216. [Google Scholar] [CrossRef]
- Plows, J.F.; Berger, P.K.; Jones, R.B.; Alderete, T.L.; Yonemitsu, C.; A Najera, J.; Khwajazada, S.; Bode, L.; I Goran, M. Longitudinal Changes in Human Milk Oligosaccharefides (HMOs) Over the Course of 24 Months of Lactation. J. Nutr. 2021, 151, 876–882. [Google Scholar] [CrossRef]
- Austin, S.; De Castro, C.A.; Bénet, T.; Hou, Y.; Sun, H.; Thakkar, S.K.; Vinyes-Pares, G.; Zhang, Y.; Wang, P. Temporal Change of the Content of 10 Oligosaccharides in the Milk of Chinese Urban Mothers. Nutrients 2016, 8, 346. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Warren, C.D.; Altaye, M.; Morrow, A.L.; Ruiz-Palacios, G.; Pickering, L.K.; Newburg, D.S. Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology 2001, 11, 365–372. [Google Scholar] [CrossRef]
- van Leeuwen, S.S.; Stoutjesdijk, E.; Kate, G.A.T.; Schaafsma, A.; Dijck-Brouwer, J.; Muskiet, F.A.J.; Dijkhuizen, L. Regional variations in human milk oligosaccharides in Vietnam suggest FucTx activity besides FucT2 and FucT3. Sci. Rep. 2018, 8, 16790. [Google Scholar] [CrossRef]
- Leo, F.; Asakuma, S.; Fukuda, K.; Senda, A.; Urashima, T. Determination of Sialyl and Neutral Oligosaccharide Levels in Transition and Mature Milks of Samoan Women, Using Anthranilic Derivatization Followed by Reverse Phase High Performance Liquid Chromatography. Biosci. Biotechnol. Biochem. 2010, 74, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.; De Castro, C.A.; Sprenger, N.; Binia, A.; Affolter, M.; Garcia-Rodenas, C.L.; Beauport, L.; Tolsa, J.-F.; Fumeaux, C.J.F. Human Milk Oligosaccharides in the Milk of Mothers Delivering Term versus Preterm Infants. Nutrients 2019, 11, 1282. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Berger, B.; Carnielli, V.P.; Ksiazyk, J.; Lagström, H.; Sanchez Luna, M.; Migacheva, N.; Mosselmans, J.-M.; Picaud, J.-C.; Possner, M.; et al. Human Milk Oligosaccharides: 2′-Fucosyllactose (2′-FL) and Lacto-N-Neotetraose (LNnT) in Infant Formula. Nutrients 2018, 10, 1161. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, M.; Guo, H.; Yan, J.; Liu, F.; Chen, J.; Li, Y.; Ren, F. Human Milk Oligosaccharides Protect against Necrotizing Enterocolitis by Inhibiting Intestinal Damage via Increasing the Proliferation of Crypt Cells. Mol. Nutr. Food Res. 2019, 63, e1900262. [Google Scholar] [CrossRef]
- Pell, L.G.; Ohuma, E.; Yonemitsu, C.; Loutet, M.G.; Ahmed, T.; Al Mahmud, A.; Azad, M.B.; Bode, L.; E Roth, D. The Human-Milk Oligosaccharide Profile of Lactating Women in Dhaka, Bangladesh. Curr. Dev. Nutr. 2021, 5, nzab137. [Google Scholar] [CrossRef]
- Menzel, P.; Vogel, M.; Austin, S.; Sprenger, N.; Grafe, N.; Hilbert, C.; Jurkutat, A.; Kiess, W.; Binia, A. Concentrations of oligosaccharides in human milk and child growth. BMC Pediatr. 2021, 21, 481. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Z.; Zhao, A.; Zhang, J.; Wu, W.; Ren, Z.; Wang, P.; Zhang, Y. Neutral Human Milk Oligosaccharides Are Associated with Multiple Fixed and Modifiable Maternal and Infant Characteristics. Nutrients 2020, 12, 826. [Google Scholar] [CrossRef]
