Nitrate and Nitrite Metabolism in Aging Rats: A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
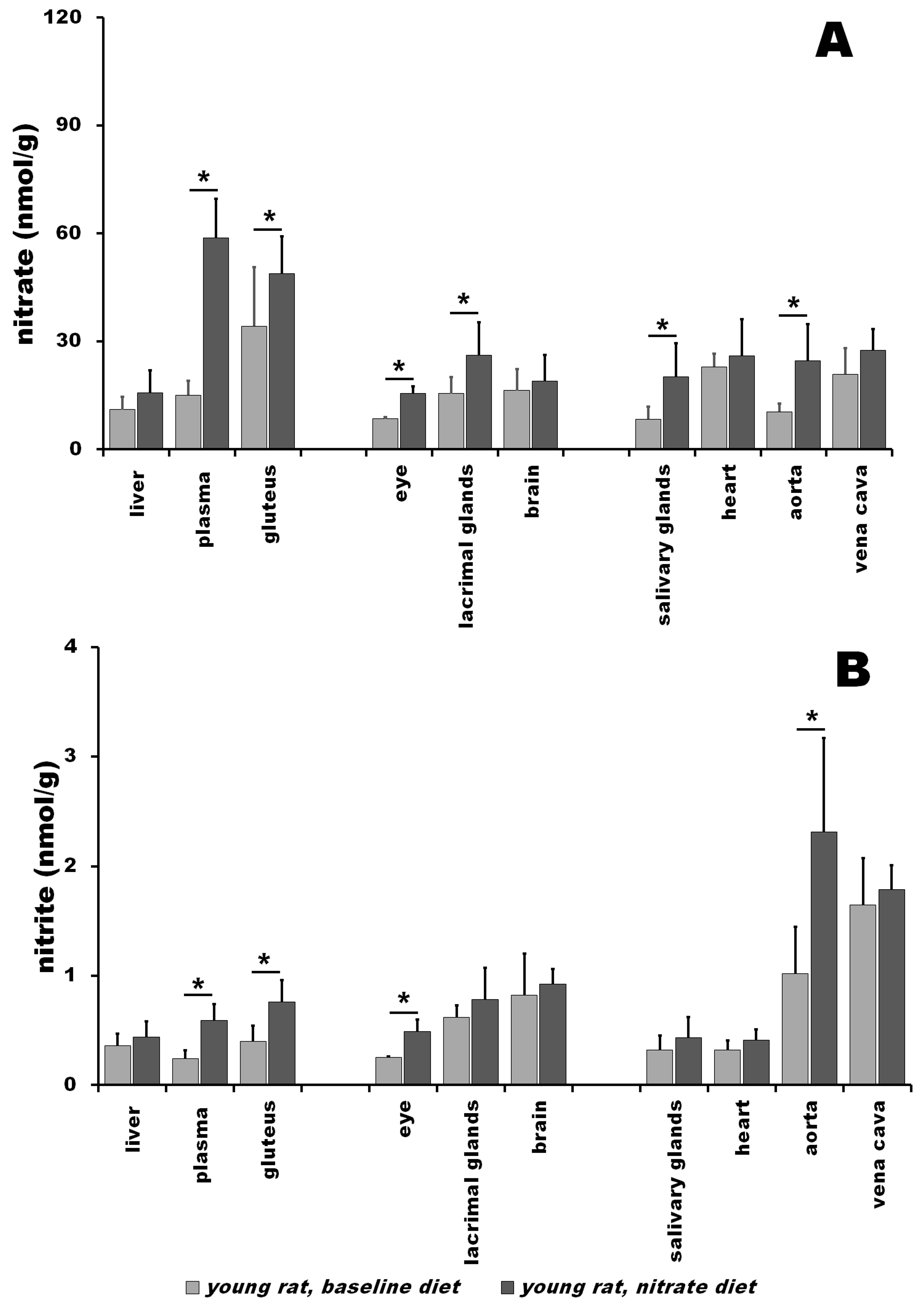
3.1. Nitrate and Nitrite in Young Rats at Baseline vs. after Nitrate Treatment
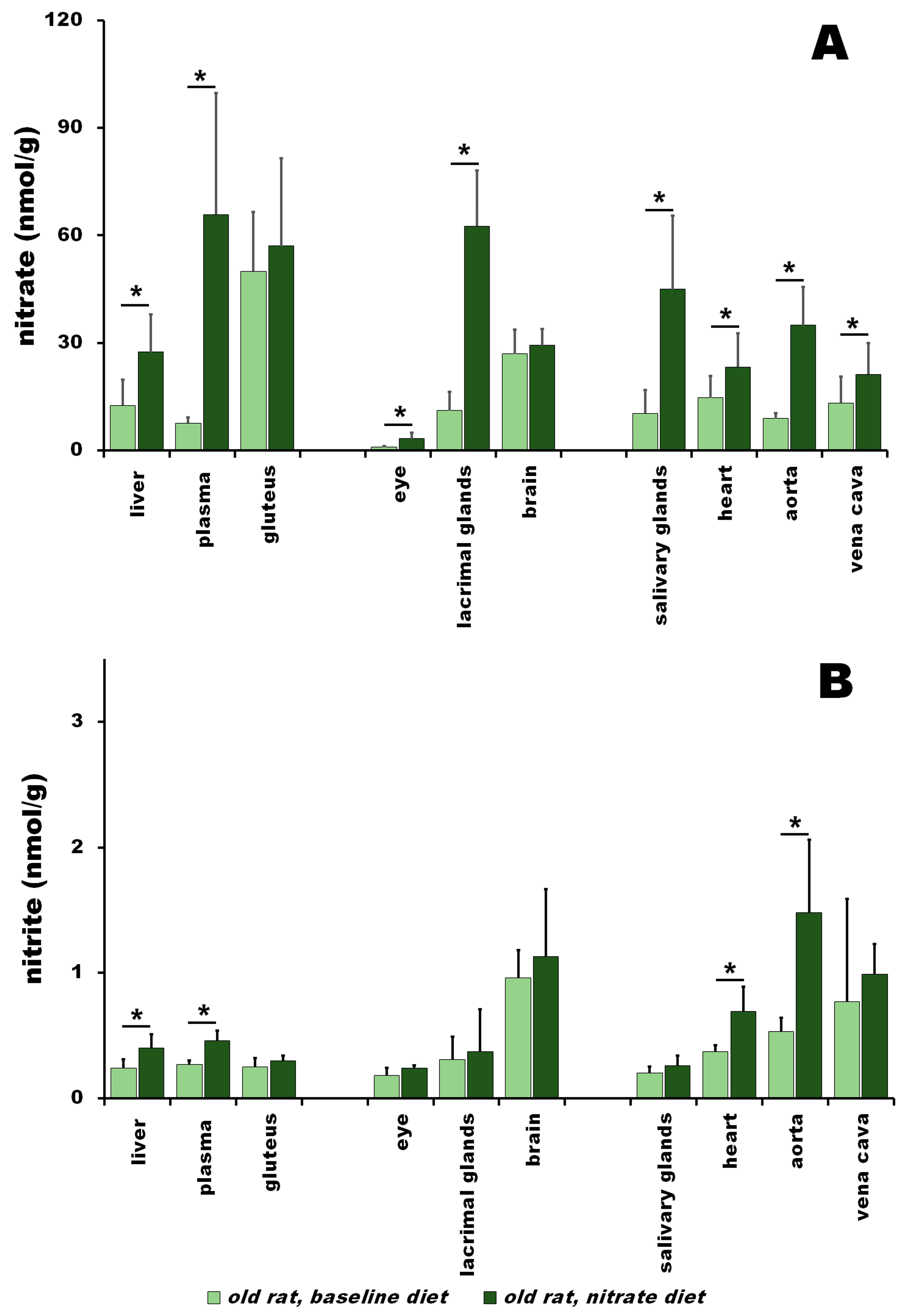
3.2. Nitrate and Nitrite in Old Rats at Baseline vs. after Nitrate Treatment
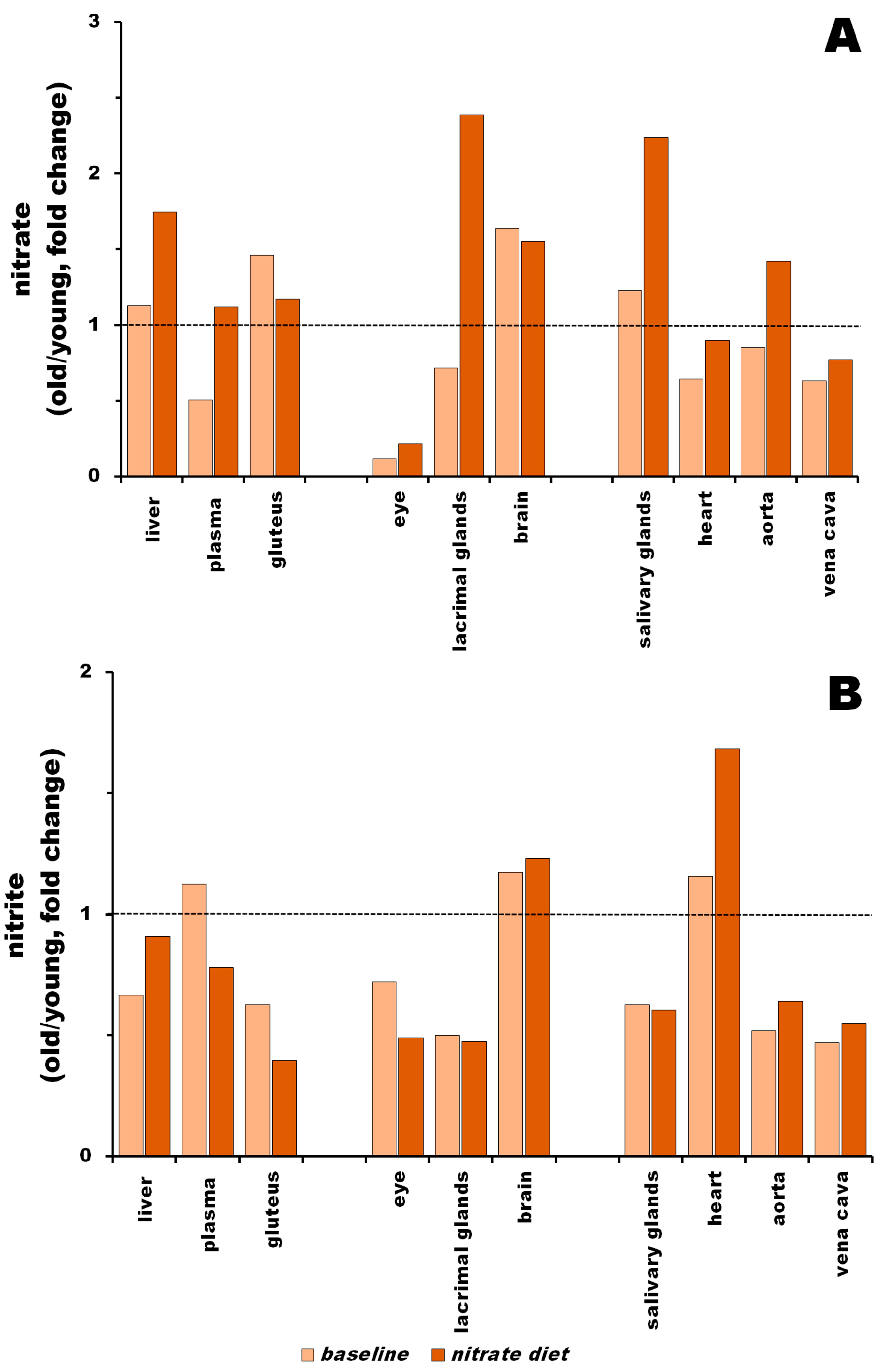
3.3. Nitrite Levels in Young vs. Old Rats at Baseline and after Nitrate Treatment
3.4. Protein Level Changes in Young and Old Rats at Baseline and after Nitrate Treatment
4. Discussion
4.1. Baseline Conditions
4.1.1. Baseline Nitrate
4.1.2. Baseline Nitrite
4.2. Changes following Dietary Nitrate Supplementation
4.2.1. Nitrate after Dietary Supplementation
4.2.2. Nitrite after Dietary Supplementation
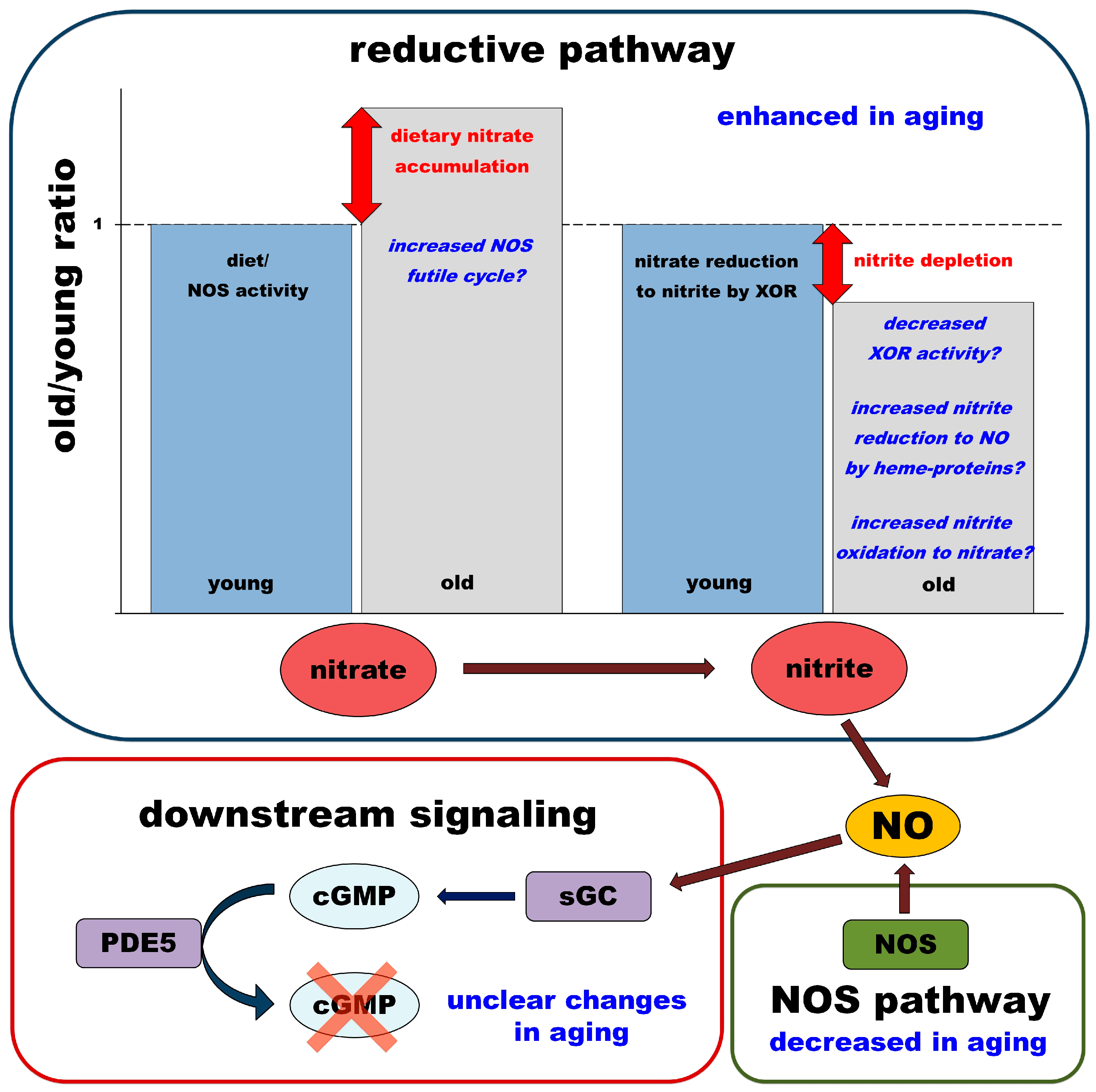
4.2.3. Summary and Hypothesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Schwarz, G.; Mendel, R.R.; Ribbe, M.W. Molybdenum cofactors, enzymes and pathways. Nature 2009, 460, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Bender, D.; Schwarz, G. Nitrite-dependent nitric oxide synthesis by molybdenum enzymes. FEBS Lett. 2018, 592, 2126–2139. [Google Scholar] [CrossRef] [PubMed]
- Carreau, A.; El Hafny-Rahbi, B.; Matejuk, A.; Grillon, C.; Kieda, C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell. Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef]
- Piknova, B.; Park, J.W.; Swanson, K.M.; Dey, S.; Noguchi, C.T.; Schechter, A.N. Skeletal muscle as an endogenous nitrate reservoir. Nitric Oxide 2015, 47, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Piknova, B.; Schechter, A.N.; Park, J.W.; Vanhatalo, A.; Jones, A.M. Skeletal Muscle Nitrate as a Regulator of Systemic Nitric Oxide Homeostasis. Exerc. Sport Sci. Rev. 2022, 50, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Jansson, E.A.; Huang, L.; Malkey, R.; Govoni, M.; Nihlen, C.; Olsson, A.; Stensdotter, M.; Petersson, J.; Holm, L.; Weitzberg, E.; et al. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat. Chem. Biol. 2008, 4, 411–417. [Google Scholar] [CrossRef]
