Fat-Soluble Vitamins Deficiency in Pediatric Cholestasis: A Scoping Review
Abstract
1. Introduction
1.1. Incidence of Fat-Soluble Vitamin Deficiency in Cholestasis
1.2. Indirect Laboratory Markers of Fat-Soluble Vitamins Deficiency
1.3. Routes of Administration
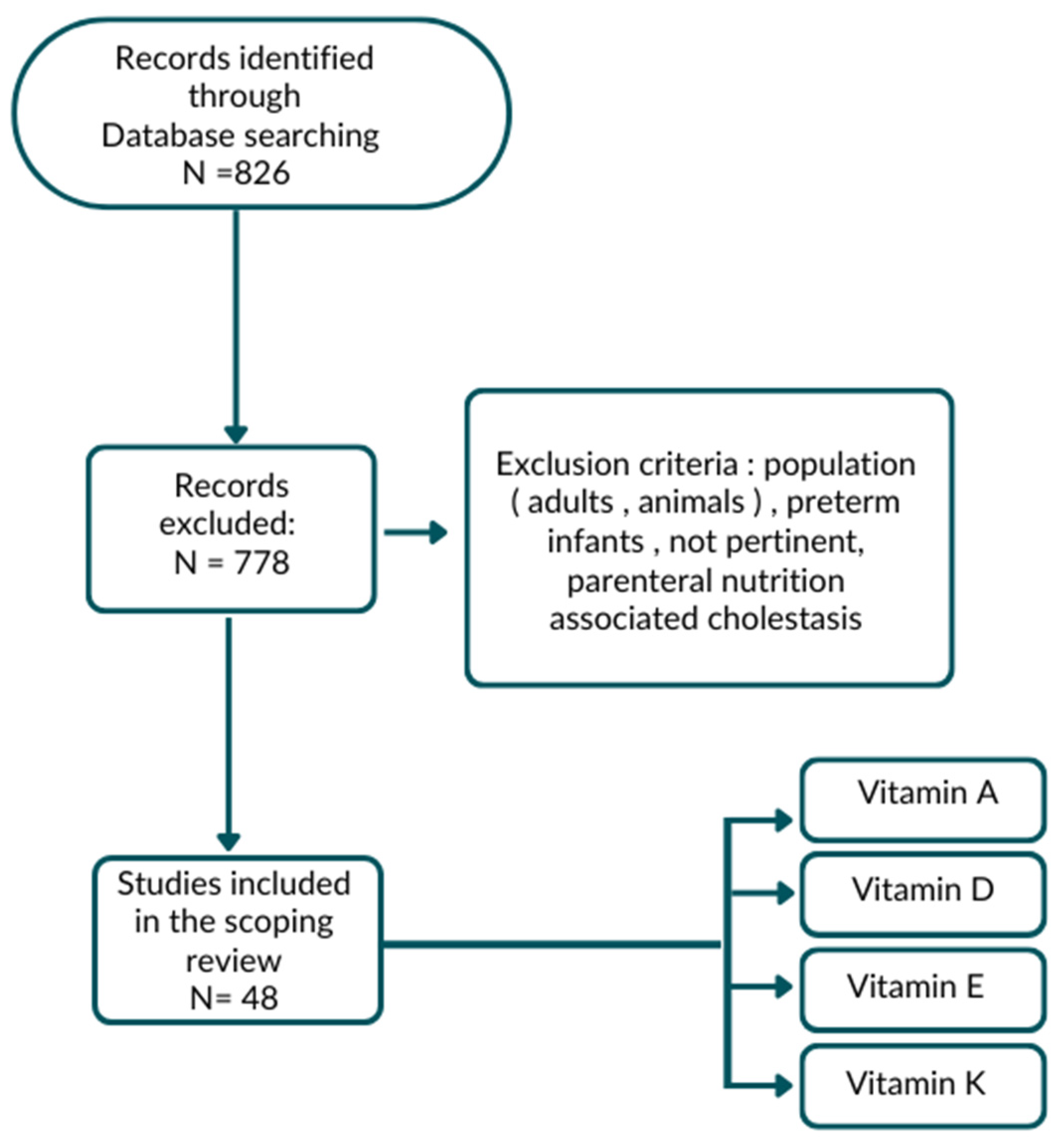
2. Materials and Methods
3. Results
3.1. Vitamin A
- Serum retinol concentration <20 μg/dL (<0.693 μmol/L) (sensitivity 90%, specificity 78.2%);
- Serum retinol-binding protein (RBP) <1 mg/dL (sensitivity 40%, specificity 91.3%);
- Retinol/RBP molar ratio <0.8 mol/mol; sufficient level between 0.8 and 2.0 (sensitivity 60%, specificity 73.9%);
- IM relative dose response test (RDR): increase in plasma level of retinol by 20% 9 h after administration of an IM dose of vitamin A;
- Modified oral relative dose response (RDR) test: increase >20% 10 h after administration of 1.500 IU (450 μg) vitamin A as a water-soluble retinyl palmitate preparation administered orally with 25 IU/kg di TPGS (sensitivity 80%, specificity 100%).
- Slit-lamp eye examination looking for signs associated with vitamin A deficiency, including xerosis, Bitot’s spots, keratomalacia and corneal ulceration;
- Tear film break-up time (TFBUT) represents the amount of time it takes for the first dry spot to form over the cornea and bulbar conjunctiva after applying a drop of 2% flour to the eye. Dry patches are denoted by black spots or lines. TFBUT denotes the time elapsed between the last blink and the onset of the first dry patch. TFBUTs shorter than 30 s are deemed abnormal;
- Schirmer test to measure tear production with a normal value >10 mm of wet paper;
- Conjunctival impression cytology (CIC).
