Effect of Curcumin Consumption on Inflammation and Oxidative Stress in Patients on Hemodialysis: A Literature Review
Abstract
1. Introduction
2. Methodology
Selection Procedure
3. Results
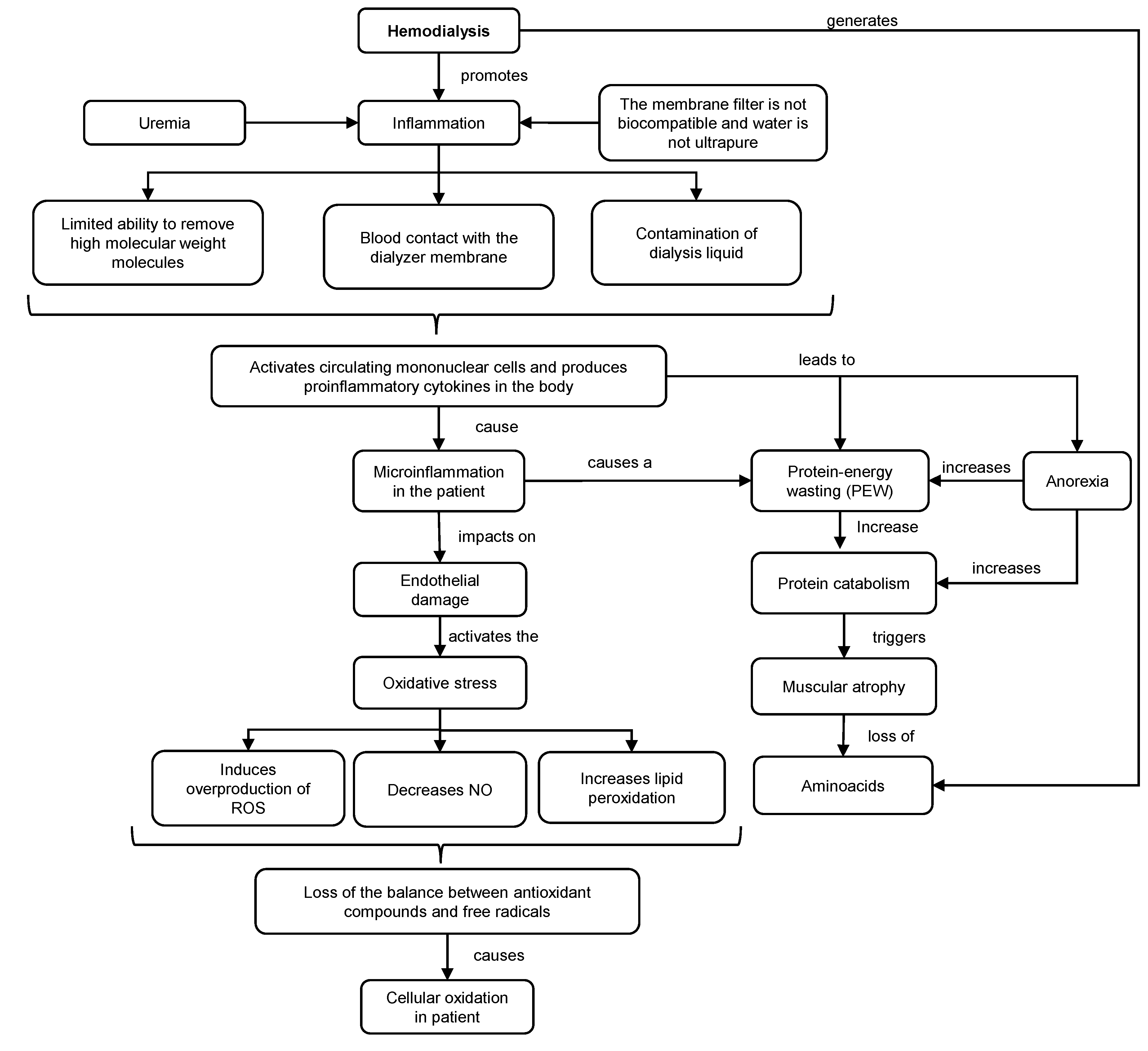
3.1. Mechanism and Consequences of HD in the Organism
3.2. Mechanism of Action and Effect of Curcumin Consumption on the Control of Oxidative and Inflammatory Parameters in HD Patients
3.3. Effect of Curcumin Bioaccessibility on Its Antioxidant and Anti-Inflammatory Effectiveness
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations and Acronyms
References
- Levey, A.S.; Schwartz, W.B.; Coresh, J. Chronic Kidney Disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P. Epidemiology of Chronic Kidney Disease: An Update 2022. In Kidney International Supplements; Elsevier: Amsterdam, The Netherlands, 2022; pp. 7–11. [Google Scholar] [CrossRef]
- Zhou, H.; Beevers, C.S.; Huang, S. Targets of Curcumin. Curr. Drug Targets 2011, 12, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease—Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2020, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Yuriy Khanin, M. Hemodyalisis. In A medication Guide to Internal Medicine Tests and Procedures; Hughes, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 33, pp. 149–152. [Google Scholar]
- Pedruzzi, L.M.; Cardozo, L.F.M.F.; Daleprane, J.B.; Stockler-Pinto, M.B.; Monteiro, E.B.; Leite, M.; Vaziri, N.D.; Mafra, D. Systemic Inflammation and Oxidative Stress in Hemodialysis Patients Are Associated with Down-Regulation of Nrf2. J. Nephrol. 2015, 28, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.R.; Leblanc, M.; Ricci, Z.; Ronco, C. Quantification and Dosing of Renal Replacement Therapy in Acute Kidney Injury: A Reappraisal. Blood Purif. 2017, 44, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Garzotto, F.; Lorenzin, A.; Zaccaria, M.; Clark, W.R. Solute and Water Transport in Hemodialysis: Dialyzers, Flow Distribution, and Cross-Filtration. In Critical Care Nephrology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 909–918.el. [Google Scholar] [CrossRef]
- Rocco, M.; Daugirdas, J.T.; Depner, T.A.; Inrig, J.; Mehrotra, R.; Rocco, M.V.; Suri, R.S.; Weiner, D.E.; Greer, N.; Ishani, A.; et al. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 Update. Am. J. Kidney Dis. 2015, 66, 884–930. [Google Scholar] [CrossRef]
- Li, P.K.-T.; Chan, G.C.-K.; Chen, J.; Chen, H.-C.; Cheng, Y.-L.; Fan, S.L.-S.; He, J.C.; Hu, W.; Lim, W.-H.; Pei, Y.; et al. Tackling Dialysis Burden around the World: A Global Challenge. Kidney Dis. 2021, 7, 167–175. [Google Scholar] [CrossRef]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.M.; Yang, C.W. Chronic Kidney Disease: Global Dimension and Perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Liyanage, T.; Ninomiya, T.; Jha, V.; Neal, B.; Patrice, H.M.; Okpechi, I.; Zhao, M.H.; Lv, J.; Garg, A.X.; Knight, J.; et al. Worldwide Access to Treatment for End-Stage Kidney Disease: A Systematic Review. Lancet 2015, 385, 1975–1982. [Google Scholar] [CrossRef]
- Poblete Badal, H.; Ortiz, M.M. XLI Cuenta de Hemodiálisis Crónica (HDC) En Chile (Al 31 de Agosto de 2021). Cuenta de Diálisis Peritoneal (Año 2020); Sociedad Chilena de Nefrología: Valparaíso, Chile, 2022; Available online: https://nefro.cl/web/biblio/registro/37.pdf (accessed on 3 May 2023).
- Guo, C.H.; Chen, P.C.; Hsu, G.S.W.; Wang, C.L. Zinc Supplementation Alters Plasma Aluminum and Selenium Status of Patients Undergoing Dialysis: A Pilot Study. Nutrients 2013, 5, 1456–1470. [Google Scholar] [CrossRef]
