Gestational Hypertension and Human Breast Milk Composition in Correlation with the Assessment of Fetal Growth—A Pilot Study
Abstract
1. Introduction
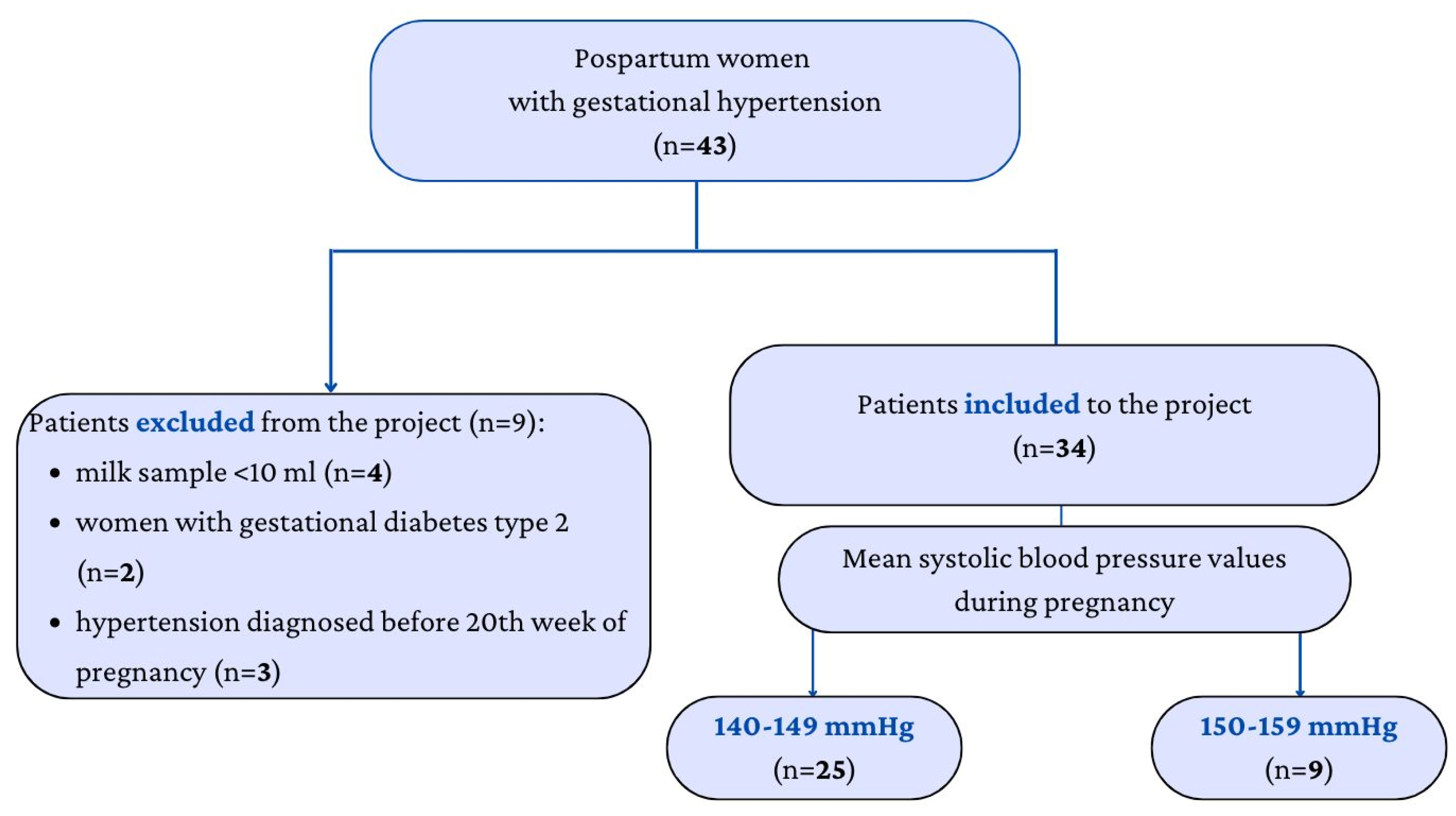
2. Materials and Methods
2.1. Collection of Samples
2.2. Laboratory Methods
2.3. Statistical Methods
3. Results
3.1. Human Milk Content
3.1.1. Protein
3.1.2. Carbohydrates
3.1.3. Fat
3.1.4. Energy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ananth, C.; Keyes, K.; Wapner, R. Preeclampsia Rates in the United States, 1980–2010: Age-Period-Cohort Analys. BMJ 2013, 347, 6564. [Google Scholar] [CrossRef]
- Abalos, E.; Cuesta, C.; Grosso, A.L.; Chou, D.; Say, L. Global and regional estimates of preeclampsia and eclampsia: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 1–7. [Google Scholar] [CrossRef]
- Jim, B.; Karumanchi, S.A. Preeclampsia: Pathogenesis, Prevention, and Long-Term Complications. Semin. Nephrol. 2017, 37, 386–397. [Google Scholar] [CrossRef]
- Preeclampsia—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26342729/ (accessed on 15 March 2023).
