Red Blood Cell Membrane Fatty Acid Composition, Dietary Fatty Acid Intake and Diet Quality as Predictors of Inflammation in a Group of Australian Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Ethics
2.2. Measurement Sessions
2.3. Dietary Intake and Diet Quality Indexes
2.3.1. The Australian Recommended Food Score (ARFS) Diet Quality Index
2.3.2. AES Modified Mediterranean Diet Score (AES-MED)
2.3.3. AES Dietary Inflammatory Index (AES-DII)
2.4. Red Blood Cell Fatty Acid Analysis
2.5. Inflammatory Marker Analysis
2.6. Statistical Analysis
3. Results
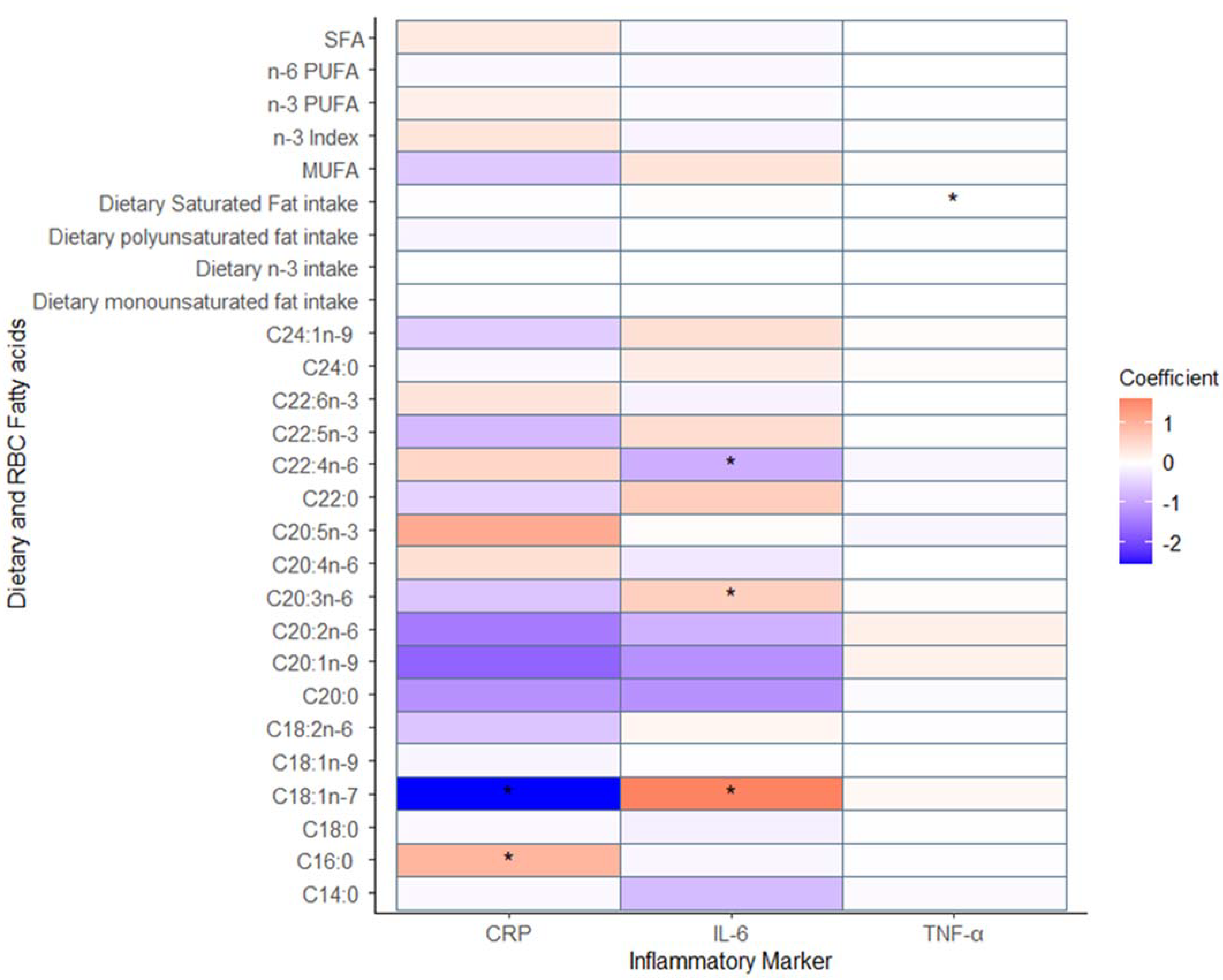
3.1. The Relationship between Inflammation and Fatty Acids Measures through Diet and Red Blood Cell Membranes
3.2. The Relationship between Inflammation and Dietary Patterns
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ridker, P.M. Residual inflammatory risk: Addressing the obverse side of the atherosclerosis prevention coin. Eur. Heart J. 2016, 37, 1720–1722. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration; Kaptoge, S.; Di Angelantonio, E.; Lowe, G.; Pepys, M.B.; Thompson, S.G.; Collins, R.; Danesh, J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet 2010, 375, 132–140. [Google Scholar] [CrossRef]