- Hassan, K.S.; Hassan, S.K.; Hijazi, E.G.; Khazim, K.O. Effects of Omega-3 on Lipid Profile and Inflammation Markers in Peritoneal Dialysis Patients. Ren. Fail. 2010, 32, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Ivey, K.L.; Lewis, J.R.; Lim, W.H.; Lim, E.M.; Hodgson, J.M.; Prince, R.L. Associations of Proanthocyanidin Intake with Renal Function and Clinical Outcomes in Elderly Women. PLoS ONE 2013, 8, 1166. [Google Scholar] [CrossRef] [PubMed]
- Korish, A.A.; Arafah, M.M. Catechin Combined with Vitamins C and E Ameliorates Insulin Resistance (IR) and Atherosclerotic Changes in Aged Rats with Chronic Renal Failure (CRF). Arch. Gerontol. Geriatr. 2008, 46, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bao, L.; Zhang, Z.; Dai, X.; Ding, Y.; Jiang, Y.; Li, Y. Effects of Grape Seed Proanthocyanidin Extract on Renal Injury in Type 2 Diabetic Rats. Mol. Med. Rep. 2015, 11, 645–652. [Google Scholar] [CrossRef]
- Naini, A.; Taheri, S.; Keyvandarian, N.; Moeinzadeh, F.; Mortazavi, M. The Effect of Omega-3 Fatty Acid Supplementation on Oxidative Stress in Continuous Ambulatory Peritoneal Dialysis Patients. Adv. Biomed. Res. 2014, 3, 142. [Google Scholar] [CrossRef]
- Abizanda, P.; López, M.D.; García, V.P.; de Estrella, J.D.; da Silva González, Á.; Vilardell, N.B.; Torres, K.A. Effects of an Oral Nutritional Supplementation plus Physical Exercise Intervention on the Physical Function, Nutritional Status, and Quality of Life in Frail Institutionalized Older Adults: The ACTIVNES Study. J. Am. Med. Dir. Assoc. 2015, 16, 439.e9–439.e16. [Google Scholar] [CrossRef]
- Pakfetrat, M.; Basiri, F.; Malekmakan, L.; Roozbeh, J. Effects of Turmeric on Uremic Pruritus in End Stage Renal Disease Patients: A Double-Blind Randomized Clinical Trial. J. Nephrol. 2014, 27, 203–207. [Google Scholar] [CrossRef]
- Pakfetrat, M.; Akmali, M.; Malekmakan, L.; Dabaghimanesh, M.; Khorsand, M. Role of Turmeric in Oxidative Modulation in End-Stage Renal Disease Patients. Hemodial. Int. 2015, 19, 124–131. [Google Scholar] [CrossRef]
- Alvarenga, L.; Salarolli, R.; Cardozo, L.F.M.F.; Santos, R.S.; de Brito, J.S.; Kemp, J.A.; Reis, D.; de Paiva, B.R.; Stenvinkel, P.; Lindholm, B.; et al. Impact of Curcumin Supplementation on Expression of Inflammatory Transcription Factors in Hemodialysis Patients: A Pilot Randomized, Double-Blind, Controlled Study. Clin. Nutr. 2020, 39, 3594–3600. [Google Scholar] [CrossRef]
- de Almeida Alvarenga, L.; de Leal, V.O.; Borges, N.A.; Silva de Aguiar, A.; Faxén-Irving, G.; Stenvinkel, P.; Lindholm, B.; Mafra, D. Curcumin—A Promising Nutritional Strategy for Chronic Kidney Disease Patients. J. Funct. Foods 2018, 715–721. [Google Scholar] [CrossRef]
- Emami, E.; Heidari-Soureshjani, S.; Sherwin, C.M. Anti-Inflammatory Response to Curcumin Supplementation in Chronic Kidney Disease and Hemodialysis Patients: A Systematic Review and Meta-Analysis. Avicenna J. Phytomed. 2022, 12, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, L.; Cardozo, L.F.M.F.; Da Cruz, B.O.; Paiva, B.R.; Fouque, D.; Mafra, D. Curcumin Supplementation Improves Oxidative Stress and Inflammation Biomarkers in Patients Undergoing Hemodialysis: A Secondary Analysis of a Randomized Controlled Trial. Int. Urol. Nephrol. 2022, 54, 2645–2652. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Yu, B.; Zhao, Y.; Zhu, W.; Li, H.; Lou, H.; Zhai, G. Enhancement of Oral Absorption of Curcumin by Self-Microemulsifying Drug Delivery Systems. Int. J. Pharm. 2009, 371, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Kocaadam, B.; Şanlier, N. Curcumin, an Active Component of Turmeric (Curcuma Longa), and Its Effects on Health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- González-Albadalejo, J.; Sanz, D.; Claramunt, R.M.; Lavandera, J.L.; Alkorta, I.; Elguero, J. Curcumin and Curcuminoids: Chemistry, Structural Studies and Biological Properties. An. Real Acad. Nac. Farm. 2015, 81, 278–310. [Google Scholar]
