Dietary Long-Chain Fatty Acids Accelerate Metabolic Dysfunction in Guinea Pigs with Non-Alcoholic Steatohepatitis
Abstract
1. Introduction
2. Materials and Methods
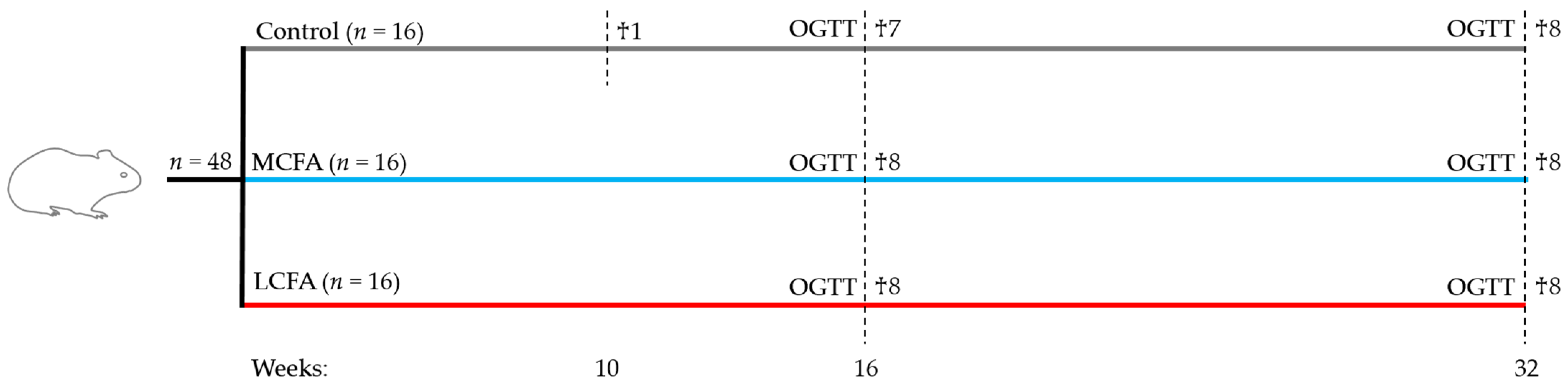
2.1. Animals and Experimental Design
2.2. Oral Glucose Tolerance Test (OGTT)
2.3. Plasma Samples
2.4. Liver Samples
2.5. Histology
2.6. Gene Expression
2.7. Statistics
3. Results
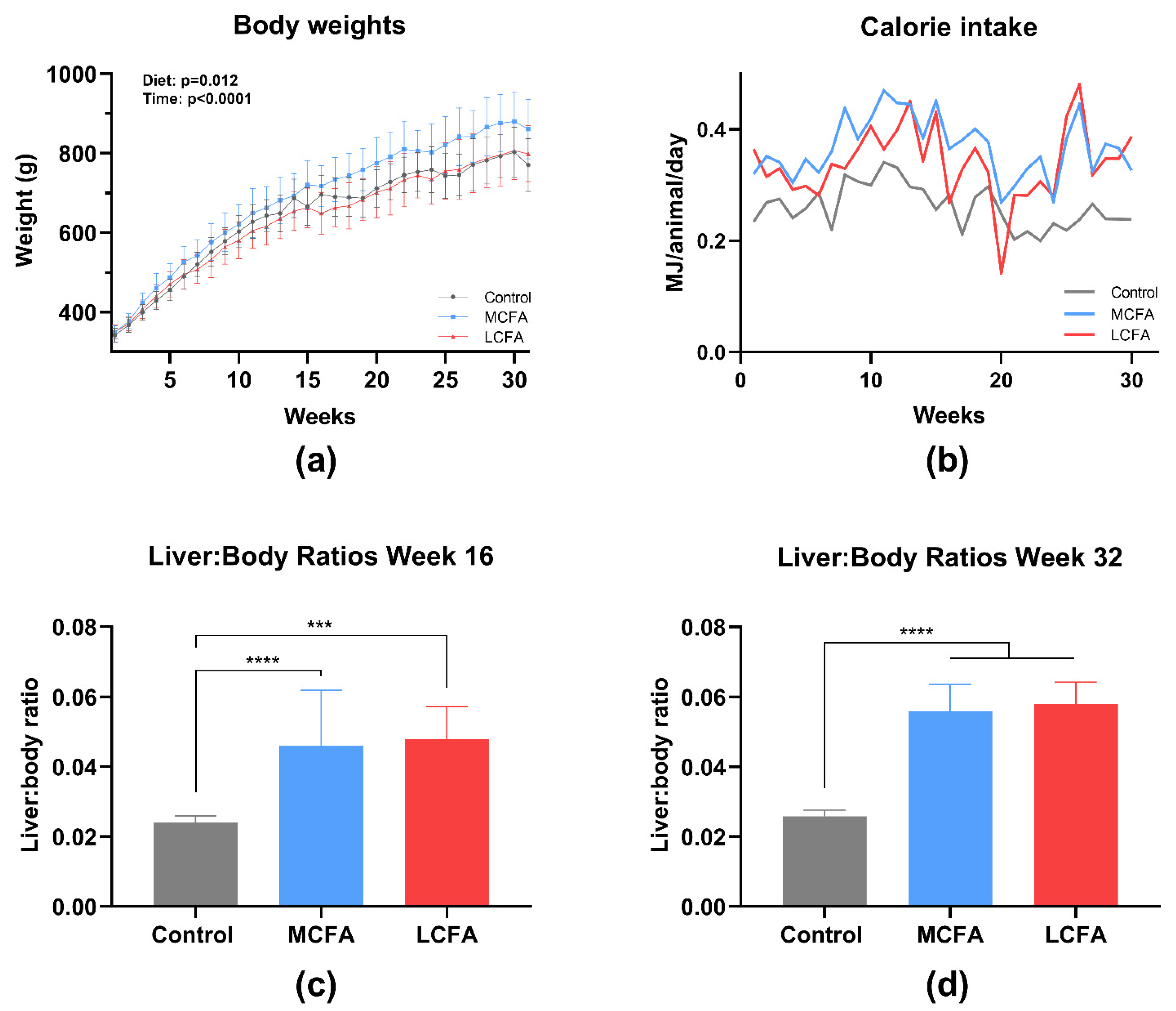
3.1. Body Weights and Liver Size
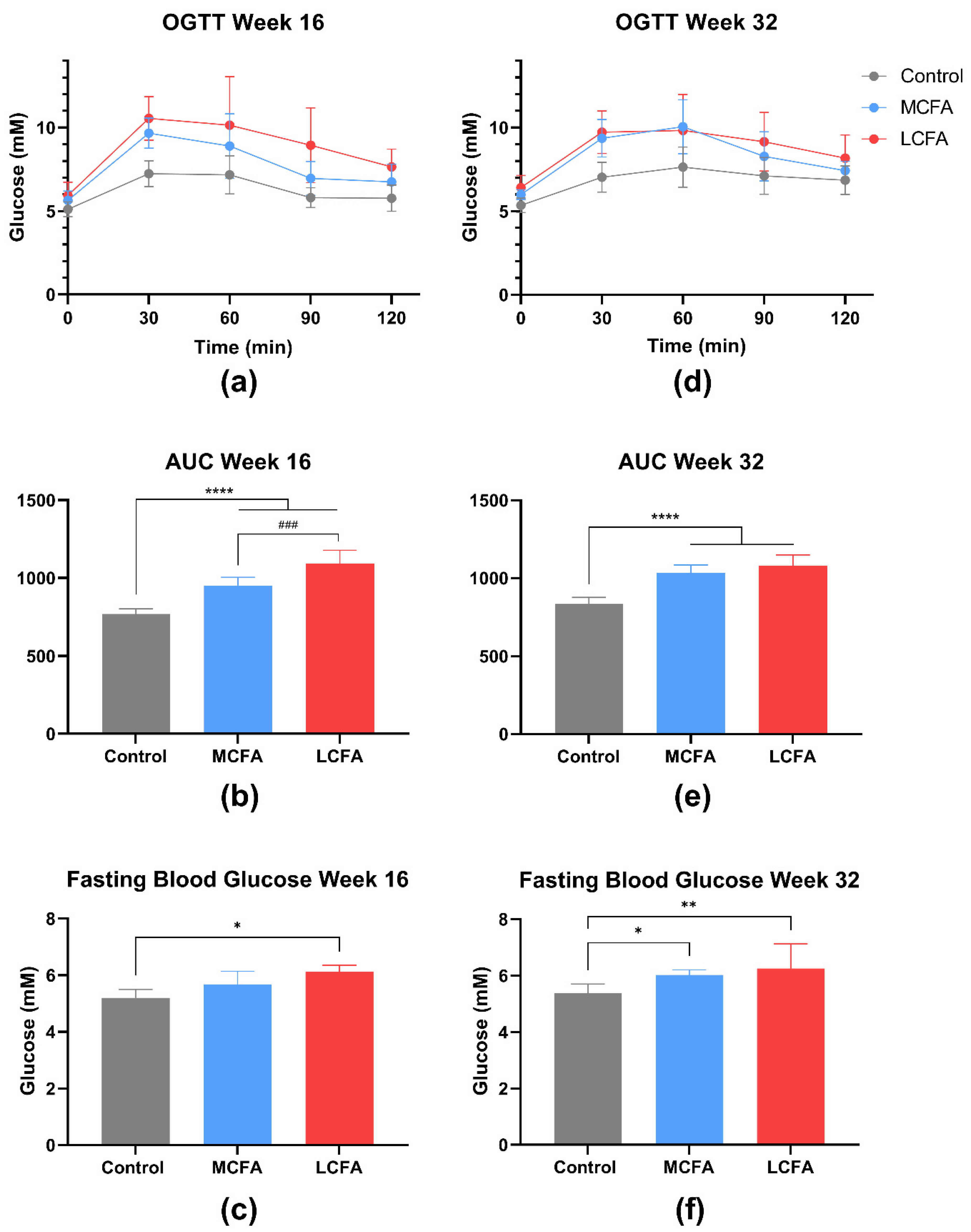
3.2. Oral Glucose Tolerance Test (OGTT)
3.3. Plasma Biochemistry
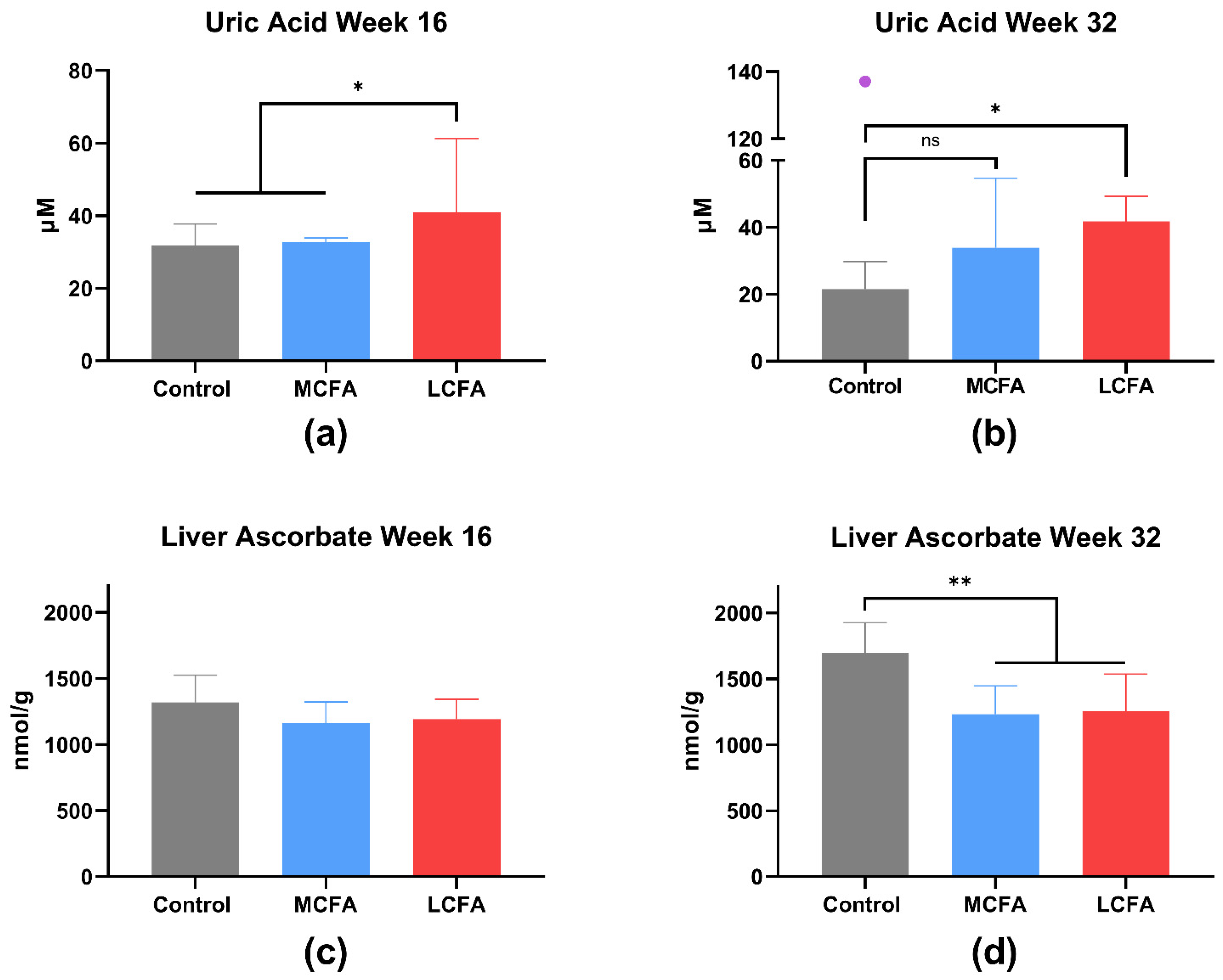
3.4. Liver Biochemistry
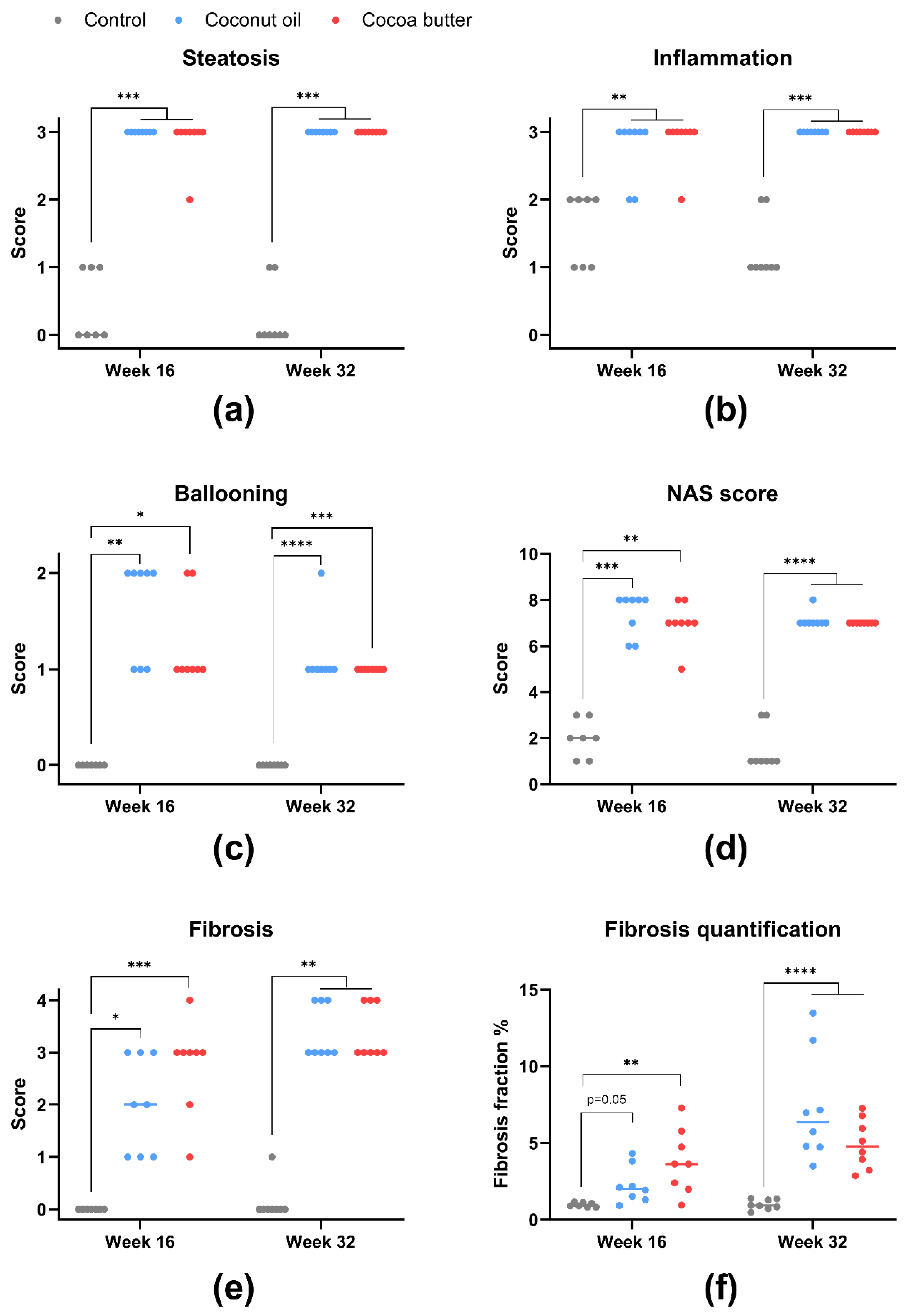
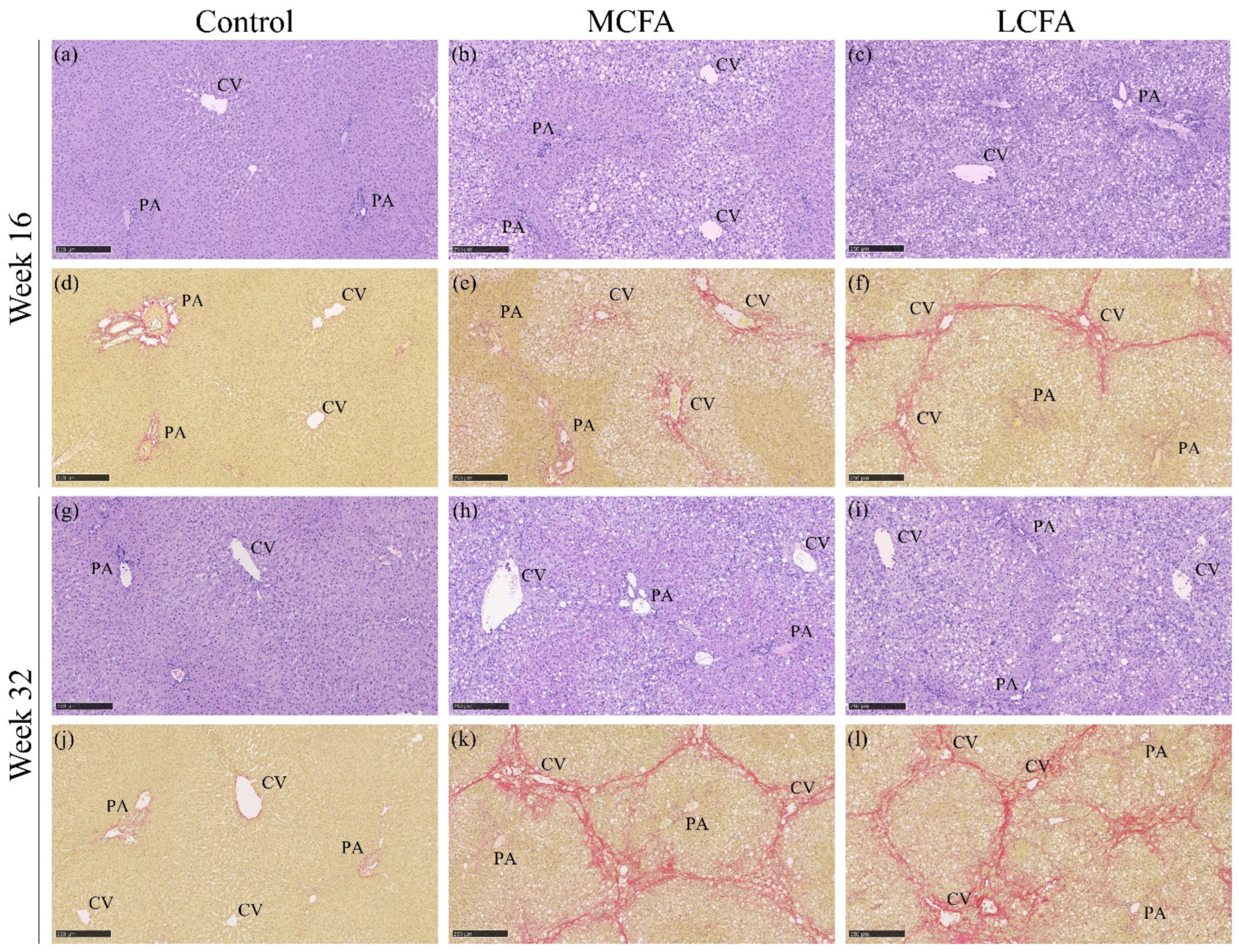
3.5. Histopathology
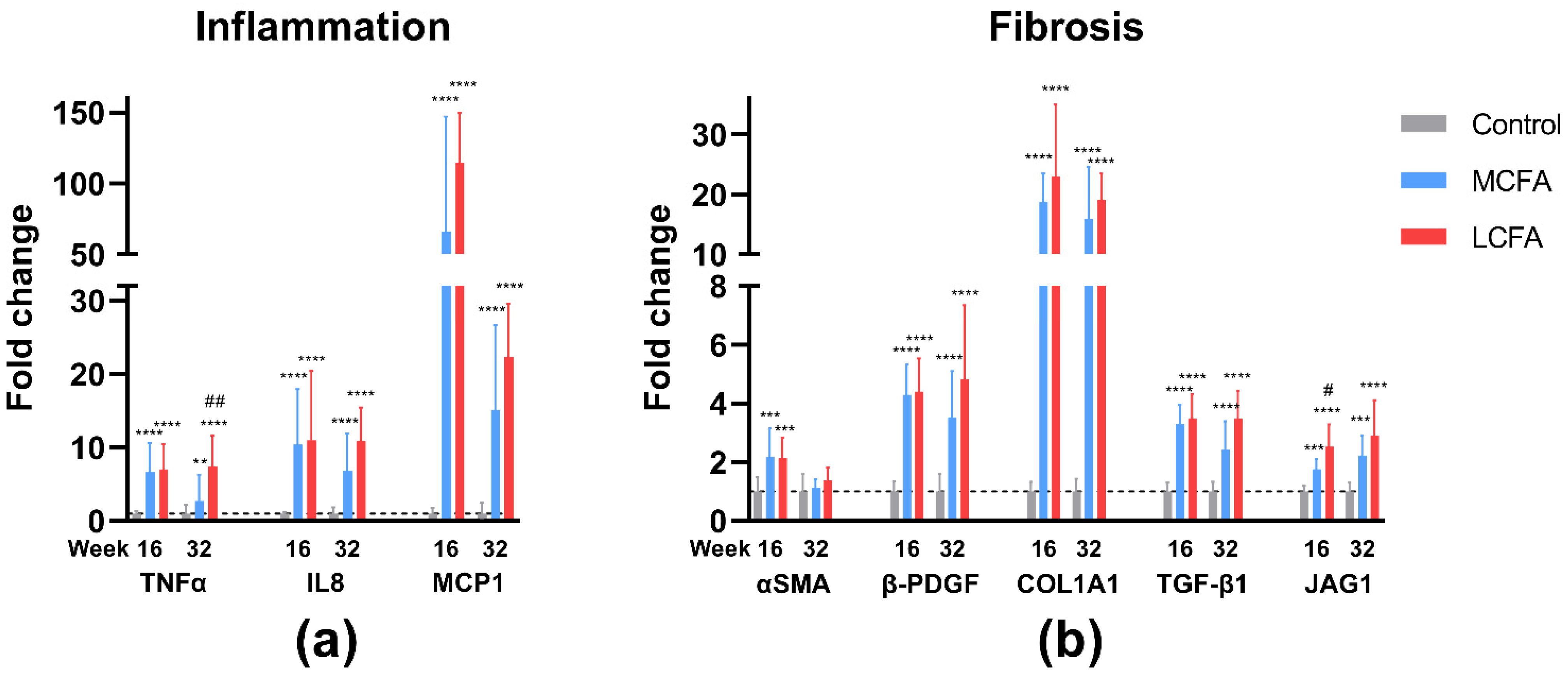
3.6. Gene Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitra, S.; De, A.; Chowdhury, A. Epidemiology of Non-Alcoholic and Alcoholic Fatty Liver Diseases. Transl. Gastroenterol. Hepatol. 2020, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Younossi, Z.M. Nonalcoholic Fatty Liver Disease: A Manifestation of the Metabolic Syndrome. Cleve. Clin. J. Med. 2008, 75, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The Diagnosis and Management of Non-Alcoholic Fatty Liver Disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
- Marjot, T.; Moolla, A.; Cobbold, J.F.; Hodson, L.; Tomlinson, J.W. Nonalcoholic Fatty Liver Disease in Adults: Current Concepts in Etiology, Outcomes, and Management. Endocr. Rev. 2020, 41, 66–117. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Hernández, E.; Chávez-Tapia, N.C.; Uribe, M.; Barbero-Becerra, V.J. Role of Bioactive Fatty Acids in Nonalcoholic Fatty Liver Disease. Nutr. J. 2016, 15, 72. [Google Scholar] [CrossRef]
- Papamandjaris, A.A.; Macdougall, D.E.; Jones, P.J.H. Medium Chain Fatty Acid Metabolism and Energy Expenditure: Obesity Treatment Implications. Life Sci. 1998, 62, 1203–1215. [Google Scholar] [CrossRef]
- Marten, B.; Pfeuffer, M.; Schrezenmeir, J. Medium-Chain Triglycerides. Int. Dairy J. 2006, 16, 1374–1382. [Google Scholar] [CrossRef]
- Newton, I.S. Long Chain Fatty Acids in Health and Nutrition. J. Food Lipids 1996, 3, 233–249. [Google Scholar] [CrossRef]
- Buettner, R.; Parhofer, K.G.; Woenckhaus, M.; Wrede, C.E.; Kunz-Schughart, L.A.; Schölmerich, J.; Bollheimer, L.C. Defining High-Fat-Diet Rat Models: Metabolic and Molecular Effects of Different Fat Types. J. Mol. Endocrinol. 2006, 36, 485–501. [Google Scholar] [CrossRef]
- Hua, J.; Ma, X.; Webb, T.; Potter, J.J.; Oelke, M.; Li, Z. Dietary Fatty Acids Modulate Antigen Presentation to Hepatic NKT Cells in Nonalcoholic Fatty Liver Disease. J. Lipid Res. 2010, 51, 1696–1703. [Google Scholar] [CrossRef]