- Wu, C.S.; Nohr, E.A.; Bech, B.H.; Vestergaard, M.; Catov, J.M.; Olsen, J. Health of children born to mothers who had preeclampsia: A population-based cohort study. Am. J. Obstet. Gynecol. 2009, 201, 269.e1–269.e10. [Google Scholar] [CrossRef]
- De Moura, M.D.R.; Margotto, P.R.; Costa, K.N.; Novaes, M.R.C.G. Hypertension induced by pregnancy and neonatal outcome: Results from a retrospective cohort study in preterm under 34 weeks. PLoS ONE 2021, 16, e0255783. [Google Scholar] [CrossRef]
- Kilander, L.; Nyman, H.; Boberg, M.; Hansson, L.; Lithell, H. Hypertension is related to cognitive impairment: A 20-year follow-up of 999 men. Hypertension 1998, 31, 780–786. [Google Scholar] [CrossRef]
- Nommsen, L.A.; Lovelady, C.A.; Heinig, M.J.; Lönnerdal, B.; Dewey, K.G. Determinants of energy, protein, lipid, and lactose concentrations in human milk during the first 12 mo of lactation: The DARLING Study. Am. J. Clin. Nutr. 1991, 53, 457–465. [Google Scholar] [CrossRef]
- Karmarkar, M.G.; Ramakrishnan, C.V. Relation between Dietary Fat, Fat Content of Milk and Concentration of Certain Enzymes in Human Milk. J. Nutr. 1959, 69, 274–276. [Google Scholar] [CrossRef]
- Anderson, S.M.; Rudolph, M.C.; McManaman, J.L.; Neville, M.C. Key stages in mammary gland development. Secretory activation in the mammary gland: It’s not just about milk protein synthesis! Breast Cancer Res. 2007, 9, 204. [Google Scholar] [CrossRef]
- Andres, A.-C.; Djonov, V. The Mammary Gland Vasculature Revisited. J. Mammary Gland. Biol. Neoplasia 2010, 15, 319–328. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, D.; Chen, L. Lipid profile and cytokines in hypertension of pregnancy: A comparison of preeclampsia therapies. J. Clin. Hypertens. 2018, 20, 394–399. [Google Scholar] [CrossRef]
- van de Woestijne, A.P. Plasma triglyceride levels increase the risk for recurrent vascular events independent of LDL-cholesterol or nonHDL-cholesterol. Int. J. Cardiol. 2013, 167, 403–408. [Google Scholar] [CrossRef]
- Stadler, J.T.; Scharnagl, H.; Wadsack, C.; Marsche, G. Preeclampsia Affects Lipid Metabolism and HDL Function in Mothers and Their Offspring. Antioxidants 2023, 12, 795. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Indicators for Assessing Infant and Young Child Feeding Practices: Definitions and Measurement Methods. Available online: https://apps.who.int/iris/handle/10665/340706 (accessed on 15 March 2023).