- Pat, P. Hemodiálisis y Diálisis Peritoneal. In Conceptos Básicos de Control de Infecciones; IFIC International Federation of Infection Control: Sheffield, UK, 2011; Chapter 19; pp. 289–302. [Google Scholar]
- Dinarello, C.A. Cytokines: Agents Provocateurs in Hemodialysis? Kidney Int. 1992, 41, 683–694. [Google Scholar] [CrossRef]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, Inflammation, and Chronic Diseases: How Are They Linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef]
- Huang, X.; Lindholm, B.; Stenvinkel, P.; Carrero, J.J. Dietary Fat Modification in Patients with Chronic Kidney Disease: N-3 Fatty Acids and Beyond. J. Nephrol. 2013, 26, 960–974. [Google Scholar] [CrossRef]
- Gracia-Iguacel, C.; González-Parra, E.; Pérez-Gómez, M.V.; Mahíllo, I.; Egido, J.; Ortiz, A.; Carrero, J.J. Prevalencia Del Síndrome de Desgaste Proteico-Energético y Su Asociación Con Mortalidad En Pacientes En Hemodiálisis En Un Centro En España. Nefrologia 2013, 33, 495–505. [Google Scholar] [CrossRef]
- Yanowsky-Escatell, F.; Pazarín-Villaseñor, L.; Andrade-Sierra, J.; Zambrano-Velarde, M.; Preciado-Figueroa, F.; Santana-Arciniega, C.; Galeno-Sánchez, R. Desgaste Proteico Energético En Pacientes Con Diálisis Peritoneal En México. Rev. Chil. Nutr. Soc. Chil. Nutr. Bromatol. Toxilogica 2017, 44, 111–112. [Google Scholar] [CrossRef]
- Carrasco, D.; Chuecas, L.; Flores, P.; Bórquez, T. Tasa de Mortalidad Por Enfermedad Renal Crónico En Chile 1997–2017, Una Enfermedad Que Acecha a La Población Chilena. Rev. Estud. Med. Sur. 2021, 9, 1–8. [Google Scholar]
- Borah, M.F.; Schoenfeld, P.Y.; Gotch, F.A.; Sargent, J.A.; Wolfsen, M.; Humphreys, M.H. Nitrogen Balance during Intermittent Dialysis Therapy of Uremia. Kidney Int. 1978, 14, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Castellano-Gasch, S.; Palomares-Sancho, I.; Molina-Núñez, M.; Ramos-Sánchez, R.; Merello-Godino, J.I.; Maduell, F. Nuevos Métodos Fiables Para Diagnosticar La Depleción Proteico-Calórica En Los Pacientes En Hemodiálisis. Nutr. Hosp. 2014, 30, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef] [PubMed]
- Tontisirin, K.; de Haen, H. Human Energy Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation. Food Nutr. Bull. 2001, 26, 166. [Google Scholar]
- Manore, M.M.; Meacham, S.L. New Dietary References Intakes Set for Enegry, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol, Proteins, and Amino Acids. ACSM Health Fit. J. 2003, 7, 25–27. [Google Scholar]
- Panstw Zakl, R.; Bogacka, A.; Sobczak-Czynsz, A.; Kucharska, E.; Madaj, M.; Stucka, K. Analysis of nutrition and nutritional status of haemodialysis patients. Rocz. Panstw. Zakl. Hig. 2018, 69, 165–174. [Google Scholar]
- Varatharajan, R.; Sattar, M.Z.A.; Chung, I.; Abdulla, M.A.; Kassim, N.M.; Abdullah, N.A. Antioxidant and Pro-Oxidant Effects of Oil Palm (Elaeis Guineensis) Leaves Extract in Experimental Diabetic Nephropathy: A Duration-Dependent Outcome. BMC Complement. Altern. Med. 2013, 13, 242. [Google Scholar] [CrossRef]
- Granata, S.; Dalla Gassa, A.; Tomei, P.; Lupo, A.; Zaza, G. Mitochondria: A New Therapeutic Target in Chronic Kidney Disease. Nutr. Metab. 2015, 12, 49. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and Anti-Inflammatory Properties of Curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Shobal, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S.S.R. Influence of Piperine on the Pharmacokinetics of Curcumin in Animals and Human Volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Silva-Silva, M.F. Estabilización de La Curcumina Mediante Su Encapsulación En Nanosistemas O/W: Estudio de La Fotólisis y Oxidación. Ph.D. Thesis, Facultad de Ciencias Químicas y Farmacéuticas, Universidad de Chile, Santiago de Chile, Chile, 2017. [Google Scholar]