- Ronis, M.J.J.; Baumgardner, J.N.; Sharma, N.; Vantrease, J.; Ferguson, M.; Tong, Y.; Wu, X.; Cleves, M.A.; Badger, T.M. Medium Chain Triglycerides Dose-Dependently Prevent Liver Pathology in a Rat Model of Non-Alcoholic Fatty Liver Disease. Exp. Biol. Med. 2013, 238, 151–162. [Google Scholar] [CrossRef] [PubMed]
- de Vogel-van den Bosch, J.; van den Berg, S.A.A.; Bijland, S.; Voshol, P.J.; Havekes, L.M.; Romijn, H.A.; Hoeks, J.; van Beurden, D.; Hesselink, M.K.C.; Schrauwen, P.; et al. High-Fat Diets Rich in Medium- versus Long-Chain Fatty Acids Induce Distinct Patterns of Tissue Specific Insulin Resistance. J. Nutr. Biochem. 2011, 22, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Chamma, C.M.d.O.; Bargut, T.C.L.; Mandarim-De-Lacerda, C.A.; Aguila, M.B. A Rich Medium-Chain Triacylglycerol Diet Benefits Adiposity but Has Adverse Effects on the Markers of Hepatic Lipogenesis and Beta-Oxidation. Food Funct. 2017, 8, 778–787. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Osborne, B.; Brown, S.H.J.; Small, L.; Mitchell, T.W.; Cooney, G.J.; Turner, N. Contrasting Metabolic Effects of Medium-versus Long-Chain Fatty Acids in Skeletal Muscle. J. Lipid Res. 2013, 54, 3322–3333. [Google Scholar] [CrossRef]
- Wang, M.E.; Singh, B.K.; Hsu, M.C.; Huang, C.; Yen, P.M.; Wu, L.S.; Jong, D.S.; Chiu, C.H. Increasing Dietary Medium-Chain Fatty Acid Ratio Mitigates High-Fat Diet-Induced Non-Alcoholic Steatohepatitis by Regulating Autophagy. Sci. Rep. 2017, 7, 13999. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Ross, R.; Parsons, W.D.; Jones, P.J.H. Medium-Chain Triglycerides Increase Energy Expenditure and Decrease Adiposity in Overweight Men. Obes. Res. 2003, 11, 395–402. [Google Scholar] [CrossRef]
- Tsuji, H.; Kasai, M.; Takeuchi, H.; Nakamura, M.; Okazaki, M.; Kondo, K. Dietary Medium-Chain Triacylglycerols Suppress Accumulation of Body Fat in a Double-Blind, Controlled Trial in Healthy Men and Women. J. Nutr. 2001, 131, 2853–2859. [Google Scholar] [CrossRef]
- Baldermann, H.; Wicklmayr, M.; Rett, K.; Banholzer, P.; Dietze, G.; Mehnert, H. Changes of Hepatic Morphology during Parenteral Nutrition with Lipid Emulsions Containing LCT or MCT/LCT Quantified by Ultrasound. J. Parenter. Enter. Nutr. 1991, 15, 601–603. [Google Scholar] [CrossRef]
- Ricchi, M.; Odoardi, M.R.; Carulli, L.; Anzivino, C.; Ballestri, S.; Pinetti, A.; Fantoni, L.I.; Marra, F.; Bertolotti, M.; Banni, S.; et al. Differential Effect of Oleic and Palmitic Acid on Lipid Accumulation and Apoptosis in Cultured Hepatocytes. J. Gastroenterol. Hepatol. 2009, 24, 830–840. [Google Scholar] [CrossRef]
- Leyton, J.; Drury, P.J.; Crawford, M.A. Differential Oxidation of Saturated and Unsaturated Fatty Acids in Vivo in the Rat. British J. Nutr. 1987, 57, 383–393. [Google Scholar] [CrossRef]
