Complex Extract of Polygonatum sibiricum and Nelumbinis semen Improves Menopause Symptoms via Regulation of Estrogen Receptor Beta in an Ovariectomized Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of PS and NS Extracts
2.2. Ovariectomy
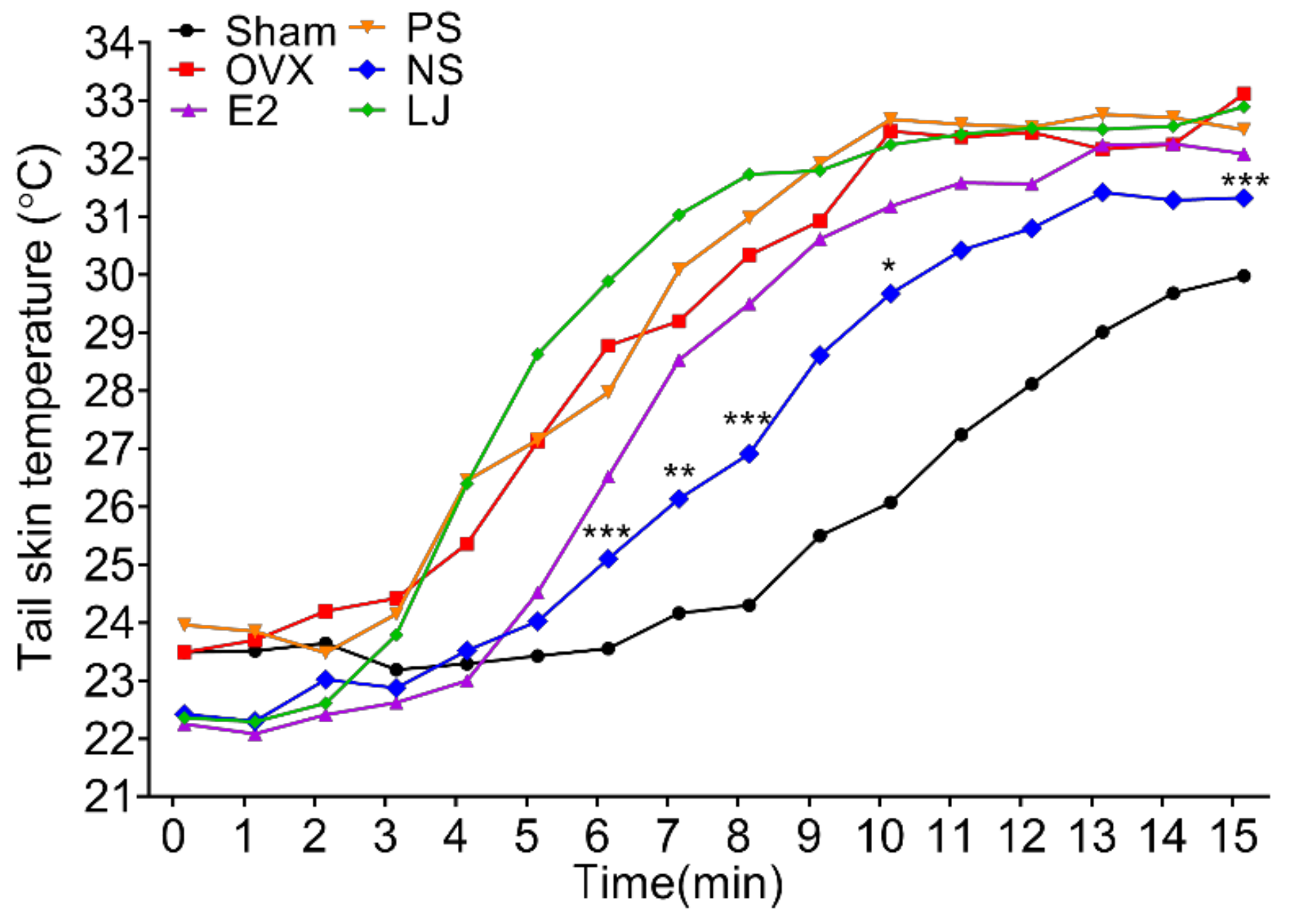
2.3. Facial Flushing Verification Test
2.4. Vaginal Smear
2.5. Histology and Immunohistochemistry
2.6. Blood Biochemical Analysis
2.7. Hypothalamus Analysis
2.8. Quantification and Statistical Analysis
3. Results
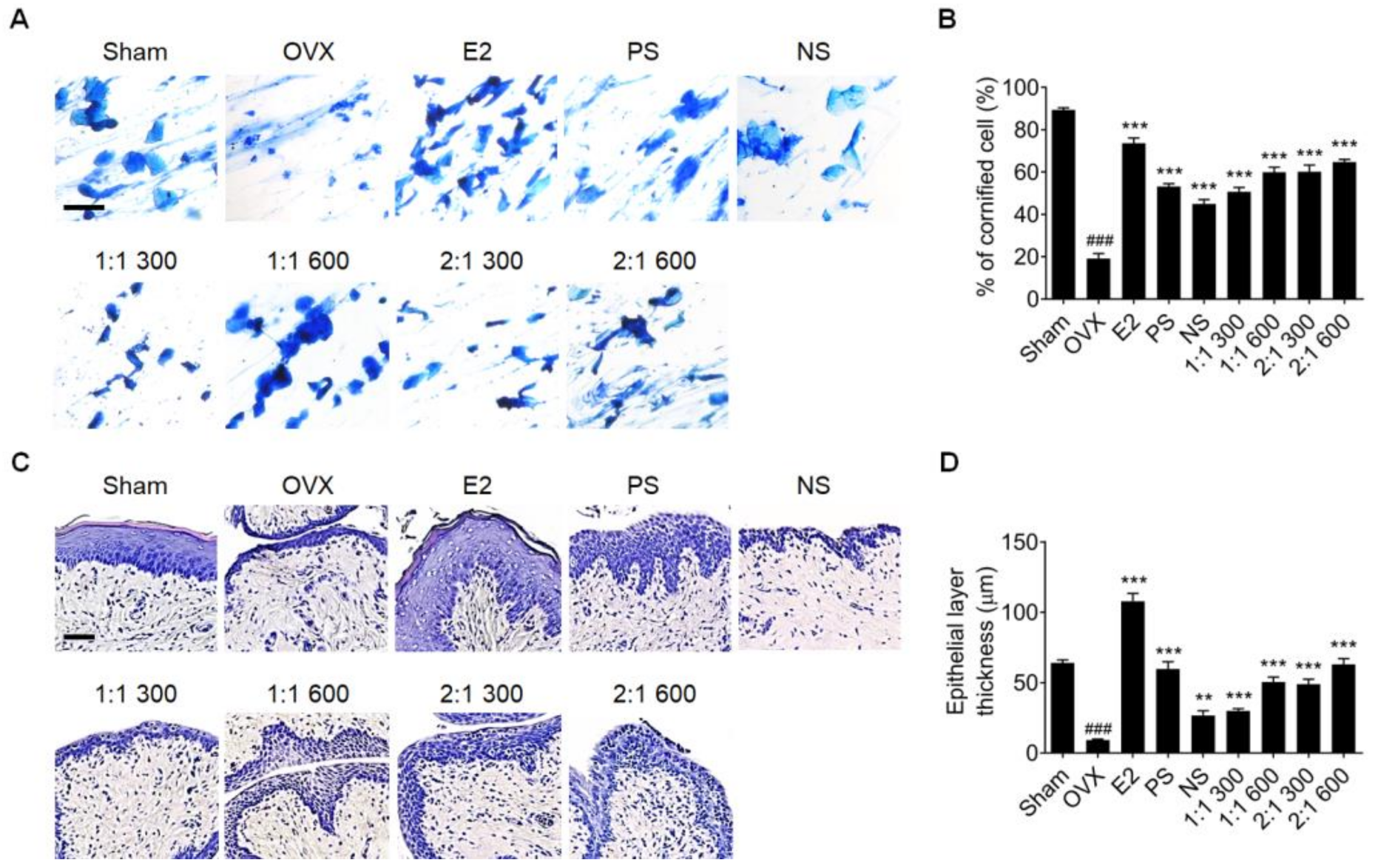
3.1. Complex Extract of PS and NS Enhanced Vaginal Epithelium Thickness in OVX Rat Models
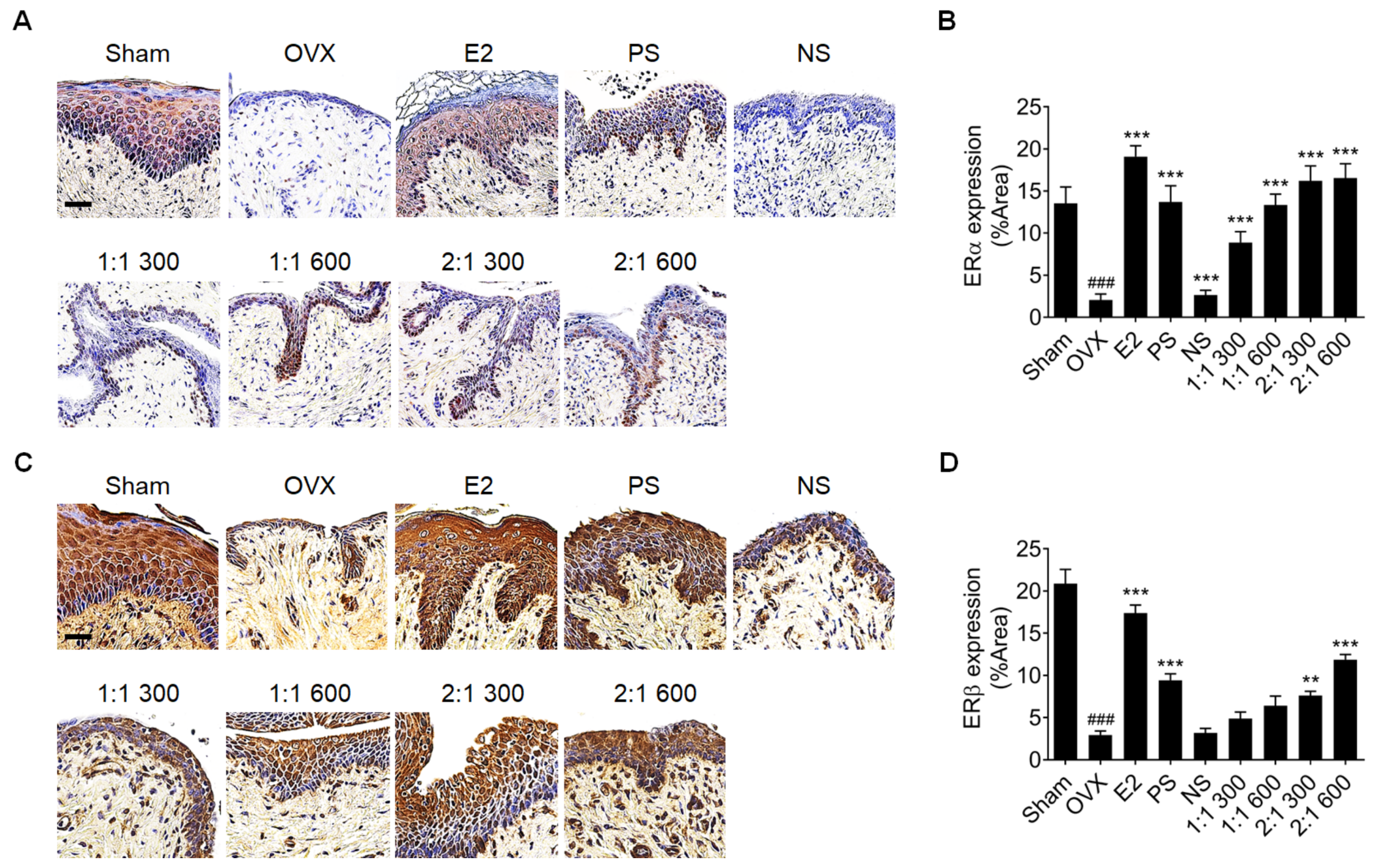
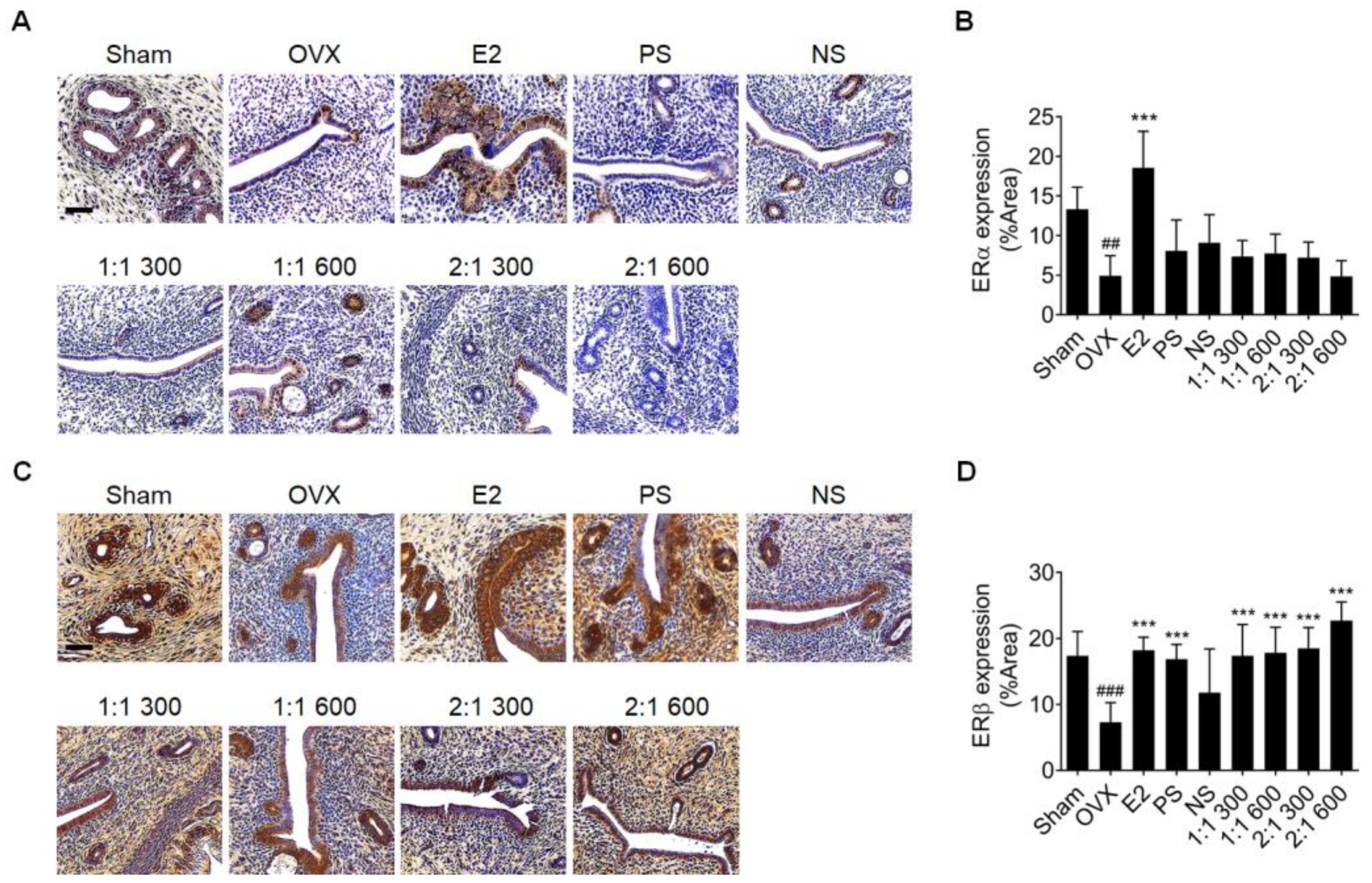
3.2. Complex Extract of PS and NS Increased ERα and ERβ Expression in Vaginal Epithelium
3.3. Complex Extract of PS and NS Ameliorated Depression in OVX Rat Models
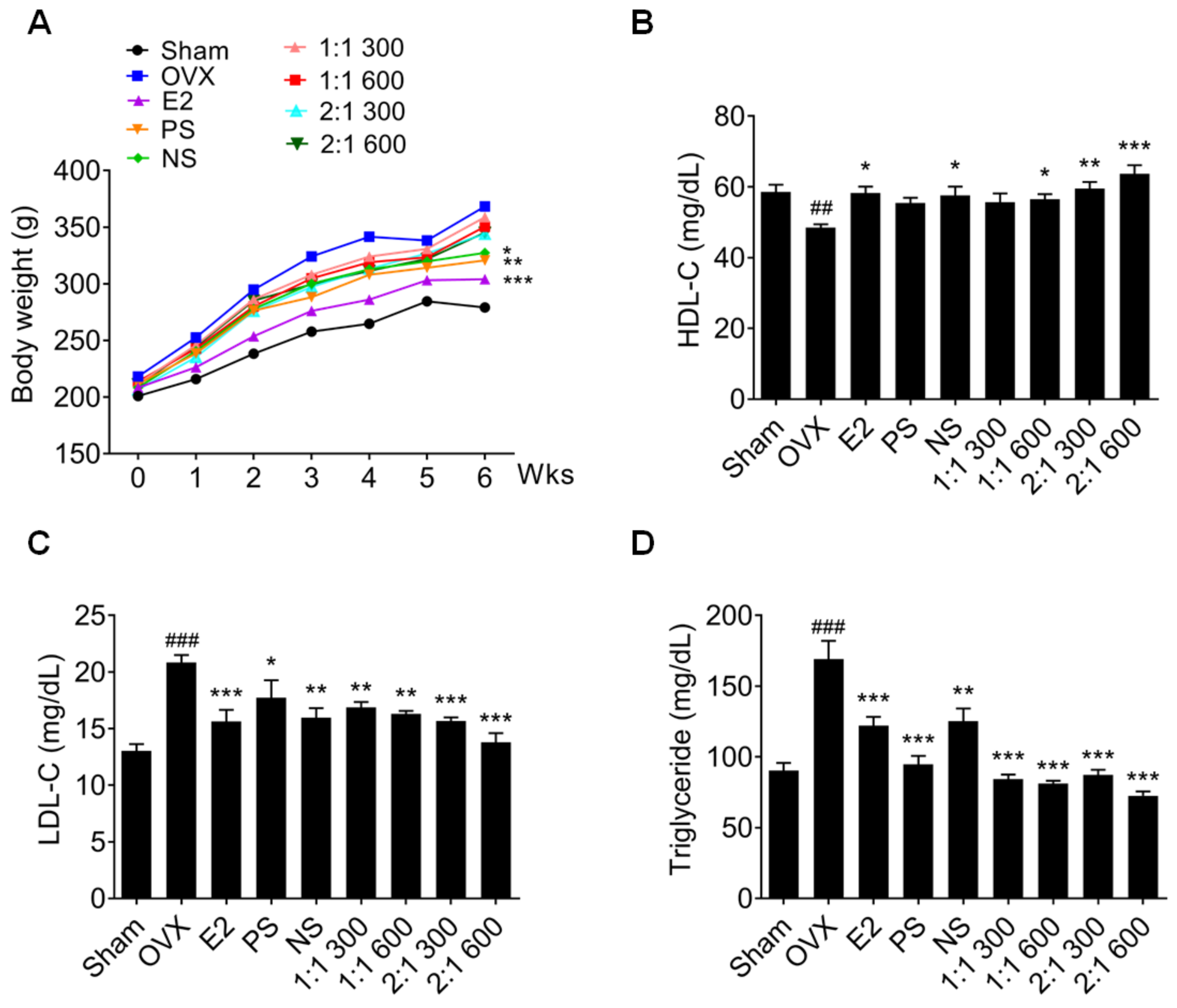
3.4. Complex Extract of PS and NS Improved Blood Lipid Metabolism in OVX Rat Models
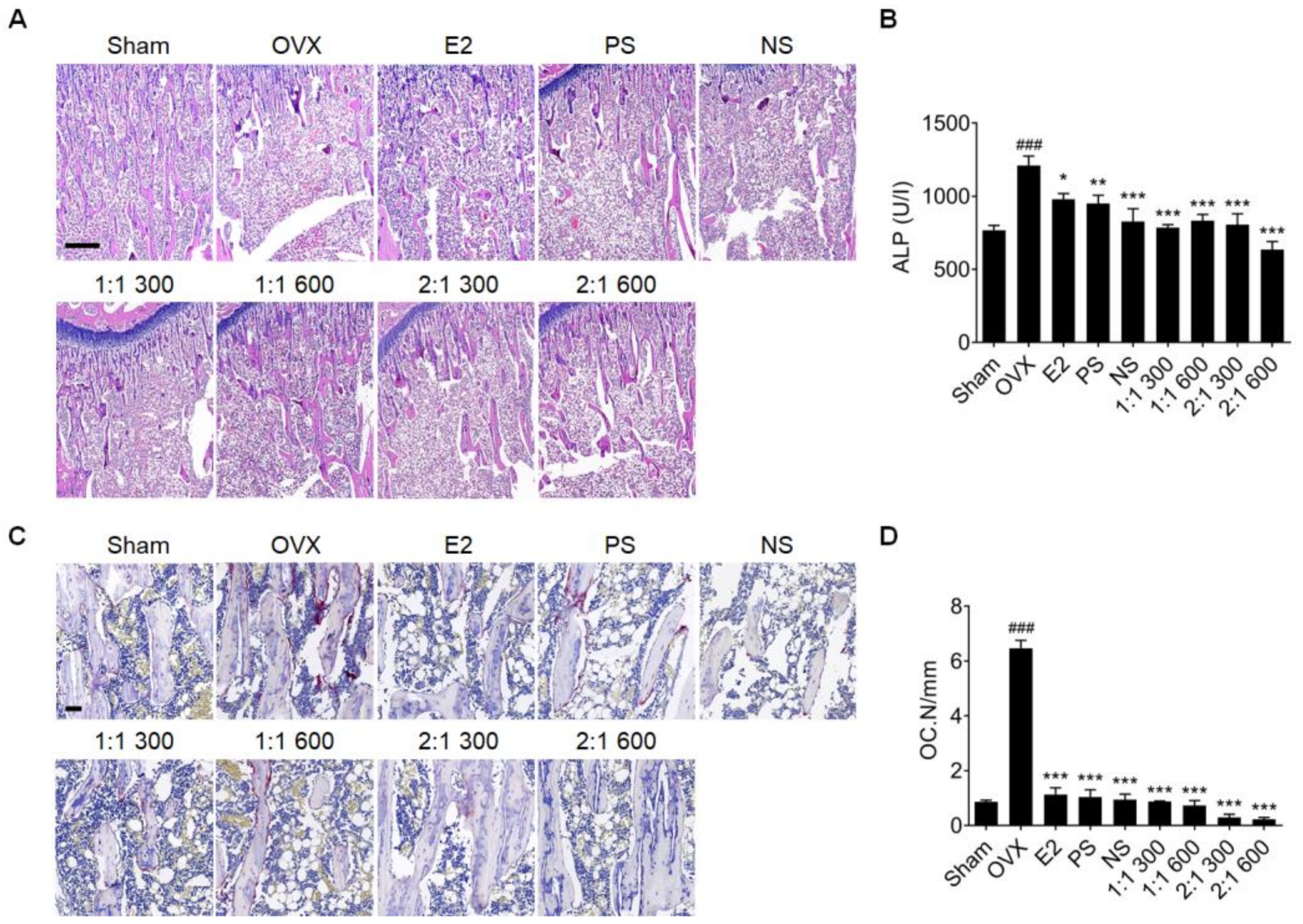
3.5. Complex Extract of PS and NS Improved Bone Loss in an OVX Rat Models
3.6. Complex Extract of PS and NS Regulated ERβ but Not ERα
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoga, L.; Rodolpho, J.; Goncalves, B.; Quirino, B. Women’s experience of menopause: A systematic review of qualitative evidence. JBI Database Syst. Rev. Implem. Rep. 2015, 13, 250–337. [Google Scholar] [CrossRef]
- Hybholt, M. Psychological and social health outcomes of physical activity around menopause: A scoping review of research. Maturitas 2022, 164, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Talaulikar, V. Menopause transition: Physiology and symptoms. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, 81, 3–7. [Google Scholar] [CrossRef] [PubMed]