- Cosby, K.; Partovi, K.S.; Crawford, J.H.; Patel, R.P.; Reiter, C.D.; Martyr, S.; Yang, B.K.; Waclawiw, M.A.; Zalos, G.; Xu, X.; et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003, 9, 1498–1505. [Google Scholar] [CrossRef]
- Shiva, S.; Huang, Z.; Grubina, R.; Sun, J.; Ringwood, L.A.; MacArthur, P.H.; Xu, X.; Murphy, E.; Darley-Usmar, V.M.; Gladwin, M.T. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ. Res. 2007, 100, 654–661. [Google Scholar] [CrossRef]
- Liu, Y.; Buerk, D.G.; Barbee, K.A.; Jaron, D. Nitric oxide release by deoxymyoglobin nitrite reduction during cardiac ischemia: A mathematical model. Microvasc. Res. 2017, 112, 79–86. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Kim-Shapiro, D.B. The functional nitrite reductase activity of the heme-globins. Blood 2008, 112, 2636–2647. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Samouilov, A.; Liu, X.; Zweier, J.L. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrate reduction: Evaluation of its role in nitrite and nitric oxide generation in anoxic tissues. Biochemistry 2003, 42, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- McNulty, P.H.; Scott, S.; Kehoe, V.; Kozak, M.; Sinoway, L.I.; Li, J. Nitrite consumption in ischemic rat heart catalyzed by distinct blood-borne and tissue factors. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2143–H2148. [Google Scholar] [CrossRef] [PubMed]
- Maia, L.B.; Moura, J.J.G. Putting xanthine oxidoreductase and aldehyde oxidase on the NO metabolism map: Nitrite reduction by molybdoenzymes. Redox. Biol. 2018, 19, 274–289. [Google Scholar] [CrossRef]
- Maia, L.B.; Pereira, V.; Mira, L.; Moura, J.J. Nitrite reductase activity of rat and human xanthine oxidase, xanthine dehydrogenase, and aldehyde oxidase: Evaluation of their contribution to NO formation in vivo. Biochemistry 2015, 54, 685–710. [Google Scholar] [CrossRef]