- Calder, P.C.; Yaqoob, P. Diet, Immunity and Inflammation; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106 (Suppl. S3), S1–S78. [Google Scholar] [CrossRef]
- Calder, P.C. Mechanisms of Action of (n-3) Fatty Acids. J. Nutr. 2012, 142, 592S–599S. [Google Scholar] [CrossRef]
- Fritsche, K. Fatty Acids as Modulators of the Immune Response. Annu. Rev. Nutr. 2006, 26, 45–73. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.E.; Boggess, M.M.; Watson, J.F.; Guest, M.; Duncanson, K.; Pezdirc, K.; Rollo, M.; Hutchesson, M.J.; Burrows, T.L. Reproducibility and comparative validity of a food frequency questionnaire for Australian adults. Clin. Nutr. 2014, 33, 906–914. [Google Scholar] [CrossRef]
- Schumacher, T.L.; Burrows, T.L.; Rollo, M.E.; Wood, L.G.; Callister, R.; Collins, C.E. Comparison of fatty acid intakes assessed by a cardiovascular-specific food frequency questionnaire with red blood cell membrane fatty acids in hyperlipidaemic Australian adults: A validation study. Eur. J. Clin. Nutr. 2016, 70, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Food Standards Australia and New Zealand. AUSNUT 2011-13 Food Nutrient Database. 2019. Available online: https://www.foodstandards.gov.au/science/monitoringnutrients/ausnut/ausnutdatafiles/pages/foodnutrient.aspx (accessed on 15 May 2023).