- McNeil, M.A.; Merriam, S.B. Menopause. Ann. Intern. Med. 2021, 174, ITC97–ITC112. [Google Scholar] [CrossRef]
- Wu, Y.J.; Zhang, W.S.; Zhu, F.; Zhu, T.; Jin, Y.L.; Pan, J.; Jiang, C.Q. Study on the relationship between the age at natural menopause and postmenopausal metabolic syndrome. Zhonghua Yu Fang Yi Xue Za Zhi 2023, 57, 433–437. [Google Scholar]
- Paciuc, J. Hormone Therapy in Menopause. Adv. Exp. Med. Biol. 2020, 1242, 89–120. [Google Scholar] [PubMed]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; Kotchen, J.M.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2022, 288, 321–333. [Google Scholar]
- Agunas, N.; Fernández-García, J.M.; Blanco, N.; Ballesta, A.; Carrillo, B.; Arevalo, M.A.; Collado, P.; Pinos, H.; Grassi, D. Organizational Effects of Estrogens and Androgens on Estrogen and Androgen Receptor Expression in Pituitary and Adrenal Glands in Adult Male and Female Rats. Front. Neuroanat. 2022, 16, 902218. [Google Scholar] [CrossRef] [PubMed]
- Dahlman-Wright, K.; Cavailles, V.; Fuqua, S.A.; Jordan, V.C.; Katzenellenbogen, J.A.; Korach, K.S.; Maggi, A.; Muramatsu, M.; Parker, M.G.; Gustafsson, J.A. International Union of Pharmacology LXIV Estrogen Receptors. Pharmacol. Rev. 2006, 58, 773–781. [Google Scholar] [CrossRef]
- Chantalat, E.; Valera, M.C.; Vaysse, C.; Noirrit, E.; Rusidze, M.; Weyl, A.; Vergriete, K.; Buscail, E.; Lluel, P.; Fontaine, C.; et al. Estrogen Receptors and Endometriosis. Int. J. Mol. Sci. 2020, 21, 2815. [Google Scholar] [CrossRef]
- Zheng, H.; Zhao, Y.; Xu, Y.; Zhang, Z.; Zhu, J.; Fan, Y.; Lin, N. Long-time qingyan formula extract treatment exerts estrogenic activities on reproductive tissues without side effects in ovariectomized rats and via active ER to ERE-independent gene regulation. Aging 2019, 11, 4032–4049. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Huang, Z.Y.; Xu, X.L.; Li, J.; Fu, X.W.; Deng, S.L. Estrogen Receptor Function: Impact on the Human Endometrium. Front. Endocrinol. 2022, 13, 827724. [Google Scholar] [CrossRef]