- Webb, A.J.; Milsom, A.B.; Rathod, K.S.; Chu, W.L.; Qureshi, S.; Lovell, M.J.; Lecomte, F.M.; Perrett, D.; Raimondo, C.; Khoshbin, E.; et al. Mechanisms underlying erythrocyte and endothelial nitrite reduction to nitric oxide in hypoxia: Role for xanthine oxidoreductase and endothelial nitric oxide synthase. Circ. Res. 2008, 103, 957–964. [Google Scholar] [CrossRef]
- Golwala, N.H.; Hodenette, C.; Murthy, S.N.; Nossaman, B.D.; Kadowitz, P.J. Vascular responses to nitrite are mediated by xanthine oxidoreductase and mitochondrial aldehyde dehydrogenase in the rat. Can. J. Physiol. Pharmacol. 2009, 87, 1095–1101. [Google Scholar] [CrossRef]
- Ortiz de Zevallos, J.; Woessner, M.N.; Kelley, E.E. Skeletal muscle as a reservoir for nitrate and nitrite: The role of xanthine oxidase reductase (XOR). Nitric Oxide 2022, 129, 102–109. [Google Scholar] [CrossRef]
- Qin, L.; Liu, X.; Sun, Q.; Fan, Z.; Xia, D.; Ding, G.; Ong, H.L.; Adams, D.; Gahl, W.A.; Zheng, C.; et al. Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane. Proc. Natl. Acad. Sci. USA 2012, 109, 13434–13439. [Google Scholar] [CrossRef]
- Jentsch, T.J.; Pusch, M. CLC Chloride Channels and Transporters: Structure, Function, Physiology, and Disease. Physiol. Rev. 2018, 98, 1493–1590. [Google Scholar] [CrossRef]
- Bergsdorf, E.Y.; Zdebik, A.A.; Jentsch, T.J. Residues important for nitrate/proton coupling in plant and mammalian CLC transporters. J. Biol. Chem. 2009, 284, 11184–11193. [Google Scholar] [CrossRef]
- Zdebik, A.A.; Zifarelli, G.; Bergsdorf, E.Y.; Soliani, P.; Scheel, O.; Jentsch, T.J.; Pusch, M. Determinants of anion-proton coupling in mammalian endosomal CLC proteins. J. Biol. Chem. 2008, 283, 4219–4227. [Google Scholar] [CrossRef]