- Dangat, K.; Upadhyay, D.; Kilari, A.; Sharma, U.; Kemse, N.; Mehendale, S.; Lalwani, S.; Wagh, G.; Joshi, S.; Jagannathan, N.R. Altered breast milk components in preeclampsia; An in-vitro proton NMR spectroscopy study. Clin. Chim. Acta 2016, 463, 75–83. [Google Scholar] [CrossRef]
- Helewa, M.E.; Burrows, R.F.; Smith, J.; Williams, K.; Brain, P.; Rabkin, S.W. Report of the Canadian Hypertension Society Consensus Conference: 1. Definitions, evaluation and classification of hypertensive disorders in pregnancy. Can. Med. Assoc. J. 1997, 157, 715–725. [Google Scholar]
- Aleksandra, P.; Dobrowolski, P.; Kosiński, P.; Bomba-Opoń, D.; Adamczak, M.; Bekiesińska-Figatowska, M.; Kądziela, J.; Konopka, A.; Kostka-Jeziorny, K.; Kurnatowska, I.; et al. Postępowanie w nadciśnieniu tętniczym u kobiet w ciąży. Zapobieganie, diagnostyka, leczenie i odległe rokowanie. Stanowisko Polskiego Towarzystwa Nadciśnienia Tętniczego, Polskiego Towarzystwa Kardiologicznego oraz Polskiego Towarzystwa Ginekologów i Położników. Ginekol. I Perinatol. Prakt. 2019, 4, 43–111. [Google Scholar]
- Groh-Wargo, S.; Valentic, J.; Khaira, S.; Super, D.M.; Collin, M. Human Milk Analysis Using Mid-Infrared Spectroscopy. Nutr. Clin. Pract. 2015, 31, 266–272. [Google Scholar] [CrossRef]
- User Manuals. Miris—Neonatal Health and Nutrition. Available online: http://www.mirissolutions.com/support/user-manuals (accessed on 3 March 2023).
- Paulaviciene, I.J.; Liubsys, A.; Molyte, A.; Eidukaite, A.; Usonis, V. Circadian changes in the composition of human milk macronutrients depending on pregnancy duration: A cross-sectional study. Int. Breastfeed. J. 2020, 15, 49. [Google Scholar] [CrossRef]
- Bzikowska-Jura, A. Do Maternal Factors and Milk Expression Patterns Affect the Composition of Donor Human Milk? Nutrients 2021, 13, 2425. [Google Scholar] [CrossRef]
- Emmett, P.M.; Rogers, I.S. Properties of human milk and their relationship with maternal nutrition. Early Hum. Dev. 1997, 49, S7–S28. [Google Scholar] [CrossRef] [PubMed]
- Horsley, K.; Chaput, K.; Da Costa, D.; Nguyen, T.; Dayan, N.; Tomfohr-Madsen, L.; Tough, S. Hypertensive disorders of pregnancy and breastfeeding practices: A secondary analysis of data from the All Our Families Cohort. Acta Obstet. Gynecol. Scand. 2022, 101, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Gerss, J. Longitudinal analysis of macronutrients and minerals in human milk produced by mothers of preterm infants. Clin. Nutr. 2011, 30, 215–220. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, Z.; Zhan, Y.; Wang, Y.; Ma, S.; Zhang, S.; Liu, J.; Wu, S.; Feng, Y.; Chen, Y.; et al. Effects of pre-pregnancy body mass index and gestational weight gain on maternal and infant complications. BMC Pregnancy Childbirth 2020, 20, 390. [Google Scholar] [CrossRef]
- James, W.P.T. WHO recognition of the global obesity epidemic. Int. J. Obes. 2008, 32 (Suppl. 7), S120–S126. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, E.; Guo, J.; Pan, L.; Li, B.; Wang, P.; Liu, J.; Wang, Y.; Liu, G.; Baccarelli, A.A.; et al. Maternal Prepregnancy Body Mass Index and Gestational Weight Gain on Pregnancy Outcomes. PLoS ONE 2013, 8, e82310. [Google Scholar] [CrossRef]
- Taoudi, F.; Laamiri, F.Z.; Barich, F.; Hasswane, N.; Aguenaou, H.; Barkat, A. Study of the Prevalence of Obesity and Its Association with Maternal and Neonatal Characteristics and Morbidity Profile in a Population of Moroccan Pregnant Women. J. Nutr. Metab. 2021, 2021, 6188847. [Google Scholar] [CrossRef]
- Macdonald-Wallis, C.; Tilling, K.; Fraser, A.; Nelson, S.M.; Lawlor, D.A.A. Gestational weight gain as a risk factor for hypertensive disorders of pregnancy. Am. J. Obstet. Gynecol. 2013, 209, 327.e1–327.e17. [Google Scholar] [CrossRef]
- Shao, Y.; Qiu, J.; Huang, H.; Mao, B.; Dai, W.; He, X.; Cui, H.; Lin, X.; Lv, L.; Wang, D.; et al. Pre-pregnancy BMI, gestational weight gain and risk of preeclampsia: A birth cohort study in Lanzhou, China. BMC Pregnancy Childbirth 2017, 17, 400. [Google Scholar] [CrossRef] [PubMed]
- Pre-Pregnancy Body Mass Index (BMI) and Delivery Outcomes in a Canadian Population—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/25528667/ (accessed on 21 March 2023).