- Samadian, F.; Dalili, N.; Poor -reza Gholi, F.; Fattah, M.; Malih, N.; Nafar, M.; Firoozan, A.; Ahmadpoor, P.; Samavat, S.; Ziaie, S. Evaluation of Curcumin’s Effect on Inflammation in Hemodialysis Patients. Clin. Nutr. ESPEN 2017, 22, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.G. A Novel Nanoparticle Drug Delivery System: The Anti-Inflammatory Activity of Curcumin Is Enhanced When Encapsulated in Exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.V. Regulation of Cox and Lox by Curcumin. Adv. Exp. Med. Biol. 2007, 595, 213–226. [Google Scholar] [CrossRef]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M.C. Curcumin and Health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef]
- Ghosh, S.; Bhattacharyya, S.; Rashid, K.; Sil, P.C. Curcumin Protects Rat Liver from Streptozotocin-Induced Diabetic Pathophysiology by Counteracting Reactive Oxygen Species and Inhibiting the Activation of P53 and MAPKs Mediated Stress Response Pathways. Toxicol. Rep. 2015, 2, 365–376. [Google Scholar] [CrossRef]
- Sharma, S.; Kulkarni, S.K.; Chopra, K. Curcumin, the Active Principle of Turmeric (Curcuma Longa), Ameliorates Diabetic Nephropathy in Rats. Clin. Exp. Pharmacol. Physiol. 2006, 33, 940–945. [Google Scholar] [CrossRef]
- Soetikno, V.; Sari, F.R.; Veeraveedu, P.T.; Thandavarayan, R.A.; Harima, M.; Sukumaran, V.; Lakshmanan, A.P.; Suzuki, K.; Kawachi, H.; Watanabe, K. Curcumin Ameliorates Macrophage Infiltration by Inhibiting NF-B Activation and Proinflammatory Cytokines in Streptozotocin Induced-Diabetic Nephropathy. Nutr. Metab. 2011, 8, 1–11. [Google Scholar] [CrossRef]
- Soetikno, V.; Watanabe, K.; Sari, F.R.; Harima, M.; Thandavarayan, R.A.; Veeraveedu, P.T.; Arozal, W.; Sukumaran, V.; Lakshmanan, A.P.; Arumugam, S.; et al. Curcumin Attenuates Diabetic Nephropathy by Inhibiting PKC-α and PKC-Β1 Activity in Streptozotocin-Induced Type I Diabetic Rats. Mol. Nutr. Food Res. 2011, 55, 1655–1665. [Google Scholar] [CrossRef]
- El-Gizawy, M.M.; Hosny, E.N.; Mourad, H.H.; Abd-El Razik, A.N. Curcumin Nanoparticles Ameliorate Hepatotoxicity and Nephrotoxicity Induced by Cisplatin in Rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Kuhad, A.; Pilkhwal, S.; Sharma, S.; Tirkey, N.; Chopra, K. Effect of Curcumin on Inflammation and Oxidative Stress in Cisplatin-Induced Experimental Nephrotoxicity. J. Agric. Food Chem. 2007, 55, 10150–10155. [Google Scholar] [CrossRef]
- Ueki, M.; Ueno, M.; Morishita, J.; Maekawa, N. Curcumin Ameliorates Cisplatin-Induced Nephrotoxicity by Inhibiting Renal Inflammation in Mice. J. Biosci. Bioeng. 2013, 115, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Soetikno, V.; Sari, F.R.; Lakshmanan, A.P.; Arumugam, S.; Harima, M.; Suzuki, K.; Kawachi, H.; Watanabe, K. Curcumin Alleviates Oxidative Stress, Inflammation, and Renal Fibrosis in Remnant Kidney through the Nrf2-Keap1 Pathway. Mol. Nutr. Food Res. 2013, 57, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, L.; Li, G.S.; Wang, J. The Anti-Inflammatory Effects of Curcumin on Renal Ischemia-Reperfusion Injury in Rats. Ren. Fail. 2018, 40, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Massey, H.D.; Krieg, R.; Fazelbhoy, Z.A.; Ghosh, S.; Sica, D.A.; Fakhry, I.; Gehr, T.W.B. Curcumin Ameliorates Renal Failure in 5/6 Nephrectomized Rats: Role of Inflammation. Am. J. Physiol. Ren. Physiol. 2009, 296, 1146–1157. [Google Scholar] [CrossRef] [PubMed]