- McGuire, M.K.; Meehan, C.L.; A McGuire, M.; Williams, J.; Foster, J.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.; et al. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am. J. Clin. Nutr. 2017, 105, 1086–1100. [Google Scholar] [CrossRef]
- Walsh, C.; Lane, J.A.; van Sinderen, D.; Hickey, R.M. From lab bench to formulated ingredient: Characterization, production, and commercialization of human milk oligosaccharides. J. Funct. Foods 2020, 72, 104052. [Google Scholar] [CrossRef]
- Liu, F.; He, S.; Yan, J.; Yan, S.; Chen, J.; Lu, Z.; Zhang, B.; Lane, J. Longitudinal changes of human milk oligosaccharides, breastmilk microbiome and infant gut microbiome are associated with maternal characteristics. Int. J. Food Sci. Technol. 2021, 57, 2793–2807. [Google Scholar] [CrossRef]
- Thurl, S.; Munzert, M.; Henker, J.; Boehm, G.; Müller-Werner, B.; Jelinek, J.; Stahl, B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br. J. Nutr. 2010, 104, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Zinger-Yosovich, K.; Iluz, D.; Sudakevitz, D.; Gilboa-Garber, N. Blocking of Pseudomonas aeruginosa and Chromobacterium violaceum lectins by diverse mammalian milks. J. Dairy Sci. 2010, 93, 473–482. [Google Scholar] [CrossRef]
- Bohari, M.H.; Yu, X.; Zick, Y.; Blanchard, H. Structure-based rationale for differential recognition of lacto- and neolacto- series glycosphingolipids by the N-terminal domain of human galectin-8. Sci. Rep. 2016, 6, 39556. [Google Scholar] [CrossRef]
- Hill, D.R.; Chow, J.M.; Buck, R.H. Multifunctional Benefits of Prevalent HMOs: Implications for Infant Health. Nutrients 2021, 13, 3364. [Google Scholar] [CrossRef]
- Bienenstock, J.; Buck, R.H.; Linke, H.; Forsythe, P.; Stanisz, A.M.; Kunze, W.A. Fucosylated but Not Sialylated Milk Oligosaccharides Diminish Colon Motor Contractions. PLoS ONE 2013, 8, e76236. [Google Scholar] [CrossRef]
- Chumpitazi, B.; Nurko, S. Pediatric gastrointestinal motility disorders: Challenges and a clinical update. Gastroenterol. Hepatol. 2008, 4, 140–148. [Google Scholar]
- Autran, C.A.; Kellman, B.P.; Kim, J.H.; Asztalos, E.; Blood, A.B.; Spence, E.C.H.; Patel, A.L.; Hou, J.; Lewis, N.E.; Bode, L. Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut 2018, 67, 1064–1070. [Google Scholar] [CrossRef]
- Masi, A.C.; Embleton, N.D.; A Lamb, C.; Young, G.; Granger, C.L.; Najera, J.; Smith, D.P.; Hoffman, K.L.; Petrosino, J.F.; Bode, L.; et al. Human milk oligosaccharide DSLNT and gut microbiome in preterm infants predicts necrotising enterocolitis. Gut 2020, 70, 2273–2282. [Google Scholar] [CrossRef]
- Jantscher-Krenn, E.; Zherebtsov, M.; Nissan, C.; Goth, K.; Guner, Y.S.; Naidu, N.; Choudhury, B.; Grishin, A.V.; Ford, H.R.; Bode, L. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut 2011, 61, 1417–1425. [Google Scholar] [CrossRef]
- Bode, L. Human Milk Oligosaccharides in the Prevention of Necrotizing Enterocolitis: A Journey From in vitro and in vivo Models to Mother-Infant Cohort Studies. Front. Pediatr. 2018, 6, 385. [Google Scholar] [CrossRef]