3.2. Vitamin D
3.3. Vitamin E
3.4. Vitamin K
- “Early” (within 24 h of life): It is rare and is typically seen in infants whose mothers have been prescribed drugs that interfere with vitamin K metabolism, either by known (oral anticoagulants such as warfarin) or uncertain mechanisms (anticonvulsants or antituberculous drugs, e.g., rifampin, isoniazid);
- “Classic” (within 1 week of birth): It is often considered idiopathic, but a known cause is inadequate nutrition and/or inadequate prophylaxis. Medications taken during pregnancy may also contribute;
- 1 mg vitamin K1 by intramuscular (IM) injection at birth;
- 3 × 2 mg of vitamin K1 orally at birth, at 4–6 days and at 4–6 weeks;
- 2 mg of vitamin K 1 orally at birth and a weekly dose of 1 mg orally for 3 months.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Venkat, V.L.; Shneider, B.L.; Magee, J.C.; Turmelle, Y.; Arnon, R.; Bezerra, J.A.; Hertel, P.M.; Karpen, S.J.; Kerkar, N.; Loomes, K.M.; et al. Total Serum Bilirubin Predicts Fat-Soluble Vitamin Deficiency Better than Serum Bile Acids in Infants with Biliary Atresia. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 702–707. [Google Scholar] [CrossRef]
- Saron, M.L.G.; Godoy, H.T.; Hessel, G. Nutritional Status of Patients with Biliary Atresia and Autoimmune Hepatitis Related to Serum Levels of Vitamins A, D and E. Arq. Gastroenterol. 2009, 46, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Veraldi, S.; Pietrobattista, A.; Liccardo, D.; Basso, M.S.; Mosca, A.; Alterio, T.; Cardile, S.; Benedetti, S.; Della Corte, C.; Candusso, M. Fat Soluble Vitamins Deficiency in Pediatric Chronic Liver Disease: The Impact of Liver Transplantation. Dig. Liver Dis. 2019, 52, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-M.; Wu, J.-F.; Hsu, H.-Y.; Ni, Y.-H.; Chang, M.-H.; Liu, Y.-W.; Lai, H.-S.; Hsu, W.-M.; Weng, H.-L.; Chen, H.-L. Oral Absorbable Fat-Soluble Vitamin Formulation in Pediatric Patients with Cholestasis. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 587–591. [Google Scholar] [CrossRef]
- Young, S.; Kwarta, E.; Azzam, R.; Sentongo, T. Nutrition Assessment and Support in Children with End-Stage Liver Disease. Nutr. Clin. Pract. 2013, 28, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Sun, S.; Liu, X.-Z.; Shen, Z.; Chen, G.; Zheng, S. Fat-Soluble Vitamin Deficiency in Pediatric Patients with Biliary Atresia. Gastroenterol. Res. Pract. 2017, 2017, 7496860. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.A. Acute and chronic liver disease. In Nutrition in Pediatrics, 3rd ed.; Walker, A., Watkins, J., Duggan, C., Eds.; BC Decker: Hamilton, ON, Canada, 2003; Chapter 40; pp. 686–698. [Google Scholar]
- Suchy, F.J.; Sokol, R.J.; Balistreri, W.F.; Bezerra, J.A.; Mack, C.L.; Shneider, B.L. Liver Disease in Children; Cambridge University Press: Cambridge, UK, 2021; ISBN 978-1-108-91137-5. [Google Scholar]
- Shneider, B.L.; Magee, J.C.; Bezerra, J.A.; Haber, B.; Karpen, S.J.; Raghunathan, T.; Rosenthal, P.; Schwarz, K.; Suchy, F.J.; Kerkar, N.; et al. Efficacy of Fat-Soluble Vitamin Supplementation in Infants with Biliary Atresia. Pediatrics 2012, 130, e607–e614. [Google Scholar] [CrossRef]
- Feldman, A.G.; Sokol, R.J. Neonatal Cholestasis. Neoreviews 2013, 14, e63–e73. [Google Scholar] [CrossRef]
- Baker, A.; Stevenson, R.; Dhawan, A.; Goncalves, I.; Socha, P.; Sokal, E. Guidelines for Nutritional Care for Infants with Cholestatic Liver Disease before Liver Transplantation. Pediatr. Transplant. 2007, 11, 825–834. [Google Scholar] [CrossRef]
- Socha, P. Nutritional Management of Cholestatic Syndromes in Childhood. Ann. Nestlé 2008, 66, 137–147. [Google Scholar] [CrossRef]
- Samra, N.M.; Emad El Abrak, S.; El Dash, H.H.; El Said El Raziky, M.; El Sheikh, M.A. Evaluation of Vitamin D Status Bone Mineral Density and Dental Health in Children with Cholestasis. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Sathe, M.N.; Patel, A.S. Update in Pediatrics: Focus on Fat-Soluble Vitamins. Nutr. Clin. Pract. 2010, 25, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, M.; Sorrentino, E.; Schiano Di Cola, G.; Colucci, A.; Vajro, P.; Mandato, C. Malnutrition in Pediatric Chronic Cholestatic Disease: An Up-to-Date Overview. Nutrients 2021, 13, 2785. [Google Scholar] [CrossRef]
- Yang, C.H.; Perumpail, B.J.; Yoo, E.R.; Ahmed, A.; Kerner, J.A., Jr. Nutritional Needs and Support for Children with Chronic Liver Disease. Nutrients 2017, 9, 1127. [Google Scholar] [CrossRef]