- Salarolli, R.T.; Alvarenga, L.; Cardozo, L.F.M.F.; Teixeira, K.T.R.; de Moreira, L.S.G.; Lima, J.D.; Rodrigues, S.D.; Nakao, L.S.; Fouque, D.; Mafra, D. Can Curcumin Supplementation Reduce Plasma Levels of Gut-Derived Uremic Toxins in Hemodialysis Patients? A Pilot Randomized, Double-Blind, Controlled Study. Int. Urol. Nephrol. 2021, 53, 1231–1238. [Google Scholar] [CrossRef]
- Vafadar Afshar, G.; Rasmi, Y.; Yagmaye, P.; Khadem-Ansari, M.-H.; Makhdomi, K.; Rasooli, J. The Effects of Nano-Curcumin Supplementation on Serum Level of Hs-CRP, Adhesion Molecules, and Lipid Profiles in Hemodialysis Patients, A Randomized Controlled Clinical Trial. J. Kidney Dis. 2020, 14, 52. [Google Scholar]
- Seddik, A.A. The Effect of Turmeric and Ginger on Oxidative Modulation in End Stage Renal Disease (ESRD) Patients. Int. J. Adv. Res. (IJAR) 2015, 3, 657–670. [Google Scholar]
- European Food Safety Authority (EFSA). Refined Exposure Assessment for Curcumin (E 100). EFSA J. 2014, 12, 3876. [Google Scholar] [CrossRef]
- Hernández-Huesca, K. Caracterización y Digestión Gastrointestinal de Cápsulas Líquidas de Curcumina. Master’s Thesis, Universidad Veracruzana, Instituto de Ciencias Básicas, Xalapa, Mexico, 2016. [Google Scholar]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Chisi Chavez, K.R.; Flores Cordoba, I.L. Efecto Antiinflamatorio de Las Combinaciones Sinérgicas de La Cúrcuma (Curcuma Longa) Extracto, Pimienta (Piper Nigrum), Yema de Huevo, En La Inflamación Aguda Sub Plantar En Ratas. Bachelor’s Thesis, Universidad Nacional de San Agustin Arequipa, Arequipa, Perú, 2017. [Google Scholar]
- Mesa, M.D.; Ramírez-Tortosa, M.C.; Aguilera, C.M.; Ramírez-Boscá, A.; Gil, A. Efectos Farmacológicos y Nutricionales de Los Extractos de Curcuma Longa L. y de Los Cucuminoides. Ars Pharm. 2000, 41, 307–321. [Google Scholar]
- Saiz De Cos, P. Cúrcuma I (Curcuma Longa L.). Reduca (Biol.) Ser. Botánica 2014, 7, 84–99. [Google Scholar]
- Faridi Esfanjani, A.; Assadpour, E.; Jafari, S.M. Improving the Bioavailability of Phenolic Compounds by Loading Them within Lipid-Based Nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66. [Google Scholar] [CrossRef]
- Bahar, B.; O’Doherty, J.V.; Smyth, T.J.; Ahmed, A.M.; Sweeney, T. A Cold Water Extract of Fucus Vesiculosus Inhibits Lipopolysaccharide (LPS) Induced pro-Inflammatory Responses in the Porcine Colon Ex-Vivo Model. Innov. Food Sci. Emerg. Technol. 2016, 37, 229–236. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-Grade Nanoemulsions: Formulation, Fabrication, Properties, Performance, Biological Fate, and Potential Toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef]
- Wu, X.; Xu, J.; Huang, X.; Wen, C. Self-Microemulsifying Drug Delivery System Improves Curcumin Dissolution and Bioavailability. Drug Dev. Ind. Pharm. 2011, 37, 15–23. [Google Scholar] [CrossRef]
- De Leo, V.; Milano, F.; Mancini, E.; Comparelli, R.; Giotta, L.; Nacci, A.; Longobardi, F.; Garbetta, A.; Agostiano, A.; Catucci, L. Encapsulation of Curcumin-Loaded Liposomes for Colonic Drug Delivery in a PH-Responsive Polymer Cluster Using a PH-Driven and Organic Solvent-Free Process. Molecules 2018, 23, 739. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Designing Food Structure and Composition to Enhance Nutraceutical Bioactivity to Support Cancer Inhibition. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2017; Volume 46, pp. 215–226. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, Protection, and Release of Hydrophilic Active Components: Potential and Limitations of Colloidal Delivery Systems. Adv. Colloid Interface Sci. 2015, 219, 27–53. [Google Scholar] [CrossRef]