- Yang, B.; Chen, R.; Liang, X.; Shi, J.; Wu, X.; Zhang, Z.; Chen, X. Estrogen enhances endometrial cancer cells proliferation by upregulation of prohibitin. J. Cancer 2019, 10, 1616–1621. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Sheng, J.; Zhu, L.T.; Zhou, T.; Huang, H. Increased expression of c-fos protein associated with increased matrix metalloproteinase-9 protein expression in the endometrium of endometriotic patients. Fertil. Steril. 2008, 90, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Stovall, D.W.; Pinkerton, J.V. MF-101, an estrogen receptor beta agonist for the treatment of vasomotor symptoms in peri- and postmenopausal women. Curr. Opin. Investig. Drugs 2009, 10, 365–371. [Google Scholar]
- Grady, D.; Sawaya, G.F.; Johnson, K.C.; Koltun, W.; Hess, R.; Vittinghoff, E.; Kristof, M.; Tagliaferri, M.; Cohen, I.; Ensrud, K.E. MF101, a selective estrogen receptor beta modulator for the treatment of menopausal hot flushes: A phase II clinical trial. Menopause 2009, 16, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Patil, S.; Qian, A.; Zhao, C. Bioactive Compounds of Polygonatum sibiricum—Therapeutic Effect and Biological Activity. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 26–37. [Google Scholar] [CrossRef]
- Lu, J.P.; Zhang, J.; Zhang, Y.Z. The function activities and application of Polygonatum sibiricum polysaccharides. J. Food Saf. Qual. 2013, 4, 273–278. [Google Scholar]
- Zhao, P.; Zhao, C.; Li, X.; Gao, Q.; Huang, L.; Xiao, P.; Gao, W. The genus Polygonatum: A review of ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2018, 214, 274–291. [Google Scholar] [CrossRef]