- Feranchak, A.P.; Gralla, J.; King, R.; Ramirez, R.O.; Corkill, M.; Narkewicz, M.R.; Sokol, R.J. Comparison of Indices of Vitamin A Status in Children with Chronic Liver Disease. Hepatology 2005, 42, 782–792. [Google Scholar] [CrossRef]
- Amedee-Manesme, O.; Mourey, M.S.; Hanck, A.; Therasse, J. Vitamin A Relative Dose Response Test: Validation by Intravenous Injection in Children with Liver Disease. Am. J. Clin. Nutr. 1987, 46, 286–289. [Google Scholar] [CrossRef]
- Amédée-Manesme, O.; Furr, H.C.; Alvarez, F.; Hadchouel, M.; Alagille, D.; Olson, J.A. Biochemical Indicators of Vitamin A Depletion in Children with Cholestasis. Hepatology 1985, 5, 1143–1148. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D Status: Measurement, Interpretation, and Clinical Application. Ann. Epidemiol. 2009, 19, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, S.; Ng, V.L. Optimizing Nutritional Management in Children with Chronic Liver Disease. Pediatr. Clin. N. Am. 2009, 56, 1161–1183. [Google Scholar] [CrossRef]
- Chongsrisawat, V.; Ruttanamongkol, P.; Chaiwatanarat, T.; Chandrakamol, B.; Poovorawan, Y. Bone Density and 25-Hydroxyvitamin D Level in Extrahepatic Biliary Atresia. Pediatr. Surg. Int. 2001, 17, 604–608. [Google Scholar] [CrossRef]
- Lee, W.S.; Jalaludin, M.Y.; Wong, S.Y.; Ong, S.Y.; Foo, H.W.; Ng, R.T. Vitamin D Non-Sufficiency Is Prevalent in Children with Chronic Liver Disease in a Tropical Country. Pediatr. Neonatol. 2019, 60, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Ruuska, S.; Laakso, S.; Leskinen, O.; Hagfors, A.; Jalanko, H.; Kolho, K.-L.; Pakarinen, M.P. Impaired Bone Health in Children With Biliary Atresia. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, B.; Najafi, M.; Farahmand, F.; Motamed, F.; Ghajarzadeh, M.; Mohammadi, J.; Eshagh Roze, M. Prevalence of Vitamin D Deficiency and Rickets in Children with Cholestasis in Iran. Acta Med. Iran. 2012, 50, 482–485. [Google Scholar]
- Bastos, M.D.; da Silveira, T.R. Blood levels of vitamin D in children and adolescents with chronic cholestasis. J. Pediatr. 2003, 79, 245–252. [Google Scholar] [CrossRef]
- Ng, J.; Paul, A.; Wright, N.; Hadzic, N.; Davenport, M. Vitamin D Levels in Infants With Biliary Atresia: Pre- and Post-Kasai Portoenterostomy. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 746–750. [Google Scholar] [CrossRef]
- de Albuquerque Taveira, A.T.; Fernandes, M.I.M.; Galvão, L.C.; Sawamura, R.; de Mello Vieira, E.; de Paula, F.J.A. Impairment of Bone Mass Development in Children with Chronic Cholestatic Liver Disease. Clin. Endocrinol. 2007, 66, 518–523. [Google Scholar] [CrossRef]
- D’Antiga, L.; Moniz, C.; Buxton-Thomas, M.; Cheeseman, P.; Gray, B.; Abraha, H.; Baker, A.J.; Heaton, N.D.; Rela, M.; Mieli-Vergani, G.; et al. Bone Mineral Density and Height Gain in Children with Chronic Cholestatic Liver Disease Undergoing Transplantation. Transplantation 2002, 73, 1788–1793. [Google Scholar] [CrossRef]
- Teisseyre, M.; Pawlowska, J.; Kryskiewicz, E.; Karczmarewicz, E.; Czubkowski, P.; Dadalski, M.; Jankowska, I.; Teisseyre, J.; Ismail, H.; Lorenc, R. Bone Mineral Metabolism in Children with Biliary Atresia after Living Related Liver Transplantation. Evaluation of Selected Parameters. Ann. Transplant. 2007, 12, 19–25. [Google Scholar]
- Okajima, H.; Shigeno, C.; Inomata, Y.; Egawa, H.; Uemoto, S.; Asonuma, K.; Kiuchi, T.; Konishi, J.; Tanaka, K. Long-Term Effects of Liver Transplantation on Bone Mineral Density in Children with End-Stage Liver Disease: A 2-Year Prospective Study. Liver Transplant. 2003, 9, 360–364. [Google Scholar] [CrossRef]
- Pawłowska, J.; Matusik, H.; Socha, P.; Ismail, H.; Ryzko, J.; Karczmarewicz, E.; Jankowska, I.; Teisseyre, M.; Lorenc, R. Beneficial Effect of Liver Transplantation on Bone Mineral Density in Small Infants with Cholestasis. Transplant. Proc. 2004, 36, 1479–1480. [Google Scholar] [CrossRef]
- Argao, E.A.; Specker, B.L.; Heubi, J.E. Bone Mineral Content in Infants and Children with Chronic Cholestatic Liver Disease. Pediatrics 1993, 91, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Mouzaki, M.; Bronsky, J.; Gupte, G.; Hojsak, I.; Jahnel, J.; Pai, N.; Quiros-Tejeira, R.E.; Wieman, R.; Sundaram, S. Nutrition Support of Children With Chronic Liver Diseases: A Joint Position Paper of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.R.; Finberg, L. Single-Day Therapy for Nutritional Vitamin D-Deficiency Rickets: A Preferred Method. J. Pediatr. 1994, 125, 487–490. [Google Scholar] [CrossRef]