- Stuehr, D.J.; Haque, M.M. Nitric oxide synthase enzymology in the 20 years after the Nobel Prize. Br. J. Pharmacol. 2019, 176, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Santolini, J.; Meade, A.L.; Stuehr, D.J. Differences in three kinetic parameters underpin the unique catalytic profiles of nitric-oxide synthases I, II, and III. J. Biol. Chem. 2001, 276, 48887–48898. [Google Scholar] [CrossRef] [PubMed]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef]
- Bailey, S.J.; Gandra, P.G.; Jones, A.M.; Hogan, M.C.; Nogueira, L. Incubation with sodium nitrite attenuates fatigue development in intact single mouse fibres at physiological PO2. J. Physiol. 2019, 597, 5429–5443. [Google Scholar] [CrossRef] [PubMed]
- Kadach, S.; Park, J.W.; Stoyanov, Z.; Black, M.I.; Vanhatalo, A.; Burnley, M.; Walter, P.J.; Cai, H.; Schechter, A.N.; Piknova, B.; et al. 15N-labelled dietary nitrate supplementation increases human skeletal muscle nitrate concentration and improves muscle torque production. Acta Physiol. 2023, 237, e13924. [Google Scholar] [CrossRef]
- Carlstrom, M.; Lundberg, J.O.; Weitzberg, E. Mechanisms underlying blood pressure reduction by dietary inorganic nitrate. Acta Physiol. 2018, 224, e13080. [Google Scholar] [CrossRef]
- Piknova, B.; Woessner, M.N.; de Zevallos, J.O.; Kraus, W.E.; VanBruggen, M.D.; Schechter, A.N.; Allen, J.D. Human skeletal muscle nitrate and nitrite in individuals with peripheral arterial disease: Effect of inorganic nitrate supplementation and exercise. Physiol. Rep. 2022, 10, e15531. [Google Scholar] [CrossRef]
- Nelson, M.D.; Rosenberry, R.; Barresi, R.; Tsimerinov, E.I.; Rader, F.; Tang, X.; Mason, O.; Schwartz, A.; Stabler, T.; Shidban, S.; et al. Sodium nitrate alleviates functional muscle ischaemia in patients with Becker muscular dystrophy. J. Physiol. 2015, 593, 5183–5200. [Google Scholar] [CrossRef]