- Peng, X.; He, J.; Zhao, J.; Wu, Y.; Shi, X.; Du, L.; Nong, M.; Zong, S.; Zeng, G. Polygonatum sibiricum polysaccharide promotes osteoblastic differentiation through the ERK/GSK-3β/β-catenin signaling pathway in vitro. Rejuvenation Res. 2017, 21, 44–52. [Google Scholar] [CrossRef]
- Li, C.Y.; Zhou, Y.Y.; Xin, Q.F.; Zhang, Z.G. Effect of Polygonati polysaccharide on superoxide dismutase, malondialdehyde and blood lipoid metabolism in serumin twelve months natural climacteric rats. Chin. J. Gerontol. 2013, 33, 6215–6216. [Google Scholar]
- Liu, B.; Tang, Y.; Song, Z.; Ge, J. Polygonatum sibiricum F. Delaroche polysaccharide ameliorates HFD-induced mouse obesity via regulation of lipid metabolism and inflammatory response. Mol. Med. Rep. 2021, 24, 501. [Google Scholar] [CrossRef] [PubMed]
- Park, D.R.; Yeo, C.H.; Yoon, J.E.; Hong, E.Y.; Choi, B.R.; Lee, Y.J.; Ha, I. Polygonatum sibiricum improves menopause symptoms by regulating hormone receptor balance in an ovariectomized mouse model. Biomed. Pharmacother. 2022, 153, 113385. [Google Scholar] [CrossRef] [PubMed]
- Arooj, M.; Imran, S.; Inam-Ur-Raheem, M.; Rajoka, M.S.R.; Sameen, A.; Siddique, R.; Sahar, A.; Tariq, S.; Riaz, A.; Hussain, A.; et al. Lotus seeds (Nelumbinis semen) as an emerging therapeutic seed: A comprehensive review. Food Sci. Nutr. 2021, 9, 3971–3987. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Ma, D.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; Christian, M.; He, Z. Alkaloids from lotus (Nelumbo nucifera): Recent advances in biosynthesis, pharmacokinetics, bioactivity, safety, and industrial applications. Crit. Rev. Food Sci. Nutr. 2021, 30, 1–34. [Google Scholar] [CrossRef]
- Chung, H.S.; Lee, H.J.; Shim, I.; Bae, H. Assessment of anti-depressant effect of nelumbinis semen on rats under chronic mild stress and its subchronic oral toxicity in rats and beagle dogs. BMC Complement. Altern. Med. 2012, 12, 68. [Google Scholar] [CrossRef]