- Shepherd, D.; Belessis, Y.; Katz, T.; Morton, J.; Field, P.; Jaffe, A. Single High-Dose Oral Vitamin D3 (Stoss) Therapy--a Solution to Vitamin D Deficiency in Children with Cystic Fibrosis? J. Cyst. Fibros. 2013, 12, 177–182. [Google Scholar] [CrossRef]
- Jensen, M.; Abu-El-Haija, M.; Bishop, W.; Rahhal, R.M. Difficulty Achieving Vitamin D Sufficiency with High-Dose Oral Repletion Therapy in Infants with Cholestasis. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 187–189. [Google Scholar] [CrossRef]
- Lal, B.B.; Alam, S.; Khanna, R.; Rawat, D. Weekly Regimen of Vitamin D Supplementation Is More Efficacious than Stoss Regimen for Treatment of Vitamin D Deficiency in Children with Chronic Liver Diseases. Eur. J. Pediatr. 2018, 177, 827–834. [Google Scholar] [CrossRef]
- Argao, E.A.; Heubi, J.E.; Hollis, B.W.; Tsang, R.C. D-Alpha-Tocopheryl Polyethylene Glycol-1000 Succinate Enhances the Absorption of Vitamin D in Chronic Cholestatic Liver Disease of Infancy and Childhood. Pediatr. Res. 1992, 31, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Sokol, R.J.; Heubi, J.E.; Iannaccone, S.T.; Bove, K.E.; Balistreri, W.F. Vitamin E Deficiency with Normal Serum Vitamin E Concentrations in Children with Chronic Cholestasis. N. Engl. J. Med. 1984, 310, 1209–1212. [Google Scholar] [CrossRef]
- Sokol, R.J.; Butler-Simon, N.; Conner, C.; Heubi, J.E.; Sinatra, F.R.; Suchy, F.J.; Heyman, M.B.; Perrault, J.; Rothbaum, R.J.; Levy, J. Multicenter Trial of D-Alpha-Tocopheryl Polyethylene Glycol 1000 Succinate for Treatment of Vitamin E Deficiency in Children with Chronic Cholestasis. Gastroenterology 1993, 104, 1727–1735. [Google Scholar] [CrossRef]
- Socha, P.; Koletzko, B.; Pawlowska, J.; Proszynska, K.; Socha, J. Treatment of Cholestatic Children with Water-Soluble Vitamin E (α-Tocopheryl Polyethylene Glycol Succinate): Effects on Serum Vitamin E, Lipid Peroxides, and Polyunsaturated Fatty Acids. J. Pediatr. Gastroenterol. Nutr. 1997, 24, 189–193. [Google Scholar] [CrossRef]
- Velasco Benítez, C.A.; Rangel de la Garza, S.D.; García Aranda, J.A. Supplementation of DL-alpha-tocopherol acetate in children with chronic cholestasis and vitamin E deficiency. Rev. Gastroenterol. Mex. 1996, 61, 14–18. [Google Scholar]
- Sokol, R.J.; Heubi, J.E.; Iannaccone, S.; Bove, K.E.; Balistreri, W.F. Mechanism Causing Vitamin E Deficiency during Chronic Childhood Cholestasis. Gastroenterology 1983, 85, 1172–1182. [Google Scholar] [CrossRef]
- Davit-Spraul, A.; Cosson, C.; Couturier, M.; Hadchouel, M.; Legrand, A.; Lemonnier, F.; Therond, P. Standard Treatment of Alpha-Tocopherol in Alagille Patients with Severe Cholestasis Is Insufficient. Pediatr. Res. 2001, 49, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Sokol, R.J.; Guggenheim, M.A.; Iannaccone, S.T.; Barkhaus, P.E.; Miller, C.; Silverman, A.; Balistreri, W.F.; Heubi, J.E. Improved Neurologic Function after Long-Term Correction of Vitamin E Deficiency in Children with Chronic Cholestasis. N. Engl. J. Med. 1985, 313, 1580–1586. [Google Scholar] [CrossRef]
- Sokol, R.J.; Heubi, J.E.; Butler-Simon, N.; McClung, H.J.; Lilly, J.R.; Silverman, A. Treatment of Vitamin E Deficiency during Chronic Childhood Cholestasis with Oral D-α-Tocopheryl Polyethylene Glycol-1000 Succinate. Gastroenterology 1987, 93, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Roongpraiwan, R.; Suthutvoravut, U.; Feungpean, B.; Phuapradit, P. Effect of Oral Vitamin E Supplementation in Children with Cholestasis. J. Med. Assoc. Thail. 2002, 85 (Suppl. S4), S1199–S1205. [Google Scholar]
- Jacquemin, E.; Hermeziu, B.; Kibleur, Y.; Friteau, I.; Mathieu, D.; Le Coz, F.; Moyse, D.; Gérardin, M.; Jacqz-Aigrain, E.; Munck, A. Bioavailability of Oral Vitamin E Formulations in Adult Volunteers and Children with Chronic Cholestasis or Cystic Fibrosis. J. Clin. Pharm. Ther. 2009, 34, 515–522. [Google Scholar] [CrossRef]
- EMA Meeting Highlights from the Committee for Medicinal Products Human Use (CHMP) 8–11 November 2021. Available online: https://www.ema.europa.eu/en/news/meeting-highlights-committee-medicinal-products-human-use-chmp-8-11-november-2021 (accessed on 1 March 2023).