- Gilliard, C.N.; Lam, J.K.; Cassel, K.S.; Park, J.W.; Schechter, A.N.; Piknova, B. Effect of dietary nitrate levels on nitrate fluxes in rat skeletal muscle and liver. Nitric Oxide 2018, 75, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Piknova, B.; Walter, P.J.; Cai, H.; Upanan, S.; Thomas, S.M.; Tunau-Spencer, K.J.; Schechter, A.N. Distribution of dietary nitrate and its metabolites in rat tissues after 15N-labeled nitrate administration. Sci. Rep. 2023, 13, 3499. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Thomas, S.M.; Schechter, A.N.; Piknova, B. Control of rat muscle nitrate levels after perturbation of steady state dietary nitrate intake. Nitric Oxide 2021, 109–110, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Arifin, W.N.; Zahiruddin, W.M. Sample Size Calculation in Animal Studies Using Resource Equation Approach. Malays. J. Med. Sci. 2017, 24, 101–105. [Google Scholar] [CrossRef]
- Serdar, C.C.; Cihan, M.; Yucel, D.; Serdar, M.A. Sample size, power and effect size revisited: Simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem. Med. 2021, 31, 010502. [Google Scholar] [CrossRef]
- Park, J.W.; Thomas, S.M.; Wylie, L.J.; Jones, A.M.; Vanhatalo, A.; Schechter, A.N.; Piknova, B. Preparation of Rat Skeletal Muscle Homogenates for Nitrate and Nitrite Measurements. J. Vis. Exp. 2021, 173, e62427. [Google Scholar] [CrossRef]
- Piknova, B.; Park, J.W.; Kwan Jeff Lam, K.; Schechter, A.N. Nitrate as a source of nitrite and nitric oxide during exercise hyperemia in rat skeletal muscle. Nitric Oxide 2016, 55–56, 54–61. [Google Scholar] [CrossRef]
- Wylie, L.J.; Park, J.W.; Vanhatalo, A.; Kadach, S.; Black, M.I.; Stoyanov, Z.; Schechter, A.N.; Jones, A.M.; Piknova, B. Human skeletal muscle nitrate store: Influence of dietary nitrate supplementation and exercise. J. Physiol. 2019, 597, 5565–5576. [Google Scholar] [CrossRef]
- Kadach, S.; Piknova, B.; Black, M.I.; Park, J.W.; Wylie, L.J.; Stoyanov, Z.; Thomas, S.M.; McMahon, N.F.; Vanhatalo, A.; Schechter, A.N.; et al. Time course of human skeletal muscle nitrate and nitrite concentration changes following dietary nitrate ingestion. Nitric Oxide 2022, 121, 1–10. [Google Scholar] [CrossRef]