- Bishayee, A.; Patel, P.A.; Sharma, P.; Thoutireddy, S.; Das, N. Lotus (Nelumbo nucifera Gaertn.) and Its Bioactive Phytocompounds: A Tribute to Cancer Prevention and Intervention. Cancers 2022, 14, 529. [Google Scholar] [CrossRef]
- Wang, M.; Hu, W.J.; Wang, Q.H.; Yang, B.Y.; Kuang, H.X. Extraction, purification, structural characteristics, biological activities, and application of the polysaccharides from Nelumbo nucifera Gaertn. (lotus): A review. Int. J. Biol. Macromol. 2023, 226, 562–579. [Google Scholar] [CrossRef]
- Park, H.J.; Jin, G.R.; Jung, J.H.; Hwang, S.B.; Lee, S.H.; Lee, B.H. Hair Growth Promotion Effect of Nelumbinis Semen Extract with High Antioxidant Activity. Evid. Based Complement. Alternat. Med. 2021, 2021, 6661373. [Google Scholar] [CrossRef]
- Park, D.; Park, C.; Choi, Y.; Lin, J.; Seo, D.; Kim, H.; Lee, S.Y.; Kang, I. A novel small-molecule PPI inhibitor targeting integrin αvβ3-osteopontin interface blocks bone resorption in vitro and prevents bone loss in mice. Biomaterials 2016, 98, 131–142. [Google Scholar] [CrossRef]
- Yousefzadeh, N.; Kashfi, K.; Jeddi, S.; Ghasemi, A. Ovariectomized rat model of osteoporosis: A practical guide. EXCLI J. 2020, 19, 89–107. [Google Scholar] [PubMed]
- Federici, L.M.; Roth, S.D.; Krier, C.; Fitz, S.D.; Skaar, T.; Shekhar, A.; Carpenter, J.S.; Johnson, P.L. Anxiogenic CO2 stimulus elicits exacerbated hot flash-like responses in a rat menopause model and hot flashes in postmenopausal women. Menopause 2016, 23, 1257–1266. [Google Scholar] [CrossRef]
- Parhizkar, S.; Latiff1, L.A.; Rahman, S.A.; Ibrahim, R.; Mohammad; Dollah, A. In vivo estrogenic activity of Nigella sativa different extracts using vaginal cornification assay. J. Med. Plants Res. 2007, 5, 6939–6945. [Google Scholar] [CrossRef]