- Valenzuela, R.; Ortiz, M.; Hernández-Rodas, M.C.; Echeverría, F.; Videla, L.A. Targeting N-3 Polyunsaturated Fatty Acids in Non-Alcoholic Fatty Liver Disease. Curr. Med. Chem. 2020, 27, 5250–5272. [Google Scholar] [CrossRef] [PubMed]
- Picklo, M.J.; Murphy, E.J. A High-Fat, High-Oleic Diet, But Not a High-Fat, Saturated Diet, Reduces Hepatic α-Linolenic Acid and Eicosapentaenoic Acid Content in Mice. Lipids 2016, 51, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Hussein, O.; Grosovski, M.; Lasri, E.; Svalb, S.; Ravid, U.; Assy, N. Monounsaturated Fat Decreases Hepatic Lipid Content in Non-Alcoholic Fatty Liver Disease in Rats. World J. Gastroenterol. 2007, 13, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Rajcic, D.; Brandt, A.; Jin, C.J.; Sánchez, V.; Engstler, A.J.; Jung, F.; Nier, A.; Baumann, A.; Bergheim, I. Exchanging Dietary Fat Source with Extra Virgin Olive Oil Does Not Prevent Progression of Diet-Induced Non-Alcoholic Fatty Liver Disease and Insulin Resistance. PLoS ONE 2020, 15, e0237946. [Google Scholar] [CrossRef]
- Tavares De Almeida, I.; Cortez-Pinto, H.; Fidalgo, G.; Rodrigues, D.; Camilo, M.E. Plasma Total and Free Fatty Acids Composition in Human Non-Alcoholic Steatohepatitis. Clin. Nutr. 2002, 21, 219–223. [Google Scholar] [CrossRef]
- Fridén, M.; Rosqvist, F.; Ahlström, H.; Niessen, H.G.; Schultheis, C.; Hockings, P.; Hulthe, J.; Gummesson, A.; Wanders, A.; Rorsman, F.; et al. Hepatic Unsaturated Fatty Acids Are Linked to Lower Degree of Fibrosis in Non-Alcoholic Fatty Liver Disease. Front. Med. 2022, 8, 814951. [Google Scholar] [CrossRef]
- Tveden-Nyborg, P.; Birck, M.M.; Ipsen, D.H.; Thiessen, T.; Feldmann, L.d.B.; Lindblad, M.M.; Jensen, H.E.; Lykkesfeldt, J. Diet-Induced Dyslipidemia Leads to Nonalcoholic Fatty Liver Disease and Oxidative Stress in Guinea Pigs. Transl. Res. 2016, 168, 146–160. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Tveden-Nyborg, P.; Rolin, B.; Rakipovski, G.; Beck, M.; Mortensen, L.W.; Færk, L.; Heegaard, P.M.H.; Møller, P.; Lykkesfeldt, J. High-Fat but Not Sucrose Intake Is Essential for Induction of Dyslipidemia and Non-Alcoholic Steatohepatitis in Guinea Pigs. Nutr. Metab. 2016, 13, 51. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Skat-Rørdam, J.; Tsamouri, M.M.; Latta, M.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular Drivers of Non-Alcoholic Steatohepatitis Are Sustained in Mild-to-Late Fibrosis Progression in a Guinea Pig Model. Mol. Genet. Genom. 2019, 294, 649–661. [Google Scholar] [CrossRef]
- Skat-Rørdam, J.; Ipsen, D.H.; Hardam, P.D.; Latta, M.; Lykkesfeldt, J.; Tveden-Nyborg, P. Differential Effects of Dietary Components on Glucose Intolerance and Non-Alcoholic Steatohepatitis. Nutrients 2021, 13, 2523. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting Animal Research: Explanation and Elaboration for the ARRIVE Guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef] [PubMed]
