Monosodium Glutamate Intake and Risk Assessment in China Nationwide, and a Comparative Analysis Worldwide
Abstract
1. Introduction
2. Materials and Methods
2.1. Food Consumption Survey and Sampling
2.2. Sample Preparation and Analysis
2.3. Estimation of Dietary Intake and Risk Assessment
3. Results
3.1. Validation of the Determination of Methodological Parameters
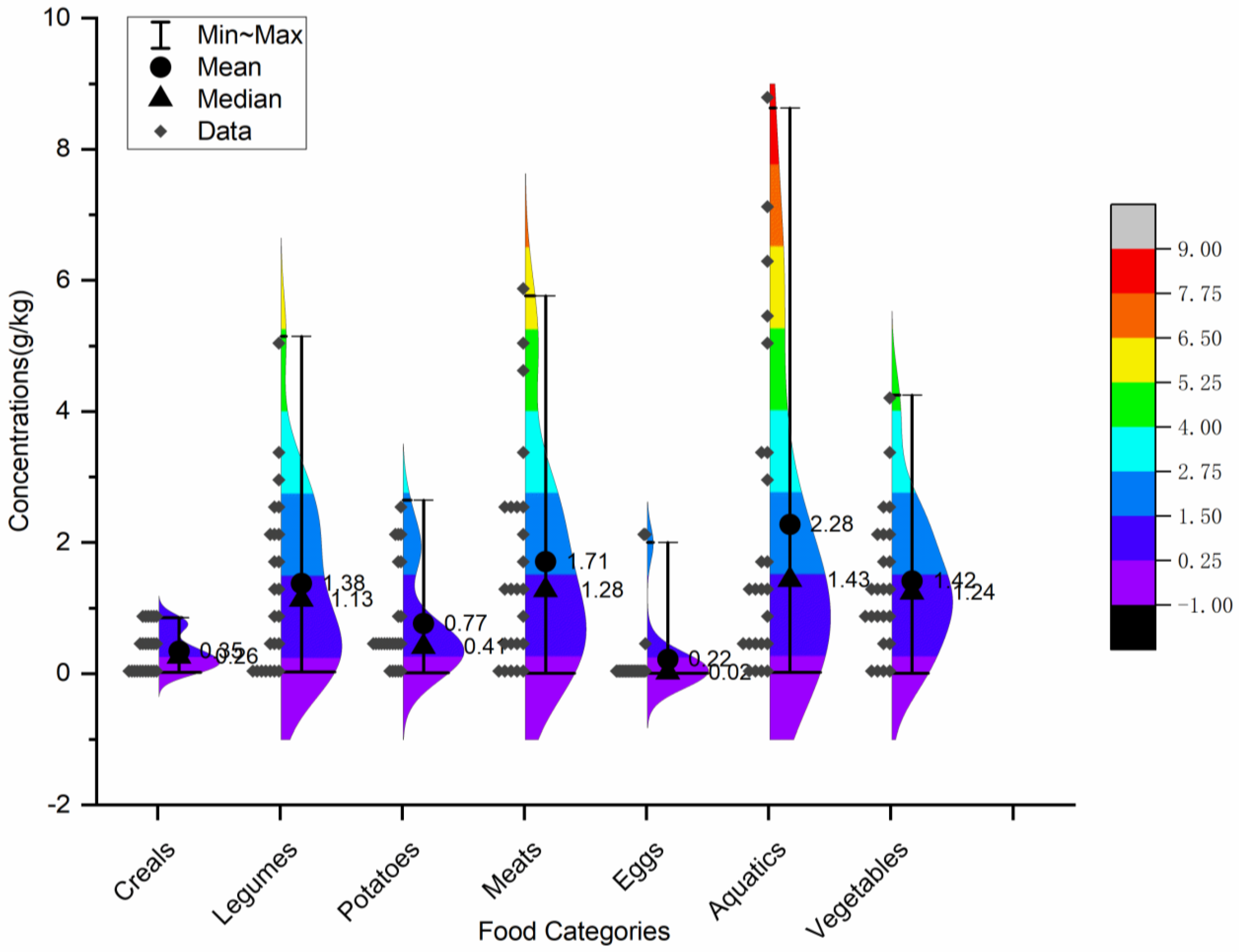
3.2. Occurrence of MSG in Chinese Dietary Foods
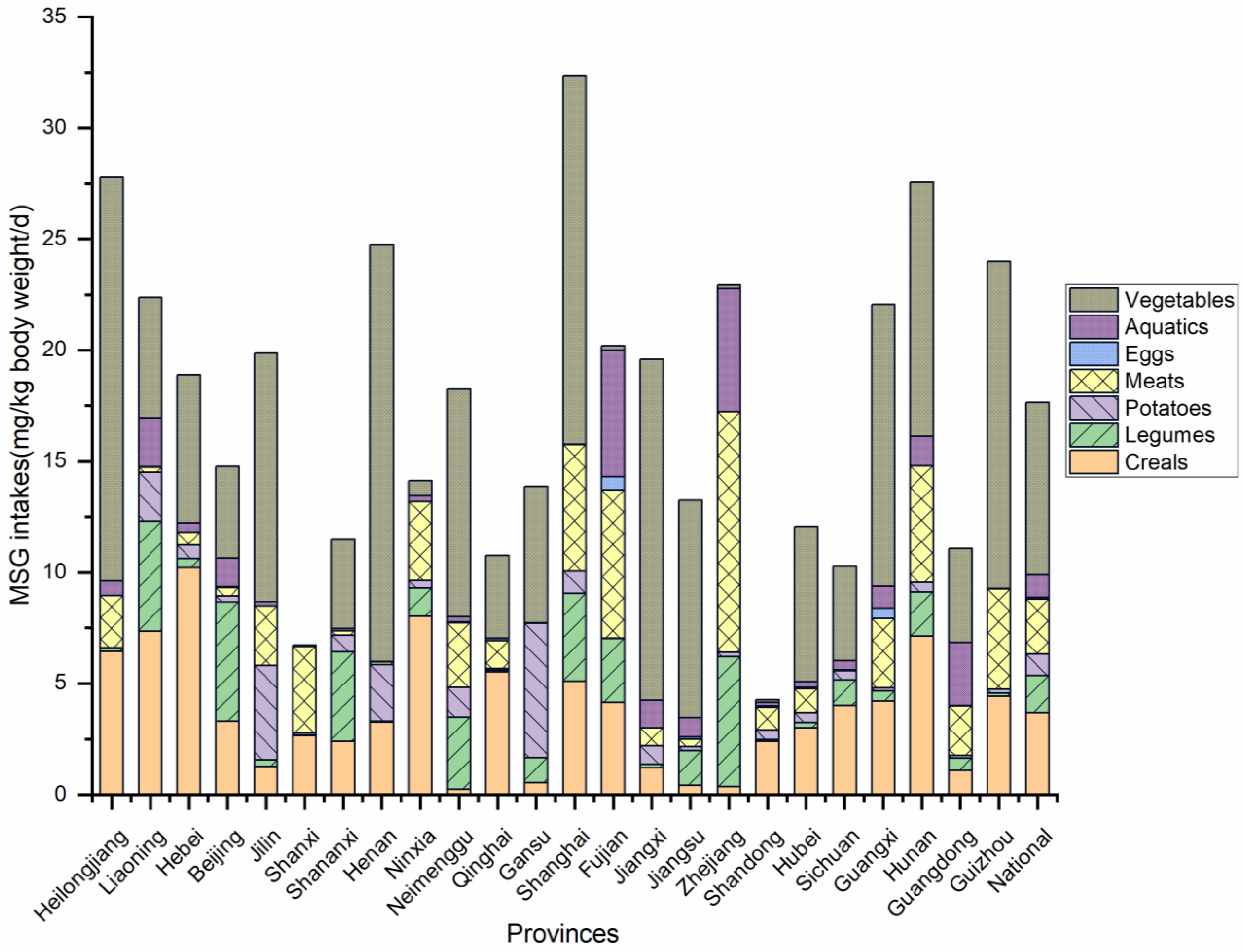
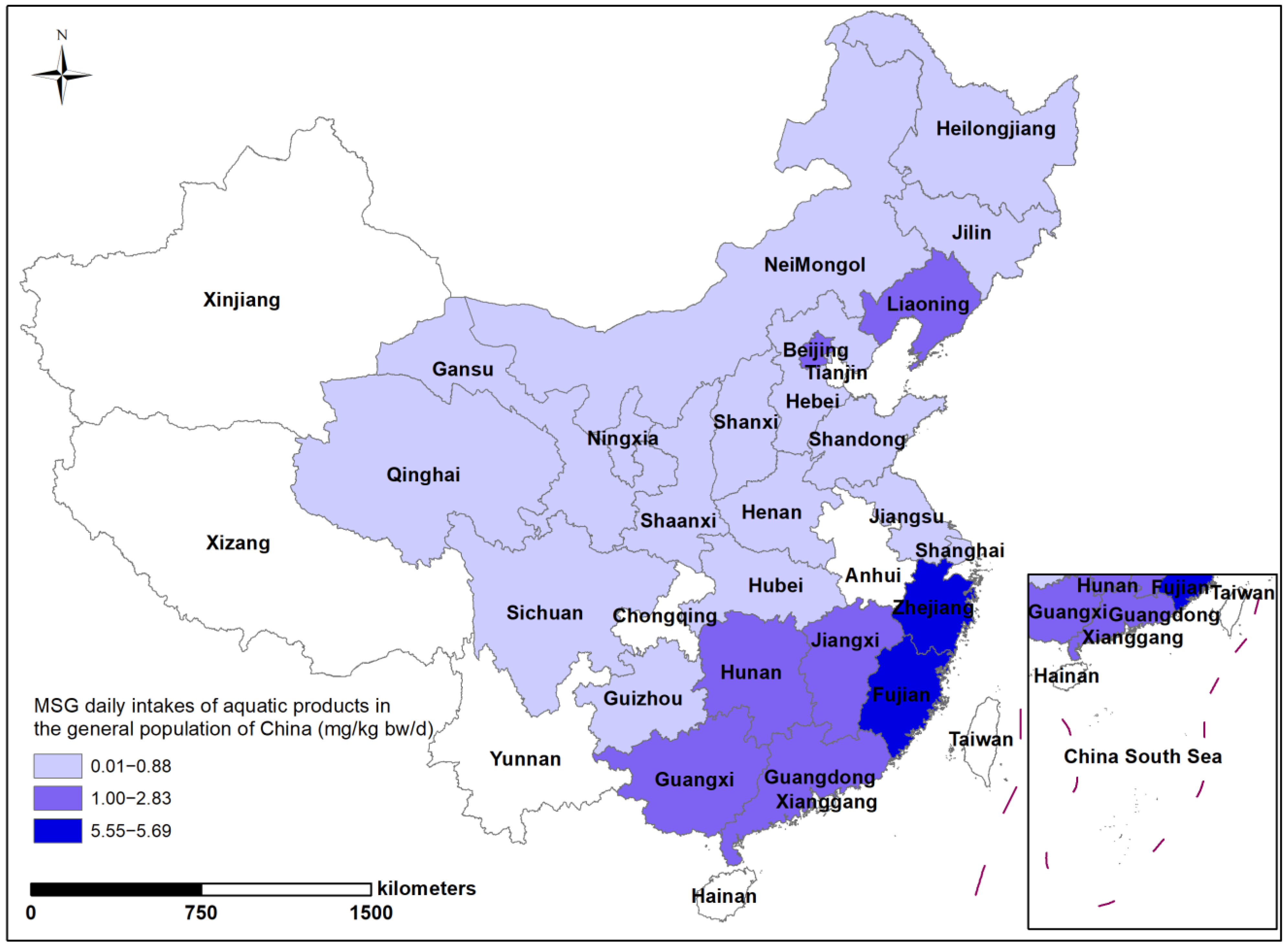
3.3. Analysis of Dietary Intake of MSG
3.4. Dietary Contribution
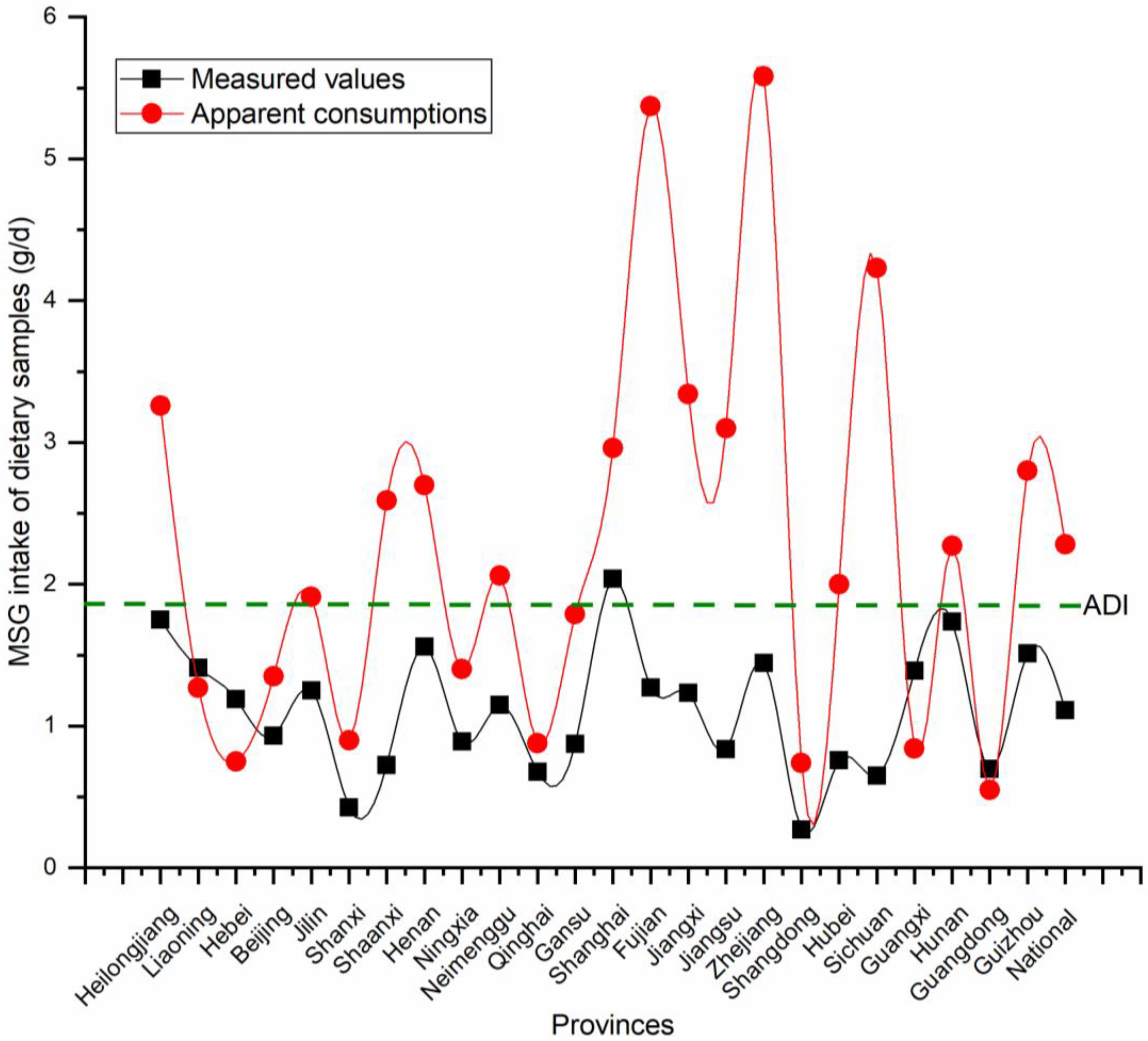
3.5. Risk Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reddy, A.; Ghoshal, J.; Sankaran, P.K.; Trivedi, G.; Ambareesha, K. Histomorphometric study on effects of monosodium glutamate in liver tissue of Wistar rats. JBCPP 2021, 32, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Morita, R.; Ohta, M.; Umeki, Y.; Nanri, A.; Tsuchihashi, T.; Hayabuchi, H. Effect of Monosodium Glutamate on Saltiness and Palatability Ratings of Low-Salt Solutions in Japanese Adults According to Their Early Salt Exposure or Salty Taste Preference. Nutrients 2021, 13, 577. [Google Scholar] [CrossRef] [PubMed]
- Kianifard, D.; Shoar, S.M.M.; Karkan, M.F.; Aly, A. Effects of monosodium glutamate on testicular structural and functional alterations induced by quinine therapy in rat: An experimental study. Int. J. Reprod. Biomed. 2021, 19, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Haque, M.; Aziz, M.; Nazira Sharmin, K. Monosodium Glutamate Level in Kid’s Food and Its Dietary Effects on Liver and Kidney Functions in Adult Rats. J. Food Nutr. 2020, 8, 32–36. [Google Scholar]
- Ervina, E.; Berget, I.; Almli, V.L. Investigating the Relationships between Basic Tastes Sensitivities, Fattiness Sensitivity, and Food Liking in 11-Year-Old Children. Foods 2020, 9, 1315. [Google Scholar] [CrossRef]
- Cebi, N.; Ozturk, T.; Ekinci Doğan, C.; Sagdic, O. Evaluation of Raman microscopy for the detection of additional monosodium glutamate in dry soup mix. Qual. Assur. 2020, 12, 1–10. [Google Scholar]
- Al-Agili, Z.H. The Effect of Food Additives (Monosodium Glutamate—MSG) On Human Health—A Critical Review. J. Almaarif Univ. Coll. 2020, 1, 362–369. [Google Scholar] [CrossRef]