- Collins, C.E.; Burrows, T.L.; Rollo, M.E.; Boggess, M.M.; Watson, J.F.; Guest, M.; Duncanson, K.; Pezdirc, K.; Hutchesson, M.J. The Comparative Validity and Reproducibility of a Diet Quality Index for Adults: The Australian Recommended Food Score. Nutrients 2015, 7, 785–798. [Google Scholar] [CrossRef]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2013, 17, 2769–2782. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Stoodley, I.; Garg, M.; Scott, H.; Macdonald-Wicks, L.; Berthon, B.; Wood, L. Higher Omega-3 Index Is Associated with Better Asthma Control and Lower Medication Dose: A Cross-Sectional Study. Nutrients 2019, 12, 74. [Google Scholar] [CrossRef]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Berthon, B.S.; McLoughlin, R.F.; Jensen, M.E.; Hosseini, B.; Williams, E.J.; Baines, K.J.; Taylor, S.L.; Rogers, G.B.; Ivey, K.L.; Morten, M.; et al. The effects of increasing fruit and vegetable intake in children with asthma: A randomized controlled trial. Clin. Exp. Allergy 2021, 51, 1144–1156. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, K.L. The Science of Fatty Acids and Inflammation. Adv. Nutr. Int. Rev. J. 2015, 6, 293S–301S. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Mukamal, K.J.; Naqvi, A.Z. Erythrocyte saturated fatty acids and systemic inflammation in adults. Nutrition 2014, 30, 1404–1408. [Google Scholar] [CrossRef]
- Santos, S.; Oliveira, A.; Lopes, C. Systematic review of saturated fatty acids on inflammation and circulating levels of adipokines. Nutr. Res. 2013, 33, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, N.; Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Rousinou, G.; Toutouza, M.; Stefanidis, C. Unsaturated fatty acids are inversely associated and n-6/n-3 ratios are positively related to inflammation and coagulation markers in plasma of apparently healthy adults. Clin. Chim. Acta. 2010, 411, 584–591. [Google Scholar] [CrossRef]
- Grosso, G.; Laudisio, D.; Frias-Toral, E.; Barrea, L.; Muscogiuri, G.; Savastano, S.; Colao, A. Anti-Inflammatory Nutrients and Obesity-Associated Metabolic-Inflammation: State of the Art and Future Direction. Nutrients 2022, 14, 1137. [Google Scholar] [CrossRef] [PubMed]