- Thijssen, D.H.; Carter, S.E.; Green, D.J. Arterial structure and function in vascular ageing: Are you as old as your arteries? J. Physiol. 2016, 594, 2275–2284. [Google Scholar] [CrossRef]
- Sullivan, J.C.; Loomis, E.D.; Collins, M.; Imig, J.D.; Inscho, E.W.; Pollock, J.S. Age-related alterations in NOS and oxidative stress in mesenteric arteries from male and female rats. J. Appl. Physiol. 2004, 97, 1268–1274. [Google Scholar] [CrossRef]
- El Assar, M.; Angulo, J.; Rodriguez-Manas, L. Oxidative stress and vascular inflammation in aging. Free Radic. Biol. Med. 2013, 65, 380–401. [Google Scholar] [CrossRef]
- Li, H.; Forstermann, U. Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr. Opin. Pharmacol. 2013, 13, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Newaz, M.A.; Yousefipour, Z.; Oyekan, A. Oxidative stress-associated vascular aging is xanthine oxidase-dependent but not NAD(P)H oxidase-dependent. J. Cardiovasc. Pharmacol. 2006, 48, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Piknova, B.; Jenkins, A.; Hellinga, D.; Parver, L.M.; Schechter, A.N. Potential roles of nitrate and nitrite in nitric oxide metabolism in the eye. Sci. Rep. 2020, 10, 13166. [Google Scholar] [CrossRef] [PubMed]
- Piknova, B.; Kocharyan, A.; Schechter, A.N.; Silva, A.C. The role of nitrite in neurovascular coupling. Brain Res. 2011, 1407, 62–68. [Google Scholar] [CrossRef]
- Presley, T.D.; Morgan, A.R.; Bechtold, E.; Clodfelter, W.; Dove, R.W.; Jennings, J.M.; Kraft, R.A.; King, S.B.; Laurienti, P.J.; Rejeski, W.J.; et al. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide 2011, 24, 34–42. [Google Scholar] [CrossRef]
- Becquet, F.; Courtois, Y.; Goureau, O. Nitric oxide in the eye: Multifaceted roles and diverse outcomes. Surv. Ophthalmol. 1997, 42, 71–82. [Google Scholar] [CrossRef]
- Karbowski, J. Scaling of brain metabolism and blood flow in relation to capillary and neural scaling. PLoS ONE 2011, 6, e26709. [Google Scholar] [CrossRef]