- Sodhi, C.P.; Wipf, P.; Yamaguchi, Y.; Fulton, W.B.; Kovler, M.; Niño, D.F.; Zhou, Q.; Banfield, E.; Werts, A.D.; Ladd, M.R.; et al. The human milk oligosaccharides 2’-fucosyllactose and 6’-sialyllactose protect against the development of necrotizing enterocolitis by inhibiting toll-like receptor 4 signaling. Pediatr. Res. 2021, 89, 91–101. [Google Scholar] [CrossRef]
- Bein, A.; Zilbershtein, A.; Golosovsky, M.; Davidov, D.; Schwartz, B. LPS Induces Hyper-Permeability of Intestinal Epithelial Cells. J. Cell. Physiol. 2017, 232, 381–390. [Google Scholar] [CrossRef] [PubMed]
- MohanKumar, K.; Namachivayam, K.; Song, T.; Cha, B.J.; Slate, A.; Hendrickson, J.E.; Pan, H.; Wickline, S.A.; Oh, J.-Y.; Patel, R.P.; et al. A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nat. Commun. 2019, 10, 3494. [Google Scholar] [CrossRef] [PubMed]
- Selma-Royo, M.; González, S.; Gueimonde, M.; Chang, M.; Fürst, A.; Martínez-Costa, C.; Bode, L.; Collado, M.C. Maternal Diet Is Associated with Human Milk Oligosaccharide Profile. Mol. Nutr. Food Res. 2022, 66, 2200058. [Google Scholar] [CrossRef] [PubMed]
- Kunz, C.; Meyer, C.; Collado, M.C.; Geiger, L.; García-Mantrana, I.; Bertua-Ríos, B.; Martínez-Costa, C.; Borsch, C.; Rudloff, S. Influence of Gestational Age, Secretor, and Lewis Blood Group Status on the Oligosaccharide Content of Human Milk. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Stepans, M.B.F.; Wilhelm, S.L.; Hertzog, M.; Rodehorst, T.K.C.; Blaney, S.; Clemens, B.; Polak, J.J.; Newburg, D.S.; Goldman, A.S.; Bunik, M.; et al. Early Consumption of Human Milk Oligosaccharides Is Inversely Related to Subsequent Risk of Respiratory and Enteric Disease in Infants. Breastfeed. Med. 2006, 1, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Molecular Mechanism Underlying Sialic Acid as an Essential Nutrient for Brain Development and Cognition. Adv. Nutr. Int. Rev. J. 2012, 3, 465S–472S. [Google Scholar] [CrossRef]
- Jacobi, S.K.; Yatsunenko, T.; Li, D.; Dasgupta, S.; Yu, R.K.; Berg, B.M.; Chichlowski, M.; Odle, J. Dietary Isomers of Sialyllactose Increase Ganglioside Sialic Acid Concentrations in the Corpus Callosum and Cerebellum and Modulate the Colonic Microbiota of Formula-Fed Piglets. J. Nutr. 2016, 146, 200–208. [Google Scholar] [CrossRef]
- Davis, J.C.C.; Lewis, Z.T.; Krishnan, S.; Bernstein, R.M.; Moore, S.E.; Prentice, A.M.; Mills, D.A.; Lebrilla, C.B.; Zivkovic, A.M. Growth and Morbidity of Gambian Infants are Influenced by Maternal Milk Oligosaccharides and Infant Gut Microbiota. Sci. Rep. 2017, 7, 40466. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Breast milk microbiota: A review of the factors that influence composition. J. Infect. 2020, 81, 17–47. [Google Scholar] [CrossRef] [PubMed]
- Tonon, K.M.; De Morais, M.B.; Abrão, A.C.F.V.; Miranda, A.; Morais, T.B. Maternal and Infant Factors Associated with Human Milk Oligosaccharides Concentrations According to Secretor and Lewis Phenotypes. Nutrients 2019, 11, 1358. [Google Scholar] [CrossRef] [PubMed]