- Skat-Rørdam, J.; Pedersen, K.; Skovsted, G.F.; Gregersen, I.; Vangsgaard, S.; Ipsen, D.H.; Latta, M.; Lykkesfeldt, J.; Tveden-Nyborg, P. Vitamin C Deficiency May Delay Diet-Induced NASH Regression in the Guinea Pig. Antioxidants 2022, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Hasselholt, S.; Tveden-Nyborg, P.; Lykkesfeldt, J. Distribution of Vitamin C Is Tissue Specific with Early Saturation of the Brain and Adrenal Glands Following Differential Oral Dose Regimens in Guinea Pigs. Br. J. Nutr. 2015, 113, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Birck, M.M.; Tveden-Nyborg, P.; Lindblad, M.M.; Lykkesfeldt, J. Non-Terminal Blood Sampling Techniques in Guinea Pigs. J. Vis. Exp. 2014, 11, e51982. [Google Scholar] [CrossRef]
- Lykkesfeldt, J. Ascorbate and Dehydroascorbic Acid as Biomarkers of Oxidative Stress: Validity of Clinical Data Depends on Vacutainer System Used. Nutr. Res. 2012, 32, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J. Determination of Ascorbic Acid and Dehydroascorbic Acid in Biological Samples by High-Performance Liquid Chromatography Using Subtraction Methods: Reliable Reduction with Tris[2-Carboxyethyl]Phosphine Hydrochloride. Anal. Biochem. 2000, 282, 89–93. [Google Scholar] [CrossRef]
- Lykkesfeldt, J. Measurement of Ascorbic Acid and Dehydroascorbic Acid in Biological Samples. In Current Protocols in Toxicology; Maines, M., Costa, L.G., Hodson, E., Reed, D.J., Sipes, I.G., Eds.; John Wiley & Sons: New York, NY, USA, 2002; pp. 7.6.1–7.6.15. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Kim, M.Y.; Cho, M.Y.; Baik, S.K.; Park, H.J.; Jeon, H.K.; Im, C.K.; Won, C.S.; Kim, J.W.; Kim, H.S.; Kwon, S.O.; et al. Histological Subclassification of Cirrhosis Using the Laennec Fibrosis Scoring System Correlates with Clinical Stage and Grade of Portal Hypertension. J. Hepatol. 2011, 55, 1004–1009. [Google Scholar] [CrossRef]
- Tveden-Nyborg, P.; Hasselholt, S.; Miyashita, N.; Moos, T.; Poulsen, H.E.; Lykkesfeldt, J. Chronic Vitamin C Deficiency Does Not Accelerate Oxidative Stress in Ageing Brains of Guinea Pigs. Basic. Clin. Pharmacol. Toxicol. 2012, 110, 524–529. [Google Scholar] [CrossRef]
- Sarr, O.; Blake, A.; Thompson, J.A.; Zhao, L.; Rabicki, K.; Walsh, J.C.; Welch, I.; Regnault, T.R.H. The Differential Effects of Low Birth Weight and Western Diet Consumption upon Early Life Hepatic Fibrosis Development in Guinea Pig. J. Physiol. 2016, 594, 1753–1772. [Google Scholar] [CrossRef]
- Balgobin, S.; Montoya, T.I.; Shi, H.; Acevedo, J.F.; Keller, P.W.; Riegel, M.; Wai, C.Y.; Word, R.A. Estrogen Alters Remodeling of the Vaginal Wall after Surgical Injury in Guinea Pigs. Biol. Reprod. 2013, 89, 138. [Google Scholar] [CrossRef] [PubMed]