- Ainslie, P.N.; Ogoh, S. Regulation of cerebral blood flow in mammals during chronic hypoxia: A matter of balance. Exp. Physiol. 2010, 95, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Damacena-Angelis, C.; Oliveira-Paula, G.H.; Pinheiro, L.C.; Crevelin, E.J.; Portella, R.L.; Moraes, L.A.B.; Tanus-Santos, J.E. Nitrate decreases xanthine oxidoreductase-mediated nitrite reductase activity and attenuates vascular and blood pressure responses to nitrite. Redox Biol. 2017, 12, 291–299. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Xu, M.; Boral, S.; Summer, H.; Lichtenberger, F.B.; Erdogan, C.; Gollasch, M.; Golz, S.; Persson, P.B.; Schleifenbaum, J.; et al. Age Impairs Soluble Guanylyl Cyclase Function in Mouse Mesenteric Arteries. Int. J. Mol. Sci. 2021, 22, 11412. [Google Scholar] [CrossRef]
- Suades, R.; Cosentino, F. Sirtuin 1/soluble guanylyl cyclase: A nitric oxide-independent pathway to rescue ageing-induced vascular dysfunction. Cardiovasc. Res. 2019, 115, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, C.; Man, A.W.C.; Bai, B.; Luo, C.; Huang, Y.; Xu, A.; Vanhoutte, P.M.; Wang, Y. Endothelial SIRT1 prevents age-induced impairment of vasodilator responses by enhancing the expression and activity of soluble guanylyl cyclase in smooth muscle cells. Cardiovasc. Res. 2019, 115, 678–690. [Google Scholar] [CrossRef]
- Tawa, M.; Okamura, T. Factors influencing the soluble guanylate cyclase heme redox state in blood vessels. Vascul. Pharmacol. 2022, 145, 107023. [Google Scholar] [CrossRef]
- Ataei Ataabadi, E.; Golshiri, K.; Juttner, A.A.; de Vries, R.; Van den Berg-Garrelds, I.; Nagtzaam, N.M.A.; Khan, H.N.; Leijten, F.P.J.; Brandt, R.M.C.; Dik, W.A.; et al. Soluble guanylate cyclase activator BAY 54-6544 improves vasomotor function and survival in an accelerated ageing mouse model. Aging Cell. 2022, 21, e13683. [Google Scholar] [CrossRef]