- Zanfirescu, A.; Ungurianu, A.; Tsatsakis, A.M.; Nitulescu, G.M.; Kouretas, D.; Veskoukis, A.; Tsoukalas, D.; Engin, A.B.; Aschner, M.; Margina, D. A review of the alleged health hazards of monosodium glutamate. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1111–1134. [Google Scholar] [CrossRef]
- Sugimoto, M.; Murakami, K.; Fujitani, S.; Matsumoto, H.; Sasaki, S. Dietary free glutamate comes from a variety of food products in the United States. Nutr. Res. 2019, 67, 67–77. [Google Scholar] [CrossRef]
- Airaodion, A.I. Toxicological Effect of Monosodium Glutamate in Seasonings on Human Health. Glob. J. Nutr. Food Sci. 2019, 1, 522. [Google Scholar] [CrossRef]
- Cebi, N.; Dogan, C.E.; Olgun, E.O.; Sagdic, O. A survey of free glutamic acid in foods using a robust LC-MS/MS method. Food Chem. 2018, 248, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Rim, K.-T. Toxicological evaluation of MSG for the manufacturing workers’ health: A literature review. Toxicol. Environ. Health Sci. 2017, 9, 1–11. [Google Scholar] [CrossRef]
- Divine Avwerosuoghene, O.; Barine Innocent, N. Monosodium glutamate alter hepatic functions, redox potential and lipid metabolism: Omega 3 fatty acids ameliorative intervention. GSC Biol. Pharm. Sci. 2020, 13, 101–110. [Google Scholar] [CrossRef]
- Ceglarek, V.M.; Coelho, M.L.; Coelho, R.L.; Almeida, D.L.; de Souza Rodrigues, W.D.N.; Camargo, R.L.; Barella, L.F.; de Freitas Mathias, P.C.; Grassiolli, S. Chronic leucine supplementation does not prevent the obesity and metabolic abnormalities induced by monosodium glutamate. Clin. Nutr. Exp. 2020, 29, 62–75. [Google Scholar] [CrossRef]
- Anderson, G.H.; Fabek, H.; Akilen, R.; Chatterjee, D.; Kubant, R. Acute effects of monosodium glutamate addition to whey protein on appetite, food intake, blood glucose, insulin and gut hormones in healthy young men. Appetite 2018, 120, 92–99. [Google Scholar] [CrossRef]
- Ismail, N.H.; Mohamad Jamil, N.; Samsulrizal, N. Gonadotoxic and Cytotoxic Effect of Induced obesity via Monosodium Glutamate on Mus musculus Testis Cytoarchitecture and Sperm Parameter. Int. J. Agric. Biosyst. Eng. 2014, 8, 1000–1003. [Google Scholar]
- Collison, K.S.; Maqbool, Z.; Saleh, S.M.; Inglis, A.; Makhoul, N.J.; Bakheet, R.; Al-Johi, M.; Al-Rabiah, R.; Zaidi, M.Z.; Al-Mohanna, F.A. Effect of dietary monosodium glutamate on trans fat-induced nonalcoholic fatty liver disease. J. Lipid Res. 2009, 50, 1521–1537. [Google Scholar] [CrossRef]