- Dror, D.K.; Allen, L.H. Overview of Nutrients in Human Milk. Adv. Nutr. Int. Rev. J. 2018, 9, 278S–294S. [Google Scholar] [CrossRef]
- Leghi, G.E.; Netting, M.J.; Middleton, P.F.; Wlodek, M.E.; Geddes, D.T.; Muhlhausler, B.S. The Impact of Maternal Obesity on Human Milk Macronutrient Composition: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 934. [Google Scholar] [CrossRef] [PubMed]
- Macronutrient Composition of Human Milk from Korean Mothers of Full Term Infants Born at 37–42 Gestational Weeks—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4523489/ (accessed on 19 March 2023).
- Kim, H.; Kang, S.; Jung, B.-M.; Yi, H.; Jung, J.A.; Chang, N. Breast milk fatty acid composition and fatty acid intake of lactating mothers in South Korea. Br. J. Nutr. 2017, 117, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Heslehurst, N.; Vieira, R.; Akhter, Z.; Bailey, H.; Slack, E.; Ngongalah, L.; Pemu, A.; Rankin, J. The association between maternal body mass index and child obesity: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002817. [Google Scholar] [CrossRef] [PubMed]
- Moussa, H.N.; Alrais, M.A.; Leon, M.G.; Abbas, E.L.; Sibai, B.M. Obesity epidemic: Impact from preconception to postpartum. Futur. Sci. OA 2016, 2, FSO137. [Google Scholar] [CrossRef]
- Borràs-Novell, C.; Barbero, A.H.; Esponera, C.B.; López-Abad, M.; Bilbao, V.A.; Renau, M.I.; Platas, I.I. Influence of maternal and perinatal factors on macronutrient content of very preterm human milk during the first weeks after birth. J. Perinatol. 2022, 43, 52–59. [Google Scholar] [CrossRef]
- Lumeng, J.C.; Kaciroti, N.; Retzloff, L.; Rosenblum, K.; Miller, A.L. Longitudinal associations between maternal feeding and overweight in low-income toddlers. Appetite 2017, 113, 23–29. [Google Scholar] [CrossRef]
- Jarvie, E.; Ramsay, J.E. Obstetric management of obesity in pregnancy. Semin. Fetal Neonatal Med. 2010, 15, 83–88. [Google Scholar] [CrossRef]
- Bakker, R.; Steegers, E.A.P.; Hofman, A.; Jaddoe, V.W.V. Blood pressure in different gestational trimesters, fetal growth, and the risk of adverse birth outcomes: The generation R study. Am. J. Epidemiol. 2011, 174, 797–806. [Google Scholar] [CrossRef]
- Cao, X.; Zu, D.; Liu, Y. Effects of Interaction Between Gestational Hypertension and History of Preterm Birth on the Risk of Preterm Birth: An Analysis Based on the National Vital Statistics System Database. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2022, 28, e935094-1–e935094-14. [Google Scholar] [CrossRef]
| Parameters | Mean ± SD | Median (IQR 1) | |||
|---|---|---|---|---|---|