- Yan, Y.D.; Kim, J.; Kwak, M.K.; Yoo, B.K.; Yong, C.S.; Choi, H.G. Enhanced Oral Bioaccessibility of Curcumin via a Solid Lipid-Based Self-Emulsifying Drug System Using a Spray Drying Technique. Biol. Pharm. Bull. 2011, 34, 1179–1186. [Google Scholar] [CrossRef]
- Mason, T.G.; Wilking, J.N.; Meleson, K.; Chang, C.B.; Graves, S.M. Nanoemulsions: Formation, Structure, and Physical Properties. J. Phys. Condens. Matter 2006, 18, R635. [Google Scholar] [CrossRef]
- Aditya, N.P.; Aditya, S.; Yang, H.; Kim, H.W.; Park, S.O.; Ko, S. Co-Delivery of Hydrophobic Curcumin and Hydrophilic Catechin by a Water-in-Oil-in-Water Double Emulsion. Food Chem. 2015, 173, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Han, S.; Wang, J.; McClements, D.J.; Liu, X.; Liu, F. Co-Delivery of Curcumin and Epigallocatechin Gallate in W/O/W Emulsions Stabilized by Protein Fibril-Cellulose Complexes. Colloids Surf. B Biointerfaces 2023, 222, 113072. [Google Scholar] [CrossRef]
- McClements, D.J. Enhanced Delivery of Lipophilic Bioactives Using Emulsions: A Review of Major Factors Affecting Vitamin, Nutraceutical, and Lipid Bioaccessibility. In Food and Function; Royal Society of Chemistry: London, UK, 2018; Volume 9, pp. 22–41. [Google Scholar] [CrossRef]
- Takahashi, M.; Uechi, S.; Takara, K.; Asikin, Y.; Wada, K. Evaluation of an Oral Carrier System in Rats: Bioavailability and Antioxidant Properties of Liposome-Encapsulated Curcumin. J. Agric. Food Chem. 2009, 57, 9141–9146. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C. Improved Bioavailability of Curcumin in Liposomes Prepared Using a PH-Driven, Organic Solvent-Free, Easily Scalable Process. RSC Adv. 2017, 7, 25978–25986. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Casagrande Sipoli, C.; de La Torre, L.G.; López-Rubio, A. Microencapsulation Structures Based on Protein-Coated Liposomes Obtained through Electrospraying for the Stabilization and Improved Bioaccessibility of Curcumin. Food Chem. 2017, 233, 343–350. [Google Scholar] [CrossRef]
- Brooks, B.R.; Brooks, C.L.; Mackerell, A.D.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The Biomolecular Simulation Program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef]
- Dhumal, D.M.; Kothari, P.R.; Kalhapure, R.S.; Akamanchi, K.G. Self-Microemulsifying Drug Delivery System of Curcumin with Enhanced Solubility and Bioavailability Using a New Semi-Synthetic Bicephalous Heterolipid: In Vitro and in Vivo Evaluation. RSC Adv. 2015, 5, 90295–90306. [Google Scholar] [CrossRef]
- Zeng, X.; Cai, D.; Zeng, Q.; Chen, Z.; Zhong, G.; Zhuo, J.; Gan, H.; Huang, X.; Zhao, Z.; Yao, N.; et al. Selective Reduction in the Expression of UGTs and SULTs, a Novel Mechanism by Which Piperine Enhances the Bioavailability of Curcumin in Rat. Biopharm. Drug Dispos. 2017, 38, 3–19. [Google Scholar] [CrossRef]
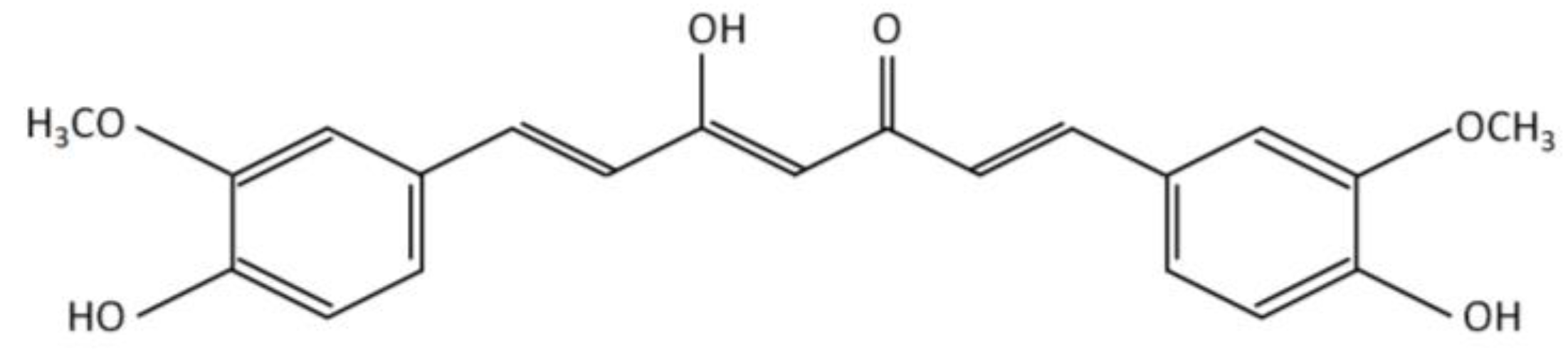

| Objective | Vehicle | Route of Administration | Dosage | Variable Response | Results | Conclusions | Reference |
|---|---|---|---|---|---|---|---|
| To explore the effect of curcumin consumption on experimental nephrotoxicity and oxidative stress induced by cisplatin | Emulsion with CMC | Oral | 15, 30, 60 mg/kg/d, 1 dose 2 days before and 3 days after cisplatin injection (CPT) | BUN, creatinine, MDA, GSD, SOD, CAT and TNF-α | (↓) the increase in BUN, creatinine, MDA, GSD, SOD, CAT, and TNF-α caused by CPT Improvement in morphological damage to the kidney | Curcumin has a protective effect on cisplatin-induced experimental nephrotoxicity due to its antioxidant and anti-inflammatory activity | [58] |