- Kayode, O.T.; Rotimi, D.E.; Olaolu, T.D.; Adeyemi, O.S. Ketogenic diet improves and restores redox status and biochemical indices in monosodium glutamate-induced rat testicular toxicity. Biomed. Pharmacother. 2020, 127, 110227. [Google Scholar] [CrossRef]
- Noel, C.A.; Finlayson, G.; Dando, R. Prolonged Exposure to Monosodium Glutamate in Healthy Young Adults Decreases Perceived Umami Taste and Diminishes Appetite for Savory Foods. J. Nutr. 2018, 148, 980–988. [Google Scholar] [CrossRef]
- Jin, L.; Lin, L.; Li, G.Y.; Liu, S.; Luo, D.J.; Feng, Q.; Sun, D.S.; Wang, W.; Liu, J.J.; Wang, Q.; et al. Monosodium glutamate exposure during the neonatal period leads to cognitive deficits in adult Sprague-Dawley rats. Neurosci. Lett. 2018, 682, 39–44. [Google Scholar] [CrossRef]
- Rivera-Carvantes, M.C.; Jarero-Basulto, J.J.; Feria-Velasco, A.I.; Beas-Zarate, C.; Navarro-Meza, M.; Gonzalez-Lopez, M.B.; Gudino-Cabrera, G.; Garcia-Rodriguez, J.C. Changes in the expression level of MAPK pathway components induced by monosodium glutamate-administration produce neuronal death in the hippocampus from neonatal rats. Neuroscience 2017, 365, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ojeda, M.; Urena-Guerrero, M.E.; Gutierrez-Barajas, P.E.; Cardenas-Castillo, J.A.; Camins, A.; Beas-Zarate, C. KB-R7943 reduces 4-aminopyridine-induced epileptiform activity in adult rats after neuronal damage induced by neonatal monosodium glutamate treatment. J. Biomed. Sci. 2017, 24, 27. [Google Scholar] [CrossRef] [PubMed]
- Collison, K.; Makhoul, N.; Inglis, A.; Al-Johi, M.; Zaidi, M.Z.; Maqbool, Z.; Saleh, S.M.; Bakheet, R.H.; Mondreal, R.; Al-Rabiah, R.; et al. Dietary trans-fat combined with monosodium glutamate induces dyslipidemia and impairs spatial memory. Physiol. Behav. 2010, 99, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Thu Hien, V.T.; Thi Lam, N.; Cong Khan, N.; Wakita, A.; Yamamoto, S. Monosodium glutamate is not associated with overweight in Vietnamese adults. Public. Health Nutr. 2013, 16, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Luscombe-Marsh, N.D.; Wittert, G.A.; Yuan, B.; Dai, Y.; Pan, X.; Taylor, A.W. Monosodium glutamate is not associated with obesity or a greater prevalence of weight gain over 5 years: Findings from the Jiangsu Nutrition Study of Chinese adults. Br. J. Nutr. 2010, 104, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Ataseven, N.; Yuzbasioglu, D.; Keskin, A.C.; Unal, F. Genotoxicity of monosodium glutamate. Food Chem. Toxicol. 