- Terenius, L. The Allen-Doisy test for estrogens reinvestigated. Steroids 1971, 17, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Go, M.; Shin, E.; Jang, S.Y.; Nam, M.; Hwang, G.S.; Lee, S.Y. BCAT1 promotes osteoclast maturation by regulating branched-chain amino acid metabolism. Exp. Mol. Med. 2022, 54, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.R.; Lambrinoudaki, I.; Lumsden, M.; Mishra, G.D.; Pal, L.; Rees, M.; Santoro, N.; Simoncini, T. Menopause. Nat. Rev. Dis. Primers 2015, 1, 15004. [Google Scholar] [CrossRef]
- Gu, C.; Duluc, D.; Wiest, M.; Xue, Y.; Yi, J.; Gorvel, J.P.; Joo, H.; Oh, S. Cell type-specific expression of estrogen and progesterone receptors in the human vaginal mucosa. Clin. Immunol. 2021, 232, 108874. [Google Scholar] [CrossRef]
- Morssinkhof, M.W.L.; van Wylick, D.W.; Priester-Vink, S.; van der Werf, Y.D.; den Heijer, M.; van den Heuvel, O.A.; Broekman, B.F.P. Associations between sex hormones, sleep problems and depression: A systematic review. Neurosci. Biobehav. Rev. 2020, 118, 669–680. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Shang, X.; Pan, H.; Wang, X.; He, H.; Li, M. Leonurus japonicus Houtt.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2014, 152, 14–32. [Google Scholar] [CrossRef]
- Huang, L.; Xu, D.Q.; Chen, Y.Y.; Yue, S.J.; Tang, Y.P. Leonurine, a potential drug for the treatment of cardiovascular system and central nervous system diseases. Brain Behav. 2021, 11, e01995. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, N.S.; de Lima, A.B.F.; de Brito, J.C.R.; Sarmento, A.C.A.; Gonçalves, A.K.S.; Eleutério, J., Jr. Postmenopausal Vaginal Microbiome and Microbiota. Front. Reprod. Health 2022, 3, 780931. [Google Scholar] [CrossRef] [PubMed]