- Muka, T.; Jong, J.C.K.-D.; Hofman, A.; Dehghan, A.; Rivadeneira, F.; Franco, O.H. Polyunsaturated Fatty Acids and Serum C-Reactive Protein: The Rotterdam Study. Am. J. Epidemiol. 2015, 181, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Gao, H.-K.; Vatanparast, H.; Kengne, A.P. Impact of the dietary fatty acid intake on C-reactive protein levels in US adults. Medicine 2017, 96, e5736. [Google Scholar] [CrossRef]
- Julia, C.; Touvier, M.; Meunier, N.; Papet, I.; Galan, P.; Hercberg, S.; Kesse-Guyot, E. Intakes of PUFAs Were Inversely Associated with Plasma C-Reactive Protein 12 Years Later in a Middle-Aged Population with Vitamin E Intake as an Effect Modifier. J. Nutr. 2013, 143, 1760–1766. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Huerta, O.D.; Aguilera, C.M.; Mesa, M.D.; Gil, A. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: A systematic review of randomised clinical trials. Br. J. Nutr. 2012, 107 (Suppl. 2), S159–S170. [Google Scholar] [CrossRef] [PubMed]
- Kiecolt-Glaser, J.K.; Belury, M.A.; Andridge, R.; Malarkey, W.B.; Hwang, B.S.; Glaser, R. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: A randomized controlled trial. Brain Behav. Immun. 2012, 26, 988–995. [Google Scholar] [CrossRef]
- Ferrucci, L.; Cherubini, A.; Bandinelli, S.; Bartali, B.; Corsi, A.; Lauretani, F.; Martin, A.; Andres-Lacueva, C.; Senin, U.; Guralnik, J.M. Relationship of Plasma Polyunsaturated Fatty Acids to Circulating Inflammatory Markers. J. Clin. Endocrinol. Metab. 2006, 91, 439–446. [Google Scholar] [CrossRef]
- Perreault, M.; Roke, K.; Badawi, A.; Nielsen, D.E.; Abdelmagid, S.A.; El-Sohemy, A.; Ma, D.; Mutch, D. Plasma Levels of 14:0, 16:0, 16:1n-7, and 20:3n-6 are Positively Associated, but 18:0 and 18:2n-6 are Inversely Associated with Markers of Inflammation in Young Healthy Adults. Lipids 2013, 49, 255–263. [Google Scholar] [CrossRef]
- Willett, W. Nutritional Epidemiology, 2nd ed.; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Hodson, L.; Skeaff, C.M.; Fielding, B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid Res. 2008, 47, 348–380. [Google Scholar] [CrossRef]
- Hart, M.J.; Torres, S.J.; McNaughton, S.A.; Milte, C.M. Dietary patterns and associations with biomarkers of inflammation in adults: A systematic review of observational studies. Nutr. J. 2021, 20, 1–14. [Google Scholar] [CrossRef]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, L.; Cicerale, S. The Health Benefiting Mechanisms of Virgin Olive Oil Phenolic Compounds. Molecules 2016, 21, 1734. [Google Scholar] [CrossRef] [PubMed]