- Lyons, M.J.; Yoshimura, T.; McMurray, D.N. Mycobacterium Bovis BCG Vaccination Augments Interleukin-8 MRNA Expression and Protein Production in Guinea Pig Alveolar Macrophages Infected with Mycobacterium Tuberculosis. Infect. Immun. 2002, 70, 5471–5478. [Google Scholar] [CrossRef] [PubMed]
- Sarr, O.; Thompson, J.A.; Zhao, L.; Lee, T.Y.; Regnault, T.R.H. Low Birth Weight Male Guinea Pig Offspring Display Increased Visceral Adiposity in Early Adulthood. PLoS ONE 2014, 9, e98433. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hotta, K.; Kikuchi, M.; Kitamoto, T.; Kitamoto, A.; Ogawa, Y.; Honda, Y.; Kessoku, T.; Kobayashi, K.; Yoneda, M.; Imajo, K.; et al. Identification of Core Gene Networks and Hub Genes Associated with Progression of Non-Alcoholic Fatty Liver Disease by RNA Sequencing. Hepatol. Res. 2017, 47, 1445–1458. [Google Scholar] [CrossRef]
- Xu, H.; Wang, L. The Role of Notch Signaling Pathway in Non-Alcoholic Fatty Liver Disease. Front. Mol. Biosci. 2021, 8, 792667. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Hariharan, K.; TidAng, J.; Frangioudakis, G.; Beale, S.M.; Wright, L.E.; Zeng, X.Y.; Leslie, S.J.; Li, J.Y.; Kraegen, E.W.; et al. Enhancement of Muscle Mitochondrial Oxidative Capacity and Alterations in Insulin Action Are Lipid Species Dependent: Potent Tissue-Specific Effects of Medium-Chain Fatty Acids. Diabetes 2009, 58, 2547–2554. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; Bourque, C.; Jones, P.; Ross, R.; Parsons, W.E. Medium-versus Long-Chain Triglycerides for 27 Days Increases Fat Oxidation and Energy Expenditure without Resulting in Changes in Body Composition in Overweight Women. Int. J. Obes. 2003, 27, 95–102. [Google Scholar] [CrossRef]
- Han, J.R.; Deng, B.; Sun, J.; Chen, C.G.; Corkey, B.E.; Kirkland, J.L.; Ma, J.; Guo, W. Effects of Dietary Medium-Chain Triglyceride on Weight Loss and Insulin Sensitivity in a Group of Moderately Overweight Free-Living Type 2 Diabetic Chinese Subjects. Metabolism 2007, 56, 985–991. [Google Scholar] [CrossRef]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef]
- Kraegen, E.W.; Cooney, G.J. Free Fatty Acids and Skeletal Muscle Insulin Resistance. Curr. Opin. Lipidol. 2008, 19, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Cho, Y.; Tachibana, S.; Chiba, T.; Schneider, W.J.; Akiba, Y. Impairment of VLDL Secretion by Medium-Chain Fatty Acids in Chicken Primary Hepatocytes Is Affected by the Chain Length. J. Nutr. 2005, 135, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Wojtczak, L. Short- and Medium-Chain Fatty Acids in Energy Metabolism: The Cellular Perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular Mechanisms of Hepatic Lipid Accumulation in Non-Alcoholic Fatty Liver Disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef] [PubMed]