| To determine the protective effect of curcumin on CPT-induced nephrotoxicity | Olive oil | Intraperitoneal tube | 100 mg/kg/d, 1 dose | BUN, creatinine, TNF-α, MCP-1, ICAM-1 mRNA | (↓) the increase in BUN, creatinine, serum TNF-α, renal TNF-α, renal MCP-1 and ICAM-1 mRNA caused by CPT injection | Curcumin has a protective effect on cisplatin-induced experimental nephrotoxicity due to its antioxidant and anti-inflammatory activity | [59] |
| To explore the effect of curcumin consumption on experimental nephrotoxicity and oxidative stress induced by CPT | Nanoparticles | Oral | 50 mg/kg/d; 14 days | BUN, creatinine, MDA, NO, GSH and TNF-α | (↓) the increase in BUN, creatinine, MDA, NO and TNF-α caused by CPT Prevents the (↓) of GSH caused by CPT Improvement in morphological damage to the kidney | Curcumin has a protective effect on cisplatin-induced experimental nephrotoxicity due to its antioxidant and anti-inflammatory activity | [57] |
| To evaluate the effect of curcumin on streptozotocin (STZ)-induced diabetic nephropathy (DN) in rats | Emulsion with CMC | Oral | 15–30 mg/kg/d; 2 weeks | Creatinine, BUN, albumin, MDA, GSH, CAT, SOD | (↓) creatinine and BUN and MDA (↑) GSH, CAT, SOD in rats DN | Chronic treatment with curcumin attenuates renal dysfunction and oxidative stress in rats (DN) | [54] |
| To investigate whether curcumin reduces macrophage infiltration in STZ-induced DN rats | Emulsion with gum Arabic | Oral | 100 mg/kg/d; 2 weeks | Creatinine, BUN, NF-KB, TNF-α, IL-1, ICAM-1, TGF-β. | (↓) creatinine, BUN (↓) of NF-KB, TNF-α, IL-1, ICAM-1, TGF-β, 1 expression in DN rats (↓) of macrophage infiltration in the kidneys of DN rats | Curcumin reduces macrophage infiltration in kidneys of STZ-induced DN rats by inhibiting NF-KB activation and proinflammatory cytokines | [55] |
| To evaluate whether curcumin arrests the development of STZ-induced DN in rats, by inhibiting PKC-α and PKC-β1 activity and ERK1/2 pathway | Emulsion with gum Arabic | Oral probe | 100 mg/kg/d; 8 weeks | Creatinine, BUN, MDA, GSH, PKC-α and PKC-β1 | (↓) creatinine, BUN, and MDA β1 in DN rats (↑) GSH. β1 in DN rats (↓) of PKC-α and PKC-β1 in DN rats | Curcumin protects against the development of DN, which involves a dual blockade of PKC-α and PKC-β1 as well as the downstream pathway, ERK1/2. Probably due to its antioxidant properties, curcumin has an antifibrotic effect | [56] |
| To evaluate the effectiveness of curcumin relative to enalapril on chronic kidney disease (CKD) in 5⁄6 nephrectomized rats | Emulsion with gum Arabic | Oral probe | 75 mg/kg/d; 8 weeks | Creatinine, BUN, TNF-α, IL-1 | Curcumin was as effective as enalapril for (↓) increased BUN and creatinine (↓) levels of TNF-α and IL-1 | Curcumin was as effective as enalapril for (↓) increased BUN and creatinine (↓) levels of TNF-α and IL-1 | [62] |
| To evaluate whether curcumin, by increasing Nrf2 expression, could reduce oxidative stress, inflammation and renal fibrosis in 5⁄6 nephrectomized rats | Emulsion with gum Arabic | Oral probe | 75 mg/kg/d; 8 weeks | Creatinine, BUN, MDA, GSH, COX-2, TNF-α, TGF-β, HO-1, Keap1, Nrf2, NF-KB | (↓) creatinine and BUN (↓) MDA (↑) GSH (↓) COX-2 (↓) TNF-α and TGF-β (↑) HO-1 * (↓) Keap1 * (↑) Nrf2 * (↓) NF-KB | Curcumin effectively attenuates oxidative stress, inflammation and renal fibrosis by modulating the Nrf2-Keap1 pathway, suggesting it has promising potential for the treatment of CKD | [60] |
| To evaluate the association between the antioxidant and anti-inflammatory effect of curcumin consumption and its action on ischemia-reperfusion injury (I/R) | Emulsion with CMC | Oral | 200 mg/kg/d; 7 days | SOD, GSH, MDA, protein carbonyl (PC) and NO | (↑) GSH in serum (↓) MDA, NO and PC in serum and tissue Near normal kidney morphology | Consumption of curcumin protects the kidneys against I/R due to its antioxidant effect | [46] |