2016, 91, 8–18. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Leblanc, J.-C.; Lindtner, O.; et al. Re-evaluation of glutamic acid (E 620), sodium glutamate (E 621), potassium glutamate (E 622), calcium glutamate (E 623), ammonium glutamate (E 624) and magnesium glutamate (E 625) as food additives. EFSA J. 2017, 15, e04910. [Google Scholar] [PubMed]
- He, K.; Du, S.; Xun, P.; Sharma, S.; Wang, H.; Zhai, F.; Popkin, B. Consumption of monosodium glutamate in relation to incidence of overweight in Chinese adults: China Health and Nutrition Survey (CHNS). Am. J. Clin. Nutr. 2011, 93, 1328–1336. [Google Scholar] [CrossRef]
- Rhodes, J.; Titherley, A.C.; Norman, J.A.; Wood, R.; Lord, D.W. A survey of the monosodium glutamate content of foods and an estimation of the dietary intake of monosodium glutamate. Food Addit. Contam. 1991, 8, 265–274. [Google Scholar] [CrossRef]
- Chinna, K.; Karupaiah, T. Assessment of monosodium glutamate (MSG) intake in a rural Thai community: Questioning the methodological approach. Nutr. Metab. 2013, 10, 52. [Google Scholar] [CrossRef]
- Shi, Z.; Yuan, B.; Wittert, G.A.; Pan, X.; Dai, Y.; Adams, R.; Taylor, A.W. Monosodium glutamate intake, dietary patterns and asthma in Chinese adults. PLoS ONE 2012, 7, e51567. [Google Scholar] [CrossRef] [PubMed]
- Insawang, T.; Selmi, C.; Cha’on, U.; Pethlert, S.; Yongvanit, P.; Areejitranusorn, P.; Boonsiri, P.; Khampitak, T.; Tangrassameeprasert, R.; Pinitsoontorn, C.; et al. Monosodium glutamate (MSG) intake is associated with the prevalence of metabolic syndrome in a rural Thai population. Nutr. Metab. 2012, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Nuri, A.; Lilis, N.; Siti, M.; Hanifah, N. Free Glutamate Content of Condiment and Seasonings and Their Intake in Bogor and Jakarta, Indonesia. Food Nutr. Sci. 2011, 2, 764–769. [Google Scholar]
- He, K.; Zhao, L.; Daviglus, M.L.; Dyer, A.R.; Van Horn, L.; Garside, D.; Zhu, L.; Guo, D.; Wu, Y.; Zhou, B.; et al. Association of monosodium glutamate intake with overweight in Chinese adults: The INTERMAP Study. Obesity 2008, 16, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Populin, T.; Moret, S.; Truant, S.; Conte, L. A survey on the presence of free glutamic acid in foodstuffs, with and without added monosodium glutamate. Food Chem. 2007, 104, 1712–1717. [Google Scholar] [CrossRef]