- Listenberger, L.L.; Han, X.; Lewis, S.E.; Cases, S.; Farese, R.V.; Ory, D.S.; Schaffer, J.E. Triglyceride Accumulation Protects against Fatty Acid-Induced Lipotoxicity. Proc. Natl. Acad. Sci. USA 2003, 100, 3077–3082. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Yang, L.; McCall, S.; Huang, J.; Xing, X.Y.; Pandey, S.K.; Bhanot, S.; Monia, B.P.; Li, Y.X.; Diehl, A.M. Inhibiting Triglyceride Synthesis Improves Hepatic Steatosis but Exacerbates Liver Damage and Fibrosis in Obese Mice with Nonalcoholic Steatohepatitis. Hepatology 2007, 45, 1366–1374. [Google Scholar] [CrossRef]
- Malhi, H.; Bronk, S.F.; Werneburg, N.W.; Gores, G.J. Free Fatty Acids Induce JNK-Dependent Hepatocyte Lipoapoptosis. J. Biol. Chem. 2006, 281, 12093–12101. [Google Scholar] [CrossRef]
- Feldstein, A.E.; Werneburg, N.W.; Canbay, A.; Guicciardi, M.E.; Bronk, S.F.; Rydzewski, R.; Burgart, L.J.; Gores, G.J. Free Fatty Acids Promote Hepatic Lipotoxicity by Stimulating TNF-α Expression via a Lysosomal Pathway. Hepatology 2004, 40, 185–194. [Google Scholar] [CrossRef]
- Ryu, S.; Chang, Y.; Kim, S.G.; Cho, J.; Guallar, E. Serum Uric Acid Levels Predict Incident Nonalcoholic Fatty Liver Disease in Healthy Korean Men. Metabolism 2011, 60, 860–866. [Google Scholar] [CrossRef]
- Mosca, A.; Nobili, V.; De Vito, R.; Crudele, A.; Scorletti, E.; Villani, A.; Alisi, A.; Byrne, C.D. Serum Uric Acid Concentrations and Fructose Consumption Are Independently Associated with NASH in Children and Adolescents. J. Hepatol. 2017, 66, 1031–1036. [Google Scholar] [CrossRef]
- Liu, C.Q.; He, C.M.; Chen, N.; Wang, D.; Shi, X.; Liu, Y.; Zeng, X.; Yan, B.; Liu, S.; Yang, S.; et al. Serum Uric Acid Is Independently and Linearly Associated with Risk of Nonalcoholic Fatty Liver Disease in Obese Chinese Adults. Sci. Rep. 2016, 6, 38605. [Google Scholar] [CrossRef]
- Li, Y.; Xu, C.; Yu, C.; Xu, L.; Miao, M. Association of Serum Uric Acid Level with Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study. J. Hepatol. 2009, 50, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.; George, J.; Dillon, J.F.; Clare, K. Determining the Role for Uric Acid in Non-Alcoholic Steatohepatitis Development and the Utility of Urate Metabolites in Diagnosis: An Opinion Review. World J. Gastroenterol. 2020, 26, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Xu, C.; Lin, Y.; Lu, C.; Li, D.; Sang, J.; He, H.; Liu, X.; Li, Y.; Yu, C. Uric Acid Regulates Hepatic Steatosis and Insulin Resistance through the NLRP3 Inflammasome-Dependent Mechanism. J. Hepatol. 2016, 64, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric Acid Induces Hepatic Steatosis by Generation of Mitochondrial Oxidative Stress: Potential Role in Fructose-Dependent and -Independent Fatty Liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef]