- Pedret, A.; Catalán, U.; Fernández-Castillejo, S.; Farràs, M.; Valls, R.-M.; Rubió, L.; Canela, N.; Aragonés, G.; Romeu, M.; Castañer, O.; et al. Impact of Virgin Olive Oil and Phenol-Enriched Virgin Olive Oils on the HDL Proteome in Hypercholesterolemic Subjects: A Double Blind, Randomized, Controlled, Cross-Over Clinical Trial (VOHF Study). PLoS ONE 2015, 10, e0129160. [Google Scholar] [CrossRef]
- Murff, H.J.; Edwards, T.L. Endogenous Production of Long-Chain Polyunsaturated Fatty Acids and Metabolic Disease Risk. Curr. Cardiovasc. Risk Rep. 2014, 8, 418. [Google Scholar] [CrossRef] [PubMed]
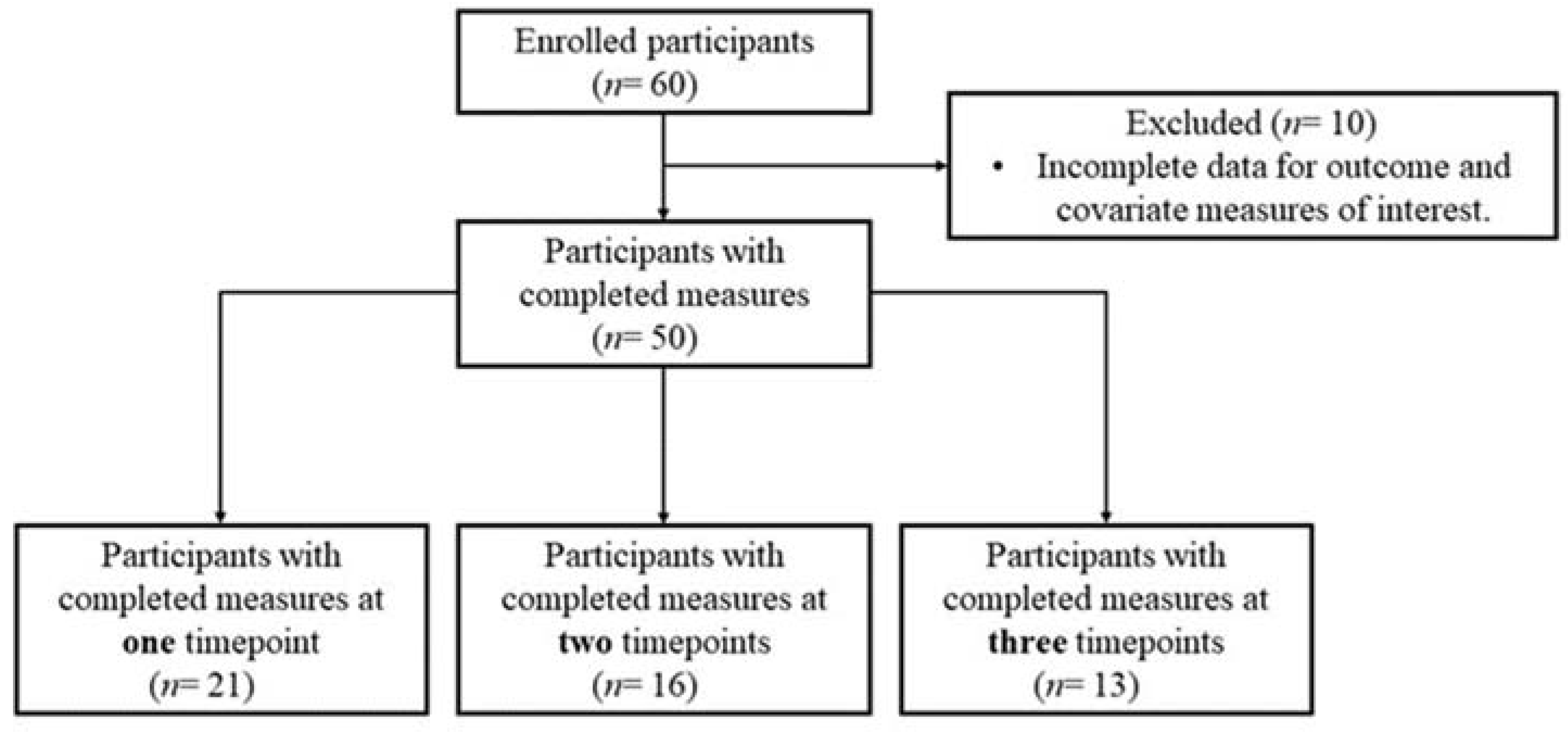
| Characteristic | N (%) | Mean (SD) |
|---|---|---|
| Gender | ||
| Female | 63 (68) | |
| Male | 29 (32) | |
| Age (years) | 37.4 (16.6) | |
| BMI (kg/m2) | 25.2 (5.2) | |
| Smoking status | ||
| Not at all | 85 (92) | |
| Yes, less than weekly | 5 (5) | |
| Yes, at least weekly | 1 (1) | |
| Yes, daily | 1 (1) | |
| Intake of anti-inflammatory supplements (a) | ||
| Yes | 7 (8) | |
| No | 85 (92) | |
| Presence of inflammatory condition (b) | ||
| Yes | 3 (3) | |
| No | 89 (97) |
| Mean (SD) | Median [IQR] | |
|---|---|---|
| AES Fatty acid intakes (g/day) | ||
| Saturated fat intake | 27.52 [14.95] | |
| Monounsaturated fat intake | 32.24 [15.76] | |
| Polyunsaturated fat intake | 11.10 [5.31] | |
| Diet Quality Scores | 40.52 (13.02) | |
| ARFS (0–73) | ||
| AES-MED (0–16) | 9.00 [3.00] | |
| AES-DII | −0.98 [1.55] | |
| Red blood cell fatty acids (%) | ||
| C14:0 | 0.12 (0.36) | |
| C16:0 | 21.45 [0.95] | |
| C18:0 | 16.43 [1.19] | |
| C18:1n-9 | 13.25 (1.19) | |
| C18:1n-7 | 1.02 [0.23] | |
| C18:2n-6 | 9.98 [1.76] | |
| C20:0 | 0.06 (1.89) | |
| C20:1n-9 | 0.02 (0.10) | |
| C20:2n-6 | 0.02 (0.11) | |
| C20:3n-6 | 1.55 [0.93] | |
| C20:4n-6 | 15.48 (1.68) | |
| C20:5n-3 | 0.95 [0.41] | |
| C22:0 | 1.47 [0.27] | |
| C22:4n-6 | 3.20 (0.67) | |
| C22:5n-3 | 2.35 (0.54) | |
| C22:6n-3 | 5.19 (1.17) | |
| C24:0 | 3.42 [1.00] | |
| C24:1n-9 | 3.89 [1.15] | |
| Total Saturated fat | 43.02 [1.73] | |
| Total Monounsaturated fat | 17.89 [1.84] | |
| Total n-6 Polyunsaturated fat | 30.22 (2.09) | |
| Total n-3 Polyunsaturated fat | 8.49 [1.93] | |
| n-3 Index | 6.14 (1.50) | |
| Inflammatory markers | ||
| IL-6 (pg/mL) | 1.17 [0.82] | |
| TNF-α (pg/mL) | 0.92 [0.46] | |
| CRP (mg/L) | 1.51 [2.36] |