- Thébaut, A.; Nemeth, A.; Le Mouhaër, J.; Scheenstra, R.; Baumann, U.; Koot, B.; Gottrand, F.; Houwen, R.; Monard, L.; de Micheaux, S.L.; et al. Oral Tocofersolan Corrects or Prevents Vitamin E Deficiency in Children With Chronic Cholestasis. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 610–615. [Google Scholar] [CrossRef]
- Araki, S.; Shirahata, A. Vitamin K Deficiency Bleeding in Infancy. Nutrients 2020, 12, 780. [Google Scholar] [CrossRef]
- Mager, D.R.; McGee, P.L.; Furuya, K.N.; Roberts, E.A. Prevalence of Vitamin K Deficiency in Children with Mild to Moderate Chronic Liver Disease. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 71–76. [Google Scholar] [CrossRef]
- Strople, J.; Lovell, G.; Heubi, J. Prevalence of Subclinical Vitamin K Deficiency in Cholestatic Liver Disease. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Mihatsch, W.A.; Braegger, C.; Bronsky, J.; Campoy, C.; Domellöf, M.; Fewtrell, M.; Mis, N.F.; Hojsak, I.; Hulst, J.; Indrio, F.; et al. Prevention of Vitamin K Deficiency Bleeding in Newborn Infants: A Position Paper by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 123–129. [Google Scholar] [CrossRef]
- Shearer, M.J. Vitamin K Deficiency Bleeding (VKDB) in Early Infancy. Blood Rev. 2009, 23, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Primhak, R.A.; Tanner, M.S. Alpha-1 Antitrypsin Deficiency. Arch. Dis. Child. 2001, 85, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Baudesson de Chanville, A.; Oudyi, M.; Bresson, V.; Bosdure, E.; Roquelaure, B.; Chambost, H.; Dubus, J.-C. Vitamin K deficiency bleeding: A case secondary to transient neonatal cholestasis. Arch Pediatr. 2013, 20, 503–506. (In French) [Google Scholar] [CrossRef]
- Committee on Nutrition. Report of Committee on Nutrition: Vitamin K Compounds and the Water-Soluble Analogues. Pediatrics 1961, 28, 501–507. [Google Scholar] [CrossRef]
- NICHD Study Supports AAP Vitamin K Policy|AAP News|American Academy of Pediatrics. Available online: https://publications.aap.org/aapnews/article-abstract/9/11/2/25601/NICHD-study-supports-AAP-vitamin-K-policy?redirectedFrom=fulltext (accessed on 1 March 2023).
- American Academy of Pediatrics Committee on Fetus and Newborn. American Academy of Pediatrics Committee on Fetus and Newborn Controversies Concerning Vitamin K and the Newborn. Pediatrics 2003, 112, 191–192. [Google Scholar] [CrossRef]
- Ijland, M.M.; Pereira, R.R.; Cornelissen, E.A.M. Incidence of Late Vitamin K Deficiency Bleeding in Newborns in the Netherlands in 2005: Evaluation of the Current Guideline. Eur. J. Pediatr. 2008, 167, 165–169. [Google Scholar] [CrossRef]
- Alatas, F.S.; Hayashida, M.; Matsuura, T.; Saeki, I.; Yanagi, Y.; Taguchi, T. Intracranial Hemorrhage Associated with Vitamin K-Deficiency Bleeding in Patients with Biliary Atresia: Focus on Long-Term Outcomes. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 552–557. [Google Scholar] [CrossRef]
- Per, H.; Arslan, D.; Gümüş, H.; Coskun, A.; Kumandaş, S. Intracranial Hemorrhages and Late Hemorrhagic Disease Associated Cholestatic Liver Disease. Neurol. Sci. 2013, 34, 51–56. [Google Scholar] [CrossRef]
- Schubiger, G.; Berger, T.M.; Weber, R.; Bänziger, O.; Laubscher, B. Swiss Paediatric Surveillance Unit Prevention of Vitamin K Deficiency Bleeding with Oral Mixed Micellar Phylloquinone: Results of a 6-Year Surveillance in Switzerland. Eur. J. Pediatr. 2003, 162, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Laubscher, B.; Bänziger, O.; Schubiger, G. Swiss Paediatric Surveillance Unit (SPSU) Prevention of Vitamin K Deficiency Bleeding with Three Oral Mixed Micellar Phylloquinone Doses: Results of a 6-Year (2005–2011) Surveillance in Switzerland. Eur. J. Pediatr. 2013, 172, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.P. Intestinal Absorption of Mixed Micellar Phylloquinone (Vitamin K1) Is Unreliable in Infants with Conjugated Hyperbilirubinaemia: Implications for Oral Prophylaxis of Vitamin K Deficiency Bleeding. Arch. Dis. Child.-Fetal Neonatal Ed. 2003, 88, 113F–118F. [Google Scholar] [CrossRef]
- von Kries, R. Oral Mixed Micellar Vitamin K for Prevention of Late Vitamin K Deficiency Bleeding. Arch. Dis. Child.-Fetal Neonatal Ed. 2003, 88, 109F–112F. [Google Scholar] [CrossRef] [PubMed]