- Nizar, N.N.A.; Marikkar, J.M.N.; Hashim, D.M. Differentiation of Lard, Chicken Fat, Beef Fat and Mutton Fat by GCMS and EA-IRMS Techniques. J. Oleo Sci. 2013, 62, 459–464. [Google Scholar] [CrossRef]
- Lipp, M.; Simoneau, C.; Ulberth, F.; Anklam, E.; Crews, C.; Brereton, P.; de Greyt, W.; Schwack, W.; Wiedmaier, C. Composition of Genuine Cocoa Butter and Cocoa Butter Equivalents. J. Food Compos. Anal. 2001, 14, 399–408. [Google Scholar] [CrossRef]
| Content | Control | MCFA | LCFA |
|---|---|---|---|
| Product number: | S9406-S042 | S9406-S0253 | S9406-S050 |
| Crude protein (%) | 17.1 | 16.9 | 16.9 |
| Crude fat (%) | 3.8 | 20.0 | 20.0 |
| Crude fiber (%) | 19.8 | 11.4 | 11.4 |
| Crude ash (%) | 7.9 | 6.6 | 6.6 |
| Starch (%) | 13.4 | 7.9 | 7.9 |
| Sugar (%) | 4.0 | 17.3 | 17.3 |
| Cholesterol (%) | 0.0 | 0.350 | 0.350 |
| ME (MJ/kg) | 11.2 | 16.8 | 16.8 |
| Ascorbic acid (mg/kg) | 2000 | 2000 | 2000 |
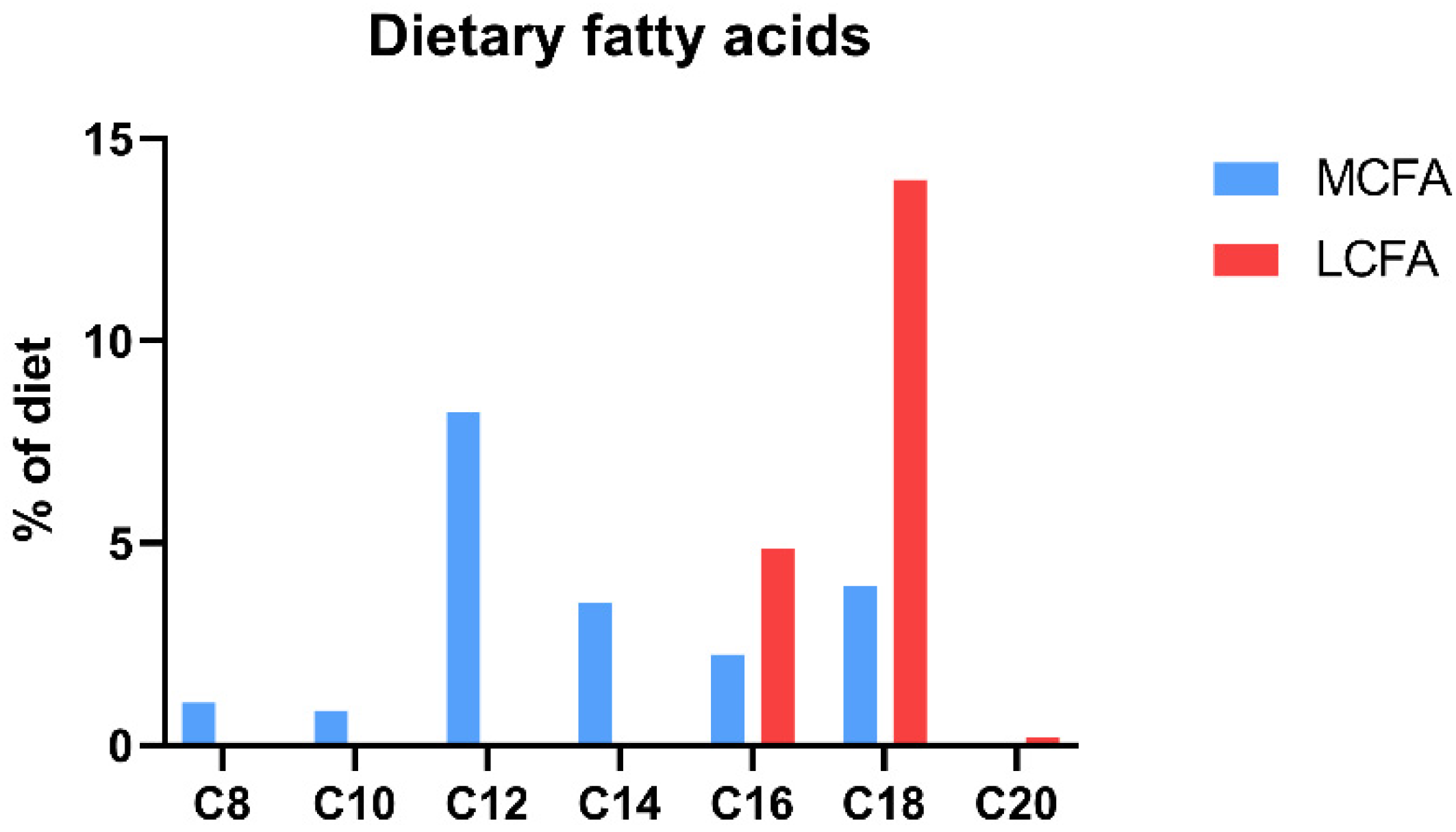
| Fatty Acids | Control | MCFA | LCFA |
|---|---|---|---|
| C8:0 (%) | - | 1.06 | - |
| C10:0 (%) | - | 0.86 | - |
| C12:0 (%) | - | 8.22 | - |
| C14:0 (%) | 0.01 | 3.53 | 0.02 |
| C16:0 (%) | 0.64 | 2.23 | 4.80 |
| C18:0 (%) | 0.11 | 2.32 | 6.27 |
| C20:0 (%) | 0.01 | 0.03 | 0.19 |
| C16:1 (%) | 0.02 | 0.01 | 0.07 |
| C18:1 (%) | 0.67 | 0.44 | 6.13 |
| C18:2 (%) | 1.89 | 0.96 | 1.31 |
| C18:3 (%) | 0.35 | 0.22 | 0.27 |
| C20:1 (%) | <0.01 | <0.01 | <0.01 |
| Total MCFAs (%) | - | 10.14 | - |
| Total LCFAs (%) | 3.70 | 9.74 | 19.06 |
| Total saturated fatty acids (%) | 0.77 | 18.25 | 11.28 |
| Total unsaturated fatty acids (%) | 2.93 | 1.63 | 7.78 |
| Gene | Accession No. | Forward | Reverse | Product (bp) |
|---|---|---|---|---|
| ACTA2 (αSMA) [41] | ENSCPOT00000011693.2 | GACATCAAGGAGAAGCTGTG | GCTGTTGTAGGTGGTTTCAT | 273 |
| COL1A1 [41] | XM_003466865.2 | CTGGACAGCGTGGTGTAGTC | TCCAGAAGGACCTTGTTTGC | 104 |
| PDGFB (β-PDGF) [29] | XM_013153075.1 | CCCCTCCAGCAGATGAAGTT | GGTCTCAATCCAGGGTCCAA | 199 |
| TGFB1 (TGF-β1) [42] | NM_001173023.1 | AACCCGAGCCGGACTACTATG | TGCTTTTATAGATATTGTGGC TGTTGT | 78 |
| CXCL8 (IL8) [43] | NM_001173399.2 | GGCAGCCTTCCTGCTCTCT | CAGCTCCGAGACCAACTTTGT | 67 |
| TNF (TNFα) [44] | NM_001173025.1 | GCCGTCTCCTACCCGGAAAA | TAGATCTGCCCGGAATCGGC | 203 |
| CCL2 (MCP1) [29] | NM_001172926.1 | TGCCAAACTGGACCAGAGAA | CGAATGTTCAAAGGCTTT GAAGT | 75 |
| JAG1 | XM_003476557.4 | ACAGGACAACAGGGACTTGG | AGTGCCCTCCGATTCTACCT | 105 |
| ACTB (β-ACTIN) [40] | AF508792 | GTAAGGACCTCTATGCCAACACA | ATGCCAATCTCATCTCGTTTTCT | 346 |
| DCTN5 | XM_003477819.4 | TTGACGGGATTCTGAGGTGC | CACAACACTGACTGGCGACT | 122 |