- Acebal, C.C.; Lista, A.G.; Fernández Band, B.S. Simultaneous determination of flavor enhancers in stock cube samples by using spectrophotometric data and multivariate calibration. Food Chem. 2008, 106, 811–815. [Google Scholar] [CrossRef]
- Lau, O.-W.; Mok, C.-S. Indirect conductometric detection of amino acids after liquid chromatographic separation. Part II. determination of monosodium glutamate in foods. Anal. Chim. Acta 1995, 302, 45–52. [Google Scholar] [CrossRef]
- Skurray, G.R.; Pucar, N. l-glutamic acid content of fresh and processed foods. Food Chem. 1988, 27, 177–180. [Google Scholar] [CrossRef]
- Bodor, R.; Žúborová, M.; Ölvecká, E.; Madajová, V.; Masár, M.; Kaniansky, D.; Stanislawski, B. Isotachophoresis and isotachophoresis-zone electrophoresis of food additives on a chip with column-coupling separation channels. J. Sep. Sci. 2001, 24, 802–809. [Google Scholar] [CrossRef]
- Isa, I.M.; Ab Ghani, S. A non-plasticized chitosan based solid state electrode for flow injection analysis of glutamate in food samples. Food Chem. 2009, 112, 756–759. [Google Scholar] [CrossRef]
- Krishna, V.N.; Karthika, D.; Surya, D.M.; Rubini, M.; Vishalini, M.; Pradeepa, Y. Analysis of Monosodium l-Glutamate in Food Products by High-Performance Thin Layer Chromatography. J. Young Pharm. 2010, 2, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Acebal, C.C.; Insausti, M.; Pistonesi, M.F.; Lista, A.G.; Band, B.S.F. A new automated approach to determine monosodium glutamate in dehydrated broths by using the flow-batch methodology. Talanta 2010, 81, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Croitoru, M.; Fülöp, I.; Ajtay, M.; Dudutz, G.; Crăciun, O.; Dogaru, M. Glutamate determination in foodstuffs with a very simple HPLC-UV method. Acta Aliment. Hung. 2010, 39, 239–247. [Google Scholar] [CrossRef]
- Afraa, A.; Mounir, A.; Zaid, A. Colorimetric Determination of Monosodium Glutamate in Food Samples Using Colorimetric Determination of Monosodium Glutamate in Food Samples Using L-glutamate Oxidase-glutamate Oxidase. Chin. J. Appl. Environ. Biol. 2013, 19, 1069–1072. [Google Scholar] [CrossRef]
- Lee, E.H.; Lee, D.I. A Study on Intake Level of Mono Sodium Glutamate in Korean. Korean J. Environ. Health Soc. 1986, 12, 75–85. [Google Scholar]