- van Hasselt, P.M.; de Koning, T.J.; Kvist, N.; de Vries, E.; Lundin, C.R.; Berger, R.; Kimpen, J.L.L.; Houwen, R.H.J.; Jorgensen, M.H.; Verkade, H.J.; et al. Prevention of Vitamin K Deficiency Bleeding in Breastfed Infants: Lessons from the Dutch and Danish Biliary Atresia Registries. Pediatrics 2008, 121, e857–e863. [Google Scholar] [CrossRef]
- Witt, M.; Kvist, N.; Jørgensen, M.H.; Hulscher, J.B.F.; Verkade, H.J.; on behalf of the Netherlands Study group of Biliary Atresia Registry (NeSBAR). Prophylactic Dosing of Vitamin K to Prevent Bleeding. Pediatrics 2016, 137, e20154222. [Google Scholar] [CrossRef]
- Busfield, A.; Samuel, R.; McNinch, A.; Tripp, J.H. Vitamin K Deficiency Bleeding after NICE Guidance and Withdrawal of Konakion Neonatal: British Paediatric Surveillance Unit Study, 2006–2008. Arch. Dis. Child. 2013, 98, 41–47. [Google Scholar] [CrossRef]
| Population | FVS Deficiency | Reference |
|---|---|---|
| Children with BA compared with non-cholestatic children | Vit. A: 77.2% Vit. E: 50.9% Vit. D: 37.6% | Saron et al., 2009 [2] |
| 166 children with end-stage liver disease, including 126 CPP (75%) | Vit. A: 66.6%, Vit. E: 40.6% Vit. D: 36.3% | Veraldi et al., 2019 [3] |
| 23 pediatric cholestatic patients | Vit. A: 73.9%, Vit. E: 91.3% Vit. D: 81.8% Vit. K: 20.0% | Shen et al., 2012 [4] |
| 266 pediatric patients with obstructive jaundice | Vit. A: 15.2%, Vit. E: 3.9% Vit. D: 87.8% Vit. K: 5.4% | Dong et al., 2017 [5] |
| Vitamin A | Deficiency | Toxicity | Normal Levels |
|---|---|---|---|
| Measurement | Retinol/RBP ratio < 0.8 Serum RBP < 1 mg/dL Serum retinol levels < 20 µg/dL Modified oral relative dose response (RDR) test with increase > 20% after administration of vit A | Difficult to assess biochemically | Retinol
|
| Clinical signs | Dry skin, xerophthalmia, night blindness, Bitot’s spots, keratomalacia, anorexia, anemia, leucopenia, hyperkeratosis, depressed helper T cell activity and/or impaired mucus secretion | Hepatic and neurologic toxicity, long bone fractures, muscle and bone pain, cheilitis, pseudotumor cerebri, photophobia, alopecia, ataxia, conjunctivitis, hepatotoxicity and hyperlipidemia | |
| Vitamin D | |||
| Measurement | Serum 25-OH-D: <20 ng/mL: vitamin D deficiency; <30 ng/mL: vitamin D insufficiency. | Excess > 100 ng/mL Intoxication: >150 ng/mL) | Optimal level: 30–40 ng/mL |
| Clinical signs | Hypocalcemia/hypophosphatemia/tetany, osteomalacia and rickets | Hypercalcemia leading to depression of the central nervous system and ectopic calcification. Hypercalciuria leading to nephrocalcinosis Weakness, fatigue, diarrhea, anorexia, headache, confusion, psychosis, tremor | |
| Vitamin E | |||
| Measurement | Vit E/total lipid ratio Deficiency of vit E: <0.6 mg/g (age < 1 year) <0.8 mg/g (age > 1 year) | Serum α-tocopherol >0.7 mg/dL | |
| Clinical signs | Hypo- or a-reflexia ataxia, hemolytic anemia, impaired vibratory sensation, proximal muscle weakness, ophthalmoplegia, degenerative lesions of the retina | Worsening of vit K deficiency coagulopathy Diarrhea Hyperosmolality (TPGS) | |
| Vitamin K | |||
| Measurement | Prothrombin time International normalized ratio Protein induced in vit K absence II (PIVKA II): <3 ng/mL | Prothrombin time (11–15 s) PIVKA II > 3 ng/mL | |
| Clinical signs | Hemorrhage | No major toxicity |
| Subjects | Deficiency (D) and Insufficiency (I) | Rickets | Fractures | Low Bone Mineral Density | Author |
|---|---|---|---|---|---|
| N = 50 Controls (30) | Hypovitaminosis 56% D = 30% (N = 15) I = 26% (N = 13) | N = 28 (56%) | N = 6 (12%) | N = 19 (67.8%) | Samra et al., 2018 [13] |
| N = 59 (N = 17 (30%) with bilirubin ≥34 μmol/L) | Hypovitaminosis 28% D = 14% (N = 8) I = 14% (N = 8) | N = 13 (22%) | Lee et al., 2018 [23] | ||
| N = 48 | Hypovitaminosis 64% D = 10.4% (N = 5) I = 54.1% (N = 26) | N = 22 (45.8%) | Mohammad et al., 2012 [25] | ||
| N = 22 Controls 17 | Hypovitaminosis(<9 ng/mL) = 36% | Bastos e da Silveira, 2003 [26] | |||
| N = 49 | Hypovitaminosis 64% D = 10.4% (N = 5) I = 54.1% (N = 26) | N = 7 (14%) | N = 11 (72%) | Ruuska et al., 2020 [24] | |
| N = 92 | Hypovitaminosis 98.9% D = 81.5% (N= 75) I = 17.4% (N = 16) | Ng et al., 2016 [27] |
| Vitamin D Supplementation | Population | Comments | |
|---|---|---|---|
| High-dose oral vitamin D therapy (HDR) “stoss therapy”: 300,000 IU ergocalciferol | 32 children with cholestasis | All patients remained in deficient of vitamin D. | [37] |
| Comparison of weekly regimens versus stoss therapy | 67 children with CLD and hypovitaminosis D | Increased 25(OH)D levels with weekly regimen. | [38] |