- Cesarini, V.; Guida, E.; Campolo, F.; Crescioli, C.; Di Baldassarre, A.; Pisano, C.; Balistreri, C.R.; Ruvolo, G.; Jannini, E.A.; Dolci, S. Type 5 phosphodiesterase (PDE5) and the vascular tree: From embryogenesis to aging and disease. Mech. Ageing Dev. 2020, 190, 111311. [Google Scholar] [CrossRef] [PubMed]
- Srihirun, S.; Park, J.W.; Teng, R.; Sawaengdee, W.; Piknova, B.; Schechter, A.N. Nitrate uptake and metabolism in human skeletal muscle cell cultures. Nitric Oxide 2020, 94, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, M.F.; Sundqvist, M.L.; Nihlen, C.; Hezel, M.; Carlstrom, M.; Weitzberg, E.; Lundberg, J.O. Profound differences between humans and rodents in the ability to concentrate salivary nitrate: Implications for translational research. Redox Biol. 2016, 10, 206–210. [Google Scholar] [CrossRef]
- Golshiri, K.; Ataei Ataabadi, E.; Portilla Fernandez, E.C.; Jan Danser, A.H.; Roks, A.J.M. The importance of the nitric oxide-cGMP pathway in age-related cardiovascular disease: Focus on phosphodiesterase-1 and soluble guanylate cyclase. Basic Clin. Pharmacol. Toxicol. 2020, 127, 67–80. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piknova, B.; Park, J.W.; Thomas, S.M.; Tunau-Spencer, K.J.; Schechter, A.N. Nitrate and Nitrite Metabolism in Aging Rats: A Comparative Study. Nutrients 2023, 15, 2490. https://doi.org/10.3390/nu15112490
Piknova B, Park JW, Thomas SM, Tunau-Spencer KJ, Schechter AN. Nitrate and Nitrite Metabolism in Aging Rats: A Comparative Study. Nutrients. 2023; 15(11):2490. https://doi.org/10.3390/nu15112490
Chicago/Turabian StylePiknova, Barbora, Ji Won Park, Samantha M. Thomas, Khalid J. Tunau-Spencer, and Alan N. Schechter. 2023. "Nitrate and Nitrite Metabolism in Aging Rats: A Comparative Study" Nutrients 15, no. 11: 2490. https://doi.org/10.3390/nu15112490
APA StylePiknova, B., Park, J. W., Thomas, S. M., Tunau-Spencer, K. J., & Schechter, A. N. (2023). Nitrate and Nitrite Metabolism in Aging Rats: A Comparative Study. Nutrients, 15(11), 2490. https://doi.org/10.3390/nu15112490