| Food Matrix | LODs (μg/kg) | LOQs (μg/kg) | Recoveries (n = 6) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Theoretical (μg/kg) | Measured (μg/kg) | Recoveries (%) | RSD% | Theoretical (μg/kg) | Measured (μg/kg) | Recoveries (%) | RSD % | Theoretical (μg/kg) | Measured (μg/kg) | Recoveries (%) | RSD % | |||
| Cereals | 1.5 | 5 | 10 | 9.6 | 96.0 | 2.3 | 100 | 103.5 | 103.5 | 3.7 | 1000 | 1009.8 | 101.0 | 5.6 |
| Legume | 1.5 | 5 | 10 | 10.2 | 102.0 | 4.1 | 100 | 112.2 | 112.2 | 2.2 | 1000 | 1024.2 | 102.4 | 4.7 |
| Potatoes | 1.5 | 5 | 10 | 9.8 | 98.0 | 3.4 | 100 | 115.7 | 115.7 | 6.4 | 1000 | 1120.3 | 112.0 | 6.5 |
| Meats | 3.5 | 10 | 10 | 7.9 | 79.0 | 4.7 | 100 | 80.1 | 80.1 | 9.2 | 1000 | 956.3 | 95.6 | 12.5 |
| Eggs | 3.5 | 10 | 10 | 8.2 | 82.0 | 3.2 | 100 | 107.8 | 107.8 | 5.5 | 1000 | 1120.2 | 112.0 | 2.2 |
| Aquatic | 3.5 | 10 | 10 | 9.2 | 92.0 | 5.4 | 100 | 101.0 | 101.0 | 6.1 | 1000 | 1104.3 | 110.0 | 9.8 |
| Vegetable | 3.5 | 10 | 10 | 7.7 | 77.0 | 6.0 | 100 | 122.1 | 122.1 | 8.9 | 1000 | 1190.2 | 119.0 | 14.3 |
| Literature | Countries | Food Matrixes and the Concentrations |
|---|---|---|
| Skurray G.R. et al. (1988) [38] | Austria | Fresh foods (0.077−7.57 g kg−1) a; processed foods (0.0006 to 78.51 g kg−1) a |
| Rhodes J. et al. (1991) [29] | United Kingdom | Meat and meat products (0.03–0.81%) b; fish (0.39%) b; vegetables (0.14–2.68%) b; fruits (0.48%) b; cereals (0.17–0.29%) b; pizza (0.92–2.06%) b; miscellaneous (0.33–8.70%) b |
| Bodor R. et al. (2001) [39] | Slovakia | Beef stock (148.8 g kg−1) c; meat stock (71 g kg−1) c; vegetable soup (52.4 g kg−1) c |
| Populin T. et al. (2007) [35] | Italy | Broths, soups, sauces, and salad dressings (1.29 g kg 1) d |
| Acebal C.C. et al. (2008) [36] | Argentina | Beef stocks, chicken stock, stew stock (2.63 ± 0.09–11.93 ± 0.65 g dm −3) a |
| Isa I. et al. (2009) [40] | Malaysia | Seasoning (4.8 ± 0.2–21.3 ± 0.4%) a; chicken soup (5.8 ± 0.2%) c; Chinese soup (7.8 ± 0.3%) c; mushroom soup (2.8 ± 0.2%) c |
| Krishna V.N. (2010) [41] | India | Masala (49.66 ± 1.34 g kg−1) c; soup (24.59 ± 1.47 g kg−1) c; cubes (133.50 ± 0.84 g kg−1) c |
| Acebal C.C. et al. (2010) [42] | Argentina | Dehydrated meat broths, dehydrated vegetable broths (0.17 ± 0.01–1.65 ± 0.02 g dm−3) a |