| Mothers | PIH 2 | NR 3 | PIH 2 | NR 3 | p-Value |
| Age | 30.6 ± 4.3 | 30.1 ± 4.5 | 30.5 (28–34) | 31 (27–33) | 0.584 |
| Pre-pregnancy BMI (kg/m2) | 26.3 ± 5.1 | 21.5 ± 8.4 | 24.7 (22.0–30.0) | 22.4 (20.3–25.8) | 0.005 |
| Pregnancy weight gain (kg) | 14.5 ± 6.1 | 13.1 ± 5.3 | 15.0 (11.3–19.8) | 12.5 (10.0–17.0) | 0.323 |
| Pregnancy BMI (kg/m2) | 31.5 ± 5.1 | 29.1 ± 4.4 | 29.7 (27.8–35.1) | 28.7 (25.9–31.2) | 0.033 |
| Newborn | PIH 2 | NR 3 | PIH 2 | NR 3 | p-Value |
| Birth weight (g) | 2951 ± 682 | 3311 ± 511 | 3070 (2752–3315) | 3320 (3003–3590) | 0.013 |
| Body length (cm) | 52.1 ± 4.5 | 54.0 ± 2.4 | 53.0 (51.0–54.8) | 54.0 (52.5–56.0) | 0.030 |
| Head circumference (cm) | 33.2 ± 2.4 | 33.8 ± 1.8 | 33.5 (32–35) | 34.0 (33–34.8) | 0.312 |
| Coefficients | Wald Test | |||||
|---|---|---|---|---|---|---|
| Estimate | Standard Error | z | Wald Statistic | df | p | |
| (Intercept) | −8.986 | 13.283 | −0.677 | 0.458 | 1 | 0.499 |
| Fat | −18.258 | 15.206 | −1.201 | 1.442 | 1 | 0.230 |
| Carbohydrates | −7.482 | 6.647 | −1.126 | 1.267 | 1 | 0.260 |
| Calories | 2.100 | 1.675 | 1.254 | 1.573 | 1 | 0.210 |
| True protein | −12.766 | 9.457 | −1.350 | 1.822 | 1 | 0.177 |
| Coefficients | ||||||
|---|---|---|---|---|---|---|
| Wald Test | ||||||
| Estimate | Standard Error | z | Wald Statistic | df | p | |
| (Intercept) | 10.225 | 16.154 | 0.633 | 0.401 | 1 | 0.527 |
| Fat | −21.476 | 18.540 | −1.158 | 1.342 | 1 | 0.247 |
| Carbohydrates | −10.762 | 8.618 | −1.249 | 1.559 | 1 | 0.212 |
| Calories | 2.337 | 2.025 | 1.154 | 1.332 | 1 | 0.248 |
| True protein | −13.768 | 11.588 | −1.188 | 1.412 | 1 | 0.235 |
| Descriptive Statistics | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fat | Carbohydrates | Calories | True Protein | |||||
| NR | GH | NR | GH | NR | GH | NR | GH | |
| Valid | 38 | 34 | 38 | 34 | 38 | 34 | 38 | 34 |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mean | 1.9 | 2.5 | 7.3 | 7.7 | 58.0 | 63.2 | 1.9 | 1.7 |
| Std. Deviation | 0.9 | 0.9 | 0.5 | 0.3 | 8.6 | 8.0 | 0.5 | 0.3 |
| Skewness | 0.49 | 0.34 | −1.81 | −0.39 | 0.47 | 0.01 | 1.64 | 1.43 |
| Std. Error of Skewness | 0.38 | 0.40 | 0.38 | 0.40 | 0.38 | 0.40 | 0.38 | 0.40 |
| Kurtosis | −0.57 | 0.30 | 4.50 | −1.09 | −0.10 | −0.09 | 3.70 | 1.65 |
| Std. Error of Kurtosis | 0.75 | 0.79 | 0.75 | 0.79 | 0.75 | 0.79 | 0.75 | 0.79 |
| Shapiro–Wilk | 0.95 | 0.97 | 0.85 | 0.93 | 0.97 | 0.97 | 0.87 | 0.85 |
| p-value of Shapiro–Wilk | 0.082 | 0.623 | <0.001 | 0.027 | 0.322 | 0.389 | <0.001 | <0.001 |
| Minimum | 0.6 | 1.0 | 5.5 | 7.1 | 42.0 | 49.0 | 1.2 | 1.3 |
| Maximum | 3.9 | 4.9 | 8.0 | 8.2 | 77.0 | 83.0 | 3.8 | 2.6 |