| Cointegration of vitamin D and micellar vitamin E formulation | 8 children with severe chronic cholestasis | Increased absorption of vitamin D when administered with TPGS. | [39] |
| Study | Population | Comments | Author |
|---|---|---|---|
Evaluation of 3 different dosages of oral dl-alpha-tocopheryl-acetate:
| 60 vitamin E-deficient children with chronic cholestasis | Therapy safe but not effective in maintaining levels of vitamin E. | Velasco Benitez et al., 1996 [43] |
| Efficacy of IM vitamin E injections (10 mg/kg dl-α-Tocopherol acetate) every 2 weeks (max 200 mg) Evaluation of oxidative stress in cholestatic patients with Alagille syndrome | 15 children with AGS Group 1: plasma bilirubin <100 μmol/L (n = 6); Group 2: plasma bilirubin >100 μmol/L (n = 9); Control: 15 healthy children. | IM administration of α-T (at a dose of 10 mg/kg every 2 weeks) is insufficient in patients with Alagille syndrome to prevent lipid peroxidation. | Davit-Spraul et al., 2001 [45] |
| Safety and efficacy of the TPGS form on vitamin E deficiency | 22 children (aged 7 months to 19 years) with severe cholestasis and vitamin E deficiency | Safe and effective form of vitamin E for the prevention and correction of vitamin E deficiency during childhood cholestasis. | Sokol et al., 1987 [47] |
| Therapy with 20–25 IU kg/day of oral TPGS | 60 children with chronic cholestasis | Oral TPGS therapy prevents and corrects vitamin E deficiency, replacing IM injections. | Sokol et al., 1993 [41] |
| TPGS therapy on lipid peroxidation levels | 15 children with chronic cholestasis deficient in vitamin E | Rapid normalization of serum vitamin E concentrations but no improvement on PUFA deficiency on lipid peroxidation. | Socha et al., 1997 [42] |
Supplementation of
| 11 children with biliary atresia | All 3 therapies were effective in normalizing vitamin E status in cholestatic children with direct bilirubin levels <68.4 μmol/L. With direct bilirubin >68.4 μmol/L, none of the vitamin E supplementation corrected the deficiency. | Roongpraiwan et al., 2002 [48] |
Bioavailability of two oral formulations of vitamin E
| 6 children with biliary atresia and total bilirubin >100 μmol/L | The absorption and bioavailability of oral tocofersolan was significantly greater than the reference formulation in children with chronic cholestasis. | Jacquemin et al., 2009 [49] |
| Safety/efficacy of D-α-tocopheryl polyethylene glycol 1000 succinate (Tocofersolan, Vedrop). Daily dose of 0.34 mL/kg (25 IU/kg or 17 mg/kg) body weight. | 274 children with cholestasis, including
| Safe and effective form of vitamin E that quickly restores and/or maintains adequate serum levels of vitamin E in most children with cholestasis, avoiding the need for intramuscular injections. | Thébaut et al., 2016 [51] |
| Vitamin K Supplementation | Study Population | Comments | Author |
|---|---|---|---|
| Comparison of pharmacokinetics and efficacy of mixed oral and intravenous micellar prophylaxis | 44 infants with cholestatic liver disease Group 1: 1 mg K by intravenous injection; Group 2: 2 mg oral. | Poor overall absorption in cholestatic infants with insufficient levels to provide long-term protective storage. | Pereira et al. [67], 2003 |
| Efficacy use of oral mixed micellar vitamin K3 × 2 mg (2 mg at birth, 2 mg on days 3–10 and 2 mg in weeks 4–6) compared with other preparations | Slightly lower incidence of late VKDB by 0.44/100,000 (95% CI 0.19–0.87) in children on mixed micellar vitamin K. | Von Kries et al. [68], 2003 | |
| Prevalence of vitamin K deficiency with oral supplementation according to standard dosing guidelines | 43 children with chronic cholestatic liver disease | Vitamin K deficiency prevalence of 54%; current regimens are not effective for cholestatic patients. | Mager et al. [53], 2006 |
Comparison of risk of vitamin K deficiency bleeding with different prophylactic regimens:
| 142 infants with biliary atresia, including 53 breastfed and 89 formula-fed | Risk of VKDB 8 to 10 times greater in breastfed infants with daily prophylaxis compared with weekly oral or IM prophylaxis at birth and nearly 80 times greater compared with formula-fed infants. Weekly 1 mg prophylaxis is more effective than 25 μg daily prophylaxis and is similar to IM administration. | Van Hasselt et al. [69], 2008 |