| Croitoru M. et al. (2010) [43] | Romania | Soup cubes, salamis, hams, vegetable mixes (0.37–119.95 g kg−1) a |
| Afraa A. et al. (2013) [44] | Syrian | Cream of mushroom soup, vegetable soups, lentil soups, noodle soups, hamburgers (0.93–4.9 g kg−1) a |
| Cebi N. et al. (2018) [11] | Turkey | Chips, taste cubes, sauces, soups, etc. (0.1–153.9 g kg−1) a |
| This study (2023) | China | Cereals (0.35 ± 0.28 g kg−1), legumes (1.38 ± 1.33 g kg−1), potatoes (0.77 ± 0.79 g kg−1), meats (1.71 ± 1.64 g kg−1), eggs (0.22 ± 0.55 g kg−1), aquatic foods (2.28 ± 2.47 g kg−1), and vegetables (1.42 ± 1.11 g kg−1) e |
| Literature | Countries | Study Type | Number of Volunteers | Protocol | MSG Intake (g/d) |
|---|---|---|---|---|---|
| Mortensen A. et al. [27] | Austria | ASNS_Adults | Volunteers comprise an unknown number of people of 6 age groups from 19 European countries. | Dietary exposures were estimated by combining individual food consumption data and maximum permitted levels (MPLs) from the EFSA European Integrated Food Consumption Database. ADI values were obtained from rodent experiments previously, and the daily MSG intake for humans was calculated at 70 kg per kg body weight. | 1.41 a |
| Belgium | Diet_National (2004) | 1.00 a | |||
| Czech Republic | SISP (2004) | 1.09 a | |||
| Denmark | DANSDA (2005-08) | 0.35 a | |||
| Finland | FINDIET (2012) | 0.97 a | |||
| France | INCA2 | 0.98 a | |||
| Germany | National_Nutrition_Survey_II | 0.94 a | |||
| Hungary | National_Repr_Surv | 0.39 a | |||
| Ireland | NANS (2012) | 0.99 a | |||
| Italy | INRAN_SCAI (2005_06) | 0.54 a | |||
| Latvia | EFSA_TEST | 1.23 a | |||
| Netherlands | VCPBasis_AVL (2007_2010) | 1.05 a | |||
| Romania | Dieta_Pilot_Adults | 0.52 a | |||
| Spain | AESAN | 0.63 a | |||
| Sweden | Riksmaten (2010) | 1.10 a | |||
| United Kingdom | NDNS–RollingProgrammeYears 1–3 | 0.99 a | |||
| Rhodes J. et al. [29] | United Kingdom | National Food Survey (1987) | School children (aged 10–15) Young adults (aged 15–25) | MSG intake was obtained by measuring food content in combination with consumption. | 0.58 b |
| Sugimoto M. et al. [9] | USA | The National Health and Nutrition Examination Survey (2009–2014) | 8597 children (aged 2–19) and 13,969 adults (age ≥ 20) | Food items, population information, and dietary intake of free glutamate were obtained from various national databases | 0.32 c |