| Independent Samples t-Test | ||||
|---|---|---|---|---|
| Test | Statistic | df | p | |
| True protein | Student | 1.470 | 70.000 | 0.073 |
| Welch | 1.510 | 60.958 | 0.068 | |
| Independent Samples t-Test | ||||
|---|---|---|---|---|
| Test | Statistic | df | p | |
| Carbohydrates | Student | −3.532 | 70.000 | <0.001 |
| Welch | −3.605 | 65.559 | <0.001 | |
| Independent Samples t-Test | ||||
|---|---|---|---|---|
| Test | Statistic | df | p | |
| Fat | Student | −2.666 | 70.000 | 0.005 |
| Welch | −2.670 | 69.434 | 0.005 | |
| Independent Samples t-Test | ||||
|---|---|---|---|---|
| Test | Statistic | df | p | |
| Energy content | Student | −2.656 | 70.000 | 0.005 |
| Welch | −2.665 | 69.816 | 0.005 | |
| Model | Deviance | AIC | BIC | df | Χ² | p | McFadden R² | Nagelkerke R² | Tjur R² | Cox and Snell R² | |
| H0 | 98.300 | 100.300 | 102.563 | 70 | |||||||
| H1 | 70.505 | 78.505 | 87.556 | 67 | 27.795 | <0.001 | 0.283 | 0.432 | 0.325 | 0.324 | |
| Coefficients | |||||||||||
| Wald Test | |||||||||||
| Estimate | Standard Error | z | Wald Statistic | df | p-Value | ||||||
| (Intercept) | −28.379 | 9.239 | −3.072 | 9.435 | 1 | 0.002 | |||||
| Birth weight | −0.002 | 0.001 | −2.749 | 7.557 | 1 | 0.006 | |||||
| Carbohydrates | 3.779 | 1.177 | 3.211 | 10.311 | 1 | 0.001 | |||||
| 3rd trimester BMI | 0.158 | 0.072 | 2.206 | 4.868 | 1 | 0.027 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokołowska, E.M.; Jassem-Bobowicz, J.M.; Drążkowska, I.; Świąder, Z.; Domżalska-Popadiuk, I. Gestational Hypertension and Human Breast Milk Composition in Correlation with the Assessment of Fetal Growth—A Pilot Study. Nutrients 2023, 15, 2404. https://doi.org/10.3390/nu15102404
Sokołowska EM, Jassem-Bobowicz JM, Drążkowska I, Świąder Z, Domżalska-Popadiuk I. Gestational Hypertension and Human Breast Milk Composition in Correlation with the Assessment of Fetal Growth—A Pilot Study. Nutrients. 2023; 15(10):2404. https://doi.org/10.3390/nu15102404
Chicago/Turabian StyleSokołowska, Ewa Magdalena, Joanna Maria Jassem-Bobowicz, Izabela Drążkowska, Zuzanna Świąder, and Iwona Domżalska-Popadiuk. 2023. "Gestational Hypertension and Human Breast Milk Composition in Correlation with the Assessment of Fetal Growth—A Pilot Study" Nutrients 15, no. 10: 2404. https://doi.org/10.3390/nu15102404
APA StyleSokołowska, E. M., Jassem-Bobowicz, J. M., Drążkowska, I., Świąder, Z., & Domżalska-Popadiuk, I. (2023). Gestational Hypertension and Human Breast Milk Composition in Correlation with the Assessment of Fetal Growth—A Pilot Study. Nutrients, 15(10), 2404. https://doi.org/10.3390/nu15102404