| Incidence and long-term outcome of ICH in patients with BA who previously received prophylactic vitamin K | Medical records of 88 infants with BA | All patients received prophylactic oral vitamin K (2 mg) during the neonatal period, 7 had ICH (7.95%). | Alatas et al. [63], 2012 |
| Efficacy of 2 oral doses of vitamin K (2 mg) | 475,000 births | 18 cases of late VKDB, including 13 with persistent liver disease. Two oral doses of 2 mg of a mixed micellar vitamin K preparation failed to prevent VKDB. | Schubiger et al. [65], 2003 |
| VKDB incidence after modification of Swiss guidelines, 2 to 3 oral doses (2 mg) | 458,184 born alive | 4 cases of late VKDB; all had undiagnosed cholestasis at the time of bleeding. 3/4 had refused prophylaxis and 1/4 had forgotten the third dose. | Laubscher et al. [66], 2013 |
Compared efficacy of 3 regimens:
| 91 breastfed babies with BA | A prophylactic regimen of 1 mg oral vitamin K at birth followed by oral/daily dose of 25 or 150 μg fails to prevent VKDB in neonates. Single IM administration is effective. | Witt et al. [70], 2016 |
| A | D | E | K | Author |
|---|---|---|---|---|
| Oral: <10 kg 5000 IU/day; >10 kg 10,000 IU/day. IM: 50,000 IU monthly | Oral: 25-OHD: 80–200 IU//kg/die IM: 30,000 IU 1–3 monthly | Oral: TPGS 25 IU/kg IM: 10 mg/kg (max. 200 mg) every two weeks | Oral: 2 mg/day weekly 5 mg: 5–10 kg; 10 mg: >10 kg; IM: 5–10 mg every two weeks. | Baker et al., Guidelines 2007 [11] |
| 5000–20,000 IU | 80–160 IU/kg/day | 15–25 mg/day | 2400–15,000 μg/day | ASPEN 2010 [14] |
| Guidelines ESPGHAN 2016 [55] | |||
| 5000–10,000 IU/day | Sunlight exposure; vitamin D-1-α 50 ng/kg | TPGS: 50–400 IU/day | 2.5–5.0 mg/day | Kelly et al. [7] 2003, Review |
| <10 kg: 5000 IU; >10 kg: 10,000 IU. IM: 50,000 IU | 25-OH-D: 80–200 IU/kg/die IM: 30,000 IU, 1–3 monthly | TPGS 25 IU/kg IM: 10 mg/kg (max. 200 mg) every 3 weeks | Oral: 2 mg/day Week: 5–10 kg: 5 mg; >10 kg: 10 mg; IM: 5–10 mg every 2 weeks. | Socha, 2008 [12], Review |
| 5000–25,000 IU/g | 400 IU/day, in 25-OH D3 form (in association with TPGS to improve absorption) | TPGS: 15–25 IU/kg/day | 2.5–5 mg/day | Nightingale et al., 2009 [21], Review |
| 1000 IU/kg/day to 25,000 IU (Oral) <10 kg: 5000 IU/day; >10 kg: 10,000 IU/day. | Cholecalciferol: Weight > 40 kg and serum level: <10 ng/mL: 5000 IU/day; 11–19 ng/mL: 4000 IU/day; 20–29 ng/mL: 3000 IU/day. Weight < 40 kg 120–200 IU/kg/day | TPGS: 15–25 IU/kg/day | 2.5–5.0 mg 2 to 7 times a week. 1–10 mg (intravenous if necessary) | Yang et al., 2017 [16], Review |
| <10 kg 5000 IU/day; >10 kg 10,000 IU/day. | Cholecalciferol: 2000–5000 IU/day | TPGS: 15–25 IU/kg/day | 2–5 mg/ day | Mouzaki et al., 2019 [34], Review |
| 3000–10,000 IU/day <10 kg: 5000 IU/day; >10 kg: 10,000 IU/day. IM: 50,000 IU/1–3 monthly | Oral: Cholecalciferol: 800–5000 IU/day 1.25-OH Cholecalciferol: 0.05–0.2 µg/kg/day | Oral: Alpha-tocopherol acetate: 15–25 to 25–200 IU/kg/day TPGS: 15–25 IU/kg | 2.5–5.0 mg/day from twice a week to every day Oral: 5–10 kg: 5 mg; >10 kg: 10 mg. IM: 5–10 mg/day every two weeks. | Tessitore et al., 2021 [15], Review |
| Dosage | Route of Administration | |
|---|---|---|
| Vitamin A | <10 kg 5000 IU/day; >10 kg 10,000 IU/day [12] | Oral |
| Vitamin D | Cholecalciferol 80–200 IU/kg/die oral, 30,000 IU 1–3 monthly IM [11,12] | Oral, IM |
| Vitamin E | TPGS 25 IU/kg/die [41,47] | Oral |
| Vitamin K | <5 kg: 1 mg/kg every 2 weeks; >5 kg: 10 mg every 2 weeks [11,12] | IM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degrassi, I.; Leonardi, I.; Di Profio, E.; Montanari, C.; Zuccotti, G.; Verduci, E. Fat-Soluble Vitamins Deficiency in Pediatric Cholestasis: A Scoping Review. Nutrients 2023, 15, 2491. https://doi.org/10.3390/nu15112491
Degrassi I, Leonardi I, Di Profio E, Montanari C, Zuccotti G, Verduci E. Fat-Soluble Vitamins Deficiency in Pediatric Cholestasis: A Scoping Review. Nutrients. 2023; 15(11):2491. https://doi.org/10.3390/nu15112491
Chicago/Turabian StyleDegrassi, Irene, Ilaria Leonardi, Elisabetta Di Profio, Chiara Montanari, Gianvincenzo Zuccotti, and Elvira Verduci. 2023. "Fat-Soluble Vitamins Deficiency in Pediatric Cholestasis: A Scoping Review" Nutrients 15, no. 11: 2491. https://doi.org/10.3390/nu15112491
APA StyleDegrassi, I., Leonardi, I., Di Profio, E., Montanari, C., Zuccotti, G., & Verduci, E. (2023). Fat-Soluble Vitamins Deficiency in Pediatric Cholestasis: A Scoping Review. Nutrients, 15(11), 2491. https://doi.org/10.3390/nu15112491