| Unadjusted | Simple Adjustment (a) | Multiple Adjustments (b) | ||||
|---|---|---|---|---|---|---|
| β (95% CI) | p-Value | β (95% CI) | p-Value | β (95% CI) | p-Value | |
| IL-6 and Diet Quality | ||||||
| ARFS | 0.00 (−0.04, 0.04) | 0.89 | 0.00 (−0.05, 0.06) | 0.91 | 0.02 (−0.04, 0.09) | 0.47 |
| AES-MED | −0.21 (−0.43, −0.002) | 0.048 | −0.25 (−0.46, −0.04) | 0.02 | −0.23 (−0.49, 0.03) | 0.08 |
| AES-DII | 0.00 (−0.42, 0.42) | 0.998 | 0.03 (−0.39, 0.45) | 0.88 | −0.14 (−0.66, 0.39) | 0.61 |
| TNF-α and Diet Quality | ||||||
| ARFS | 0.00 (−0.004, 0.004) | 0.98 | 0.00 (−0.01, 0.01) | 0.77 | 0.01 (−0.001, 0.02) | 0.09 |
| AES-MED | 0.00 (−0.03, 0.03) | 0.87 | −0.01 (−0.04, 0.02) | 0.67 | −0.02 (−0.06, 0.01) | 0.19 |
| AES-DII | −0.02 (−0.08, 0.04) | 0.47 | −0.02 (−0.08, 0.04) | 0.49 | −0.04 (−0.11, 0.03) | 0.31 |
| CRP and Diet Quality | ||||||
| ARFS | −0.05 (−0.10, 0.01) | 0.11 | −0.07 (−0.15, 0.02) | 0.11 | −0.04 (−0.14, 0.07) | 0.48 |
| AES-MED | −0.10 (−0.43, 0.22) | 0.52 | −0.13 (−0.45, 0.19) | 0.43 | −0.03 (−0.47, 0.40) | 0.89 |
| AES-DII | 0.38 (−0.23, 1.00) | 0.22 | 0.41 (−0.21, 1.02) | 0.20 | 0.12 (−0.72, 0.95) | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clarke, E.D.; Stanford, J.; Ferguson, J.J.A.; Wood, L.G.; Collins, C.E. Red Blood Cell Membrane Fatty Acid Composition, Dietary Fatty Acid Intake and Diet Quality as Predictors of Inflammation in a Group of Australian Adults. Nutrients 2023, 15, 2405. https://doi.org/10.3390/nu15102405
Clarke ED, Stanford J, Ferguson JJA, Wood LG, Collins CE. Red Blood Cell Membrane Fatty Acid Composition, Dietary Fatty Acid Intake and Diet Quality as Predictors of Inflammation in a Group of Australian Adults. Nutrients. 2023; 15(10):2405. https://doi.org/10.3390/nu15102405
Chicago/Turabian StyleClarke, Erin D., Jordan Stanford, Jessica J. A. Ferguson, Lisa G. Wood, and Clare E. Collins. 2023. "Red Blood Cell Membrane Fatty Acid Composition, Dietary Fatty Acid Intake and Diet Quality as Predictors of Inflammation in a Group of Australian Adults" Nutrients 15, no. 10: 2405. https://doi.org/10.3390/nu15102405
APA StyleClarke, E. D., Stanford, J., Ferguson, J. J. A., Wood, L. G., & Collins, C. E. (2023). Red Blood Cell Membrane Fatty Acid Composition, Dietary Fatty Acid Intake and Diet Quality as Predictors of Inflammation in a Group of Australian Adults. Nutrients, 15(10), 2405. https://doi.org/10.3390/nu15102405