| Insawang T. et al. [32] | Thailand | Epidemiological survey (2009–2010) | 349 Thai adults (aged 33–55) | Giving MSG as the only source for meal preparation for 10 days. | 4.00 d |
| Thu Hien V.T. et al. [24] | Vietnam | Cross-sectional survey (2008) | 1528 Vietnameseadults (age ≥ 20) | Dietary intake was obtained by the 3 d, 24 h recall survey. The consumption of MSG was assessed by weighing it for 3 consecutive days. | 2.20 d |
| Lee E.H. et al. [45] | Korea | MSG intake survey (1986) | 984 Korean (age ≥ 20) | A 2 d orientation course on MSG supervision was conducted for MSG consumption. Body weight was calculated from the “4th Revised Nutrition Investigation by Koreans”. | 1.57 d |
| He et al. [28] | China | China Health and Nutrition Survey, a prospective open-cohort study (1991–2006) | 10,095 healthyChinese adults (aged 18–65) | Dietary intake was obtained with a 3 d, 24 h recall survey. The consumption of MSG was assessed by weighing it for 3 consecutive days. | 2.20 b |
| He et al. [34] | China | Cross-sectional study INTERMAP (1997) | 752 healthy Chineseadults (aged 40–59) | 24 h recall | 0.33 d |
| This study | China | The sixth China Total Diet Study (2016–2019) | About 30,000 (aged 1–96) | See the Materials and Methods section of this article. | 1.11/2.28 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Wang, R.; Zhao, Y.; Song, Y.; Sui, H.; Wu, Y.; Miao, H.; Lyu, B. Monosodium Glutamate Intake and Risk Assessment in China Nationwide, and a Comparative Analysis Worldwide. Nutrients 2023, 15, 2444. https://doi.org/10.3390/nu15112444
Yu H, Wang R, Zhao Y, Song Y, Sui H, Wu Y, Miao H, Lyu B. Monosodium Glutamate Intake and Risk Assessment in China Nationwide, and a Comparative Analysis Worldwide. Nutrients. 2023; 15(11):2444. https://doi.org/10.3390/nu15112444
Chicago/Turabian StyleYu, Hangyu, Rui Wang, Yunfeng Zhao, Yan Song, Haixia Sui, Yongning Wu, Hongjian Miao, and Bing Lyu. 2023. "Monosodium Glutamate Intake and Risk Assessment in China Nationwide, and a Comparative Analysis Worldwide" Nutrients 15, no. 11: 2444. https://doi.org/10.3390/nu15112444
APA StyleYu, H., Wang, R., Zhao, Y., Song, Y., Sui, H., Wu, Y., Miao, H., & Lyu, B. (2023). Monosodium Glutamate Intake and Risk Assessment in China Nationwide, and a Comparative Analysis Worldwide. Nutrients, 15(11), 2444. https://doi.org/10.3390/nu15112444