- Saben, J.L.; Sims, C.R.; Abraham, A.; Bode, L.; Andres, A. Human Milk Oligosaccharide Concentrations and Infant Intakes Are Associated with Maternal Overweight and Obesity and Predict Infant Growth. Nutrients 2021, 13, 446. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.W.; Lind, M.V.; Laursen, R.P.; Yonemitsu, C.; Larnkjær, A.; Mølgaard, C.; Michaelsen, K.F.; Bode, L. Human Milk Oligosaccharide Composition Is Associated with Excessive Weight Gain During Exclusive Breastfeeding—An Explorative Study. Front. Pediatr. 2019, 7, 297. [Google Scholar] [CrossRef] [PubMed]
- Wejryd, E.; Martí, M.; Marchini, G.; Werme, A.; Jonsson, B.; Landberg, E.; Abrahamsson, T.R. Low Diversity of Human Milk Oligosaccharides is Associated with Necrotising Enterocolitis in Extremely Low Birth Weight Infants. Nutrients 2018, 10, 1556. [Google Scholar] [CrossRef]
- Plows, J.F.; Berger, P.K.; Jones, R.B.; Yonemitsu, C.; Ryoo, J.H.; Alderete, T.; Bode, L.; Goran, M.I. Associations between human milk oligosaccharides (HMOs) and eating behaviour in Hispanic infants at 1 and 6 months of age. Pediatr. Obes. 2020, 15, e12686. [Google Scholar] [CrossRef]
| Maternal age, year | 32.9 ± 0.9 (25–43) |
| Non-secretor | 9 (45) |
| Number of HM samples | 23 (44.2) |
| Secretor | 11 (55) |
| Number of HM samples | 29 (55.7) |
| Lewis negative | 3 (15) |
| Lewis positive | 17(85) |
| Milk group phenotype | |
| Se+Le+ | 10 (50) |
| Se−Le+ | 7 (35) |
| Se+Le− | 1 (5) |
| Se−Le− | 2 (10) |
| Maternal Pre-pregnancy BMI [kg/m−2] | 22.4 ± 0.6 (16.7–26.2) |
| Non-smoker | 18 (90) |
| Smoker before pregnancy | 2 (10) |
| Omnivorous diet | 17 (85) |
| Vegetarian diet | 3 (15) |
| Infant gestational age, week | 38.15 ± 0.5 (33–41) |
| Vaginal delivery | 17 (85) |
| C-section delivery | 3 (15) |
| Infant birth weight, g | 3084 ± 149.7 (1900–4056) |
| Male infant | 11 (55) |
| Female infant | 9 (45) |
| Not admitted to the NICU | 17 (85) |
| Admitted to the NICU | 3 (15) |
| Parity | |
| 1 | 9 (45) |
| 2 | 7 (35) |
| 3 | 4 (20) |
| Season of collected samples (n = 52) | |
| Summer (June–August) | 44 (84.6) |
| Other (September–May) | 8 (15.4) |
| 2′-FL | Variables | B-Coeff | SEM | p |
| Time 1 vs. 0 | −749.320 | 191.250 | <0.001 | |
| Time 2 vs. 0 | −838.593 | 205.317 | <0.001 | |
| Time 2 vs. 1 | −89.273 | 205.613 | 0.664 | |
| GA | 177.563 | 76.041 | 0.02 | |
| Sex | −1359.295 | 361.798 | <0.001 | |
| Diet | 957.156 | 514.795 | 0.063 | |
| 3-FL | Variables | B-Coeff | SEM | p |
| Time 1 vs. 0 | 38.032 | 50.656 | 0.453 | |
| Time 2 vs. 0 | 4.163 | 54.122 | 0.939 | |
| Time 2 vs. 1 | −33.869 | 54.229 | 0.532 | |
| Age | −6.531 | 5.692 | 0.251 | |
| ppBMI | 21.437 | 10.317 | 0.038 | |
| GA | −24.585 | 11.318 | 0.03 | |
| FUT2 | −264.571 | 53.332 | <0.001 | |
| FUT3 | 374.080 | 83.835 | <0.001 | |
| MOD | −0.614 | 66.082 | 0.993 | |
| Season | 71.280 | 69.251 | 0.303 | |
| Diet | 39.044 | 93.166 | 0.675 | |
| 3′-SL | Variables | B-Coeff | SEM | p |
| Time 1 vs. 0 | 69.642 | 8.953 | <0.001 | |
| Time 2 vs. 0 | −83.186 | 9.501 | <0.001 | |
| Time 2 vs. 1 | −13.544 | 9.539 | 0.156 | |
| Sex | 28.204 | 10.523 | 0.007 | |
| FUT2 | 30.478 | 10.113 | 0.003 | |