- Inaraja, V.; Thuissard, I.; Andreu-Vazquez, C.; Jodar, E. Lipid profile changes during the menopausal transition. Menopause 2020, 27, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Stone, N.J.; Ballantyne, C.; Bittner, V.; Criqui, M.H.; Ginsberg, H.N.; Goldberg, A.C.; Howard, W.J.; Jacobson, M.S.; Kris-Etherton, P.M.; et al. American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2011, 123, 2292–2333. [Google Scholar]
- Tomica, D.; Ramic, S.; Danolic, D.; Susnjar, L.; Peric-Balja, M.; Puljiz, M. Impact of oestrogen and progesterone receptor expression in the cancer cells and myometrium on survival of patients with endometrial cancer. J. Obstet. Gynaecol. 2018, 38, 96–102. [Google Scholar] [CrossRef]
- Ropero, A.B.; Alonso-Magdalena, P.; Quesada, I.; Nadal, A. The role of estrogen receptors in the control of energy and glucose homeostasis. Steroids 2008, 73, 874–879. [Google Scholar] [CrossRef]
- Huang, B.; Omoto, Y.; Iwase, H.; Yamashita, H.; Toyama, T.; Coombes, R.C.; Filipovic, A.; Warner, M.; Gustafsson, J.A. Differential expression of estrogen receptor α, β1, and β2 in lobular and ductal breast cancer. Proc. Natl. Acad. Sci. USA 2014, 111, 1933–1938. [Google Scholar] [CrossRef]
- McPherson, S.J.; Hussain, S.; Balanathan, P.; Hedwards, S.L.; Niranjan, B.; Grant, M.; Chandrasir, U.P.; Toivanen, R.; Wang, Y.; Taylor, R.A.; et al. Estrogen receptor-β activated apoptosis in benign hyperplasia and cancer of the prostate is androgen independent and TNFα mediated. Proc. Natl. Acad. Sci. USA 2010, 107, 3123–3128. [Google Scholar] [CrossRef]
- Mak, P.; Leav, I.; Pursell, B.; Bae, D.; Yang, X.; Taglienti, C.A.; Gouvin, L.M.; Sharma, V.M.; Mercurio, A.M. ERβ Impedes Prostate Cancer EMT by Destabilizing HIF-1α and Inhibiting VEGF-Mediated Snail Nuclear Localization: Implications for Gleason Grading. Cancer Cell 2010, 17, 319–332. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual authors and contributors and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