| Week | Control | MCFA | LCFA | |
|---|---|---|---|---|
| FFA (mmol/L) | 16 1 | 0.54 (0.49—0.62) | 0.61 (0.54–0.68) | 0.75 (0.59–0.91) * |
| 32 1 | 0.35 (0.19–0.54) | 0.59 (0.51–0.61) | 0.60 (0.50–0.84) * | |
| TG (mmol/L) | 16 1 | 0.64 (0.58–0.82) | 0.70 (0.50–0.85) | 0.75 (0.63–0.86) |
| 32 2 | 0.65 (0.54–0.81) | 0.54 (0.45–0.66) | 0.52 (0.42–0.58) * | |
| TC (mmol/L) | 16 3 | 0.52 (0.49–0.85) | 7.12 (4.98–11.23) **** | 3.17 (2.54–4.55) ****,## |
| 32 2 | 0.53 (0.43–0.66) | 7.42 (5.94–7.84) **** | 4.24 (3.78–5.17) ****,## | |
| AST (U/L) | 16 2 | 126 (30.7–329) | 398 (169–1003) * | 441 (243–492) * |
| 32 2 | 31.9 (20.4–36.2) | 470 (395–556) **** | 555 (305–718) **** | |
| ALT (U/L) | 16 1 | 29.7 (20.2–35.8) | 44.0 (33.8–62.9) | 44.0 (32.2–78.4) |
| 32 3 | 33.0 (25.2–36.5) | 66.9 (48.9–93.5) * | 72.3 (57.2–98.6) ** | |
| ALP (U/L) | 16 1 | 59 (41–68) | 53 (42–56) | 55 (39–80) |
| 32 1 | 56 (48–61) | 44 (37–49) * | 53 (47–57) | |
| HbA1c (%) | 16 1 | 3.9 (3.8–4.0) | 4.0 (3.9–4.2) | 3.9 (3.8–4.3) |
| 32 1 | 3.9 (3.8–4.0) | 4.3 (4.0–4.5) * | 4.2 (4.1–4.8) * | |
| Ascorbate (µmol/L) | 16 4 | 39.1 ± 6.1 | 35.4 ± 10.2 | 39.5 ± 8.6 |
| 32 4 | 46.6 ± 12.2 | 33.0 ± 12.6 | 44.3 ± 14.1 |
| Week | Control | MCFA | LCFA | |
|---|---|---|---|---|
| TG (µmol/g) | 16 1 | 7.06 (6.15–10.1) | 55.0 (46.3–62.7) * | 70.8 (55.3–75.3) *** |
| 32 2 | 4.79 (4.34–5.97) | 39.7 (33.4–42.5) **** | 59.3 (52.6–66.9) ****,#### | |
| TC (µmol/g) | 16 3 | 4.31 (3.91–5.05) | 32.0 (30.4–36.3) **** | 28.5 (24.4–31.4) ****,# |
| 32 3 | 4.10 (3.77–4.46) | 27.3 (24.7–32.5) **** | 28.3 (27.6–33.4) **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedersen, K.; Ipsen, D.H.; Skat-Rørdam, J.; Lykkesfeldt, J.; Tveden-Nyborg, P. Dietary Long-Chain Fatty Acids Accelerate Metabolic Dysfunction in Guinea Pigs with Non-Alcoholic Steatohepatitis. Nutrients 2023, 15, 2445. https://doi.org/10.3390/nu15112445
Pedersen K, Ipsen DH, Skat-Rørdam J, Lykkesfeldt J, Tveden-Nyborg P. Dietary Long-Chain Fatty Acids Accelerate Metabolic Dysfunction in Guinea Pigs with Non-Alcoholic Steatohepatitis. Nutrients. 2023; 15(11):2445. https://doi.org/10.3390/nu15112445
Chicago/Turabian StylePedersen, Kamilla, David Højland Ipsen, Josephine Skat-Rørdam, Jens Lykkesfeldt, and Pernille Tveden-Nyborg. 2023. "Dietary Long-Chain Fatty Acids Accelerate Metabolic Dysfunction in Guinea Pigs with Non-Alcoholic Steatohepatitis" Nutrients 15, no. 11: 2445. https://doi.org/10.3390/nu15112445
APA StylePedersen, K., Ipsen, D. H., Skat-Rørdam, J., Lykkesfeldt, J., & Tveden-Nyborg, P. (2023). Dietary Long-Chain Fatty Acids Accelerate Metabolic Dysfunction in Guinea Pigs with Non-Alcoholic Steatohepatitis. Nutrients, 15(11), 2445. https://doi.org/10.3390/nu15112445