| Smoking | −11.493 | 16.783 | 0.493 | |
| 6′-SL | Variables | B-Coeff | SEM | p |
| Time 1 vs. 0 | 60.097 | 26.095 | 0.021 | |
| Time 2 vs. 0 | −59.359 | 27.654 | 0.032 | |
| Time 2 vs. 1 | −119.455 | 28.018 | <0.001 | |
| ppBMI | 15.024 | 5.151 | 0.004 | |
| MOD | 73.711 | 34.826 | 0.034 | |
| NICU | 133.848 | 38.868 | 0.001 | |
| DSLNT | Variables | B-Coeff | SEM | p |
| Time 1 vs. 0 | 8.762 | 19.167 | 0.648 | |
| Time 2 vs. 0 | −85.223 | 20.223 | <0.001 | |
| Time 2 vs. 1 | −76.461 | 20.430 | <0.001 | |
| Sex | 49.538 | 22.483 | 0.028 | |
| FUT3 | −61.026 | 28.822 | 0.034 | |
| MOD | 47.709 | 30.700 | 0.12 | |
| LNFP I | Variables | B-Coeff | SEM | p |
| Time 1 vs. 0 | −43.467 | 180.402 | 0.810 | |
| Time 2 vs. 0 | −166.577 | 193.303 | 0.389 | |
| Time 2 vs. 1 | −120.275 | 194.590 | 0.537 | |
| Sex | −711.901 | 343.940 | 0.038 | |
| FUT3 | −327.260 | 599.268 | 0.585 | |
| Diet | 1526.702 | 599.044 | 0.011 | |
| LNFP II | Variables | B-Coeff | SEM | p |
| Time 1 vs. 0 | −114.548 | 32.480 | <0.001 | |
| Time 2 vs. 0 | 45.990 | 34.820 | 0.187 | |
| Time 2 vs. 1 | −68.558 | 34.869 | 0.049 | |
| FUT2 | −126.062 | 104.624 | 0.228 | |
| MOD | 407.329 | 140.352 | 0.004 | |
| Season | 410.123 | 130.796 | 0.002 | |
| LNFP III | Variables | B-Coeff | SEM | p |
| Time 1 vs. 0 | −47.673 | 23.150 | 0.039 | |
| Time 2 vs. 0 | −74.265 | 24.571 | 0.003 | |
| Time 2 vs. 1 | −26.592 | 24.736 | 0.282 | |
| GA | −24.039 | 6.206 | <0.001 | |
| FUT2 | −23.760 | 28.448 | 0.404 | |
| Season | 138.329 | 36.950 | <0.001 | |
| LNT | Variables | B-Coeff | SEM | p |
| Time 1 vs. 0 | −265.735 | 72.942 | <0.001 | |
| Time 2 vs. 0 | 97.095 | 77.924 | 0.213 | |
| Time 2 vs. 1 | −168.640 | 78.106 | 0.031 | |
| Sex | 233.535 | 161.251 | 0.148 | |
| MOD | 396.811 | 225.609 | 0.079 | |
| Season | 382.223 | 183.644 | 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asher, A.T.; Mangel, L.; Ari, J.B.; Gover, O.; Ahmad, W.A.; Herzlich, J.; Mandel, D.; Schwartz, B.; Lubetzky, R. Human Milk Oligosaccharide Profile across Lactation Stages in Israeli Women—A Prospective Observational Study. Nutrients 2023, 15, 2548. https://doi.org/10.3390/nu15112548
Asher AT, Mangel L, Ari JB, Gover O, Ahmad WA, Herzlich J, Mandel D, Schwartz B, Lubetzky R. Human Milk Oligosaccharide Profile across Lactation Stages in Israeli Women—A Prospective Observational Study. Nutrients. 2023; 15(11):2548. https://doi.org/10.3390/nu15112548
Chicago/Turabian StyleAsher, Adi Talan, Laurence Mangel, Julius Ben Ari, Ofer Gover, Wiessam Abu Ahmad, Jacky Herzlich, Dror Mandel, Betty Schwartz, and Ronit Lubetzky. 2023. "Human Milk Oligosaccharide Profile across Lactation Stages in Israeli Women—A Prospective Observational Study" Nutrients 15, no. 11: 2548. https://doi.org/10.3390/nu15112548
APA StyleAsher, A. T., Mangel, L., Ari, J. B., Gover, O., Ahmad, W. A., Herzlich, J., Mandel, D., Schwartz, B., & Lubetzky, R. (2023). Human Milk Oligosaccharide Profile across Lactation Stages in Israeli Women—A Prospective Observational Study. Nutrients, 15(11), 2548. https://doi.org/10.3390/nu15112548