| Antibody | Company | Dilution | Product no. |
|---|---|---|---|
| ERα | Abcam | 1:50 | ab32063 |
| ERβ | Invitrogen | 1:100 | PA1-310B |
| Variables | SHAM | OVX | E2 | PS | NS | 1:1300 | 1:1600 | 2:1300 | 2:1600 |
|---|---|---|---|---|---|---|---|---|---|
| 5-HT (pg/mL) | 25.25 ± 2.23 | 18.94 # ± 0.49 | 24.08 ± 1.41 | 24.22 ± 0.89 | 26.02 ** ± 1.54 | 25.22 *± 0.62 | 30.73 *** ± 3.83 | 31.19 ***± 2.35 | 36.48 *** ± 3.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.; Yoon, J.-E.; Choi, B.; Lee, Y.-J.; Ha, I.-H. Complex Extract of Polygonatum sibiricum and Nelumbinis semen Improves Menopause Symptoms via Regulation of Estrogen Receptor Beta in an Ovariectomized Rat Model. Nutrients 2023, 15, 2443. https://doi.org/10.3390/nu15112443
Park D, Yoon J-E, Choi B, Lee Y-J, Ha I-H. Complex Extract of Polygonatum sibiricum and Nelumbinis semen Improves Menopause Symptoms via Regulation of Estrogen Receptor Beta in an Ovariectomized Rat Model. Nutrients. 2023; 15(11):2443. https://doi.org/10.3390/nu15112443
Chicago/Turabian StylePark, Doori, Jee-Eun Yoon, Boram Choi, Yoon-Jae Lee, and In-Hyuk Ha. 2023. "Complex Extract of Polygonatum sibiricum and Nelumbinis semen Improves Menopause Symptoms via Regulation of Estrogen Receptor Beta in an Ovariectomized Rat Model" Nutrients 15, no. 11: 2443. https://doi.org/10.3390/nu15112443
APA StylePark, D., Yoon, J.-E., Choi, B., Lee, Y.-J., & Ha, I.-H. (2023). Complex Extract of Polygonatum sibiricum and Nelumbinis semen Improves Menopause Symptoms via Regulation of Estrogen Receptor Beta in an Ovariectomized Rat Model. Nutrients, 15(11), 2443. https://doi.org/10.3390/nu15112443