| To explore the effects and potential mechanisms of curcumin in modulating I/R-induced inflammatory response in rat kidney. | Emulsion with CMC | Intraperitoneal injection | 60 mg/kg, 1 dose | Creatinine, BUN, IL-8, TNF-α and IL-6 in serum; mRNA level of IL-8, TNF-α and IL-6 in kidney, expression of JAK2, p-JAK2, STAT3, p-STAT3, p65 and p-p65 in the kidney | (↓) Creatinine, BUN, IL-8, TNF-α, IL-6 and p-p65 expression. (↑) the expression of p-JAK2 and p-STAT3. Attenuate pathological kidney injury. No effect on the expression of JAK2, STAT3 and p65 | The protective effect of curcumin on I/R injury is associated with NF-KB-mediated suppression of inflammation through activation of the JAK2/STAT3 signaling pathway | [61] |
| Objective | Vehicle | Route of Administration | Dosage | Variable Response | Results | Conclusions | Reference |
|---|---|---|---|---|---|---|---|
| To evaluate the effect of curcumin juice on the expression of inflammatory markers in HD patients | Oral juice | 2.5 g of turmeric + 12 g of carrot + 100 mL orange juice | 3 times per week after HD | NF-kB and PCR | (↓) NF-kB and PCR | Oral curcumin supplementation has an anti-inflammatory effect in HD | [23] |
| To evaluate the effect of curcumin supplementation on plasma levels of uremic toxins in HD patients | Oral juice | 2.5 g of turmeric + 12 g of carrot + 100 mL orange juice | 3 times per week after HD | IS, pCS, IAA | (↓) pCS but not in IS and IAA | Oral curcumin supplementation reduces plasma pCS levels in HD patients suggesting a modulation of the gut microbiota due to decreased production of uremic toxins | [63] |
| To evaluate the effect of nano curcumin supplementation on inflammation in HD patients | Nanocapsule | 1 capsule = 40 mg curcumin | 3 capsules daily | PCR, ICAM-1, VCAM-1 | (↓) PCR and VCAM-1 | Nanocurcumin shows beneficial effects in reducing inflammation in HD patients | [64] |
| To evaluate the effect of turmeric on markers of oxidative stress in HD patients | Capsule | 1 capsule = 500 mg turmeric = 22.1 mg curcumin | 3 capsules daily | MDA, GPX, GR, CAT | (↓) MDA. (↑) GPX, GR and CAT | Curcumin significantly attenuates oxidative species and increases antioxidant markers in CKD | [22] |
| To evaluate the effects of turmeric on the reduction of inflammatory markers in HD patients | Capsule | 1 capsule = 500 mg turmeric = 21.1 mg curcumin | 3 capsules daily | BUN, creatinine, IL-6, TNF-α, and CRP | (↓) CRP, IL-6 and TNF-α in HD patients | Curcumin capsule ingestion has an anti-inflammatory effect in HD patients | [49] |
| Investigating the effect of curcumin and ginger on oxidative modulation in CKD patients | Capsule | 1 capsule = 500 mg | 3 capsules daily | MDA, GPX, GR, CAT y SOD | (↓) MDA in patients with CKD(↑) GPX and CAT in CKD patients | Curcumin and ginger ingestion showed an increase in antioxidant markers in patients with chronic renal failure | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’andurain, J.; López, V.; Arazo-Rusindo, M.; Tiscornia, C.; Aicardi, V.; Simón, L.; Mariotti-Celis, M.S. Effect of Curcumin Consumption on Inflammation and Oxidative Stress in Patients on Hemodialysis: A Literature Review. Nutrients 2023, 15, 2239. https://doi.org/10.3390/nu15102239
D’andurain J, López V, Arazo-Rusindo M, Tiscornia C, Aicardi V, Simón L, Mariotti-Celis MS. Effect of Curcumin Consumption on Inflammation and Oxidative Stress in Patients on Hemodialysis: A Literature Review. Nutrients. 2023; 15(10):2239. https://doi.org/10.3390/nu15102239
Chicago/Turabian StyleD’andurain, Javiera, Vanessa López, Migdalia Arazo-Rusindo, Caterina Tiscornia, Valeria Aicardi, Layla Simón, and María Salomé Mariotti-Celis. 2023. "Effect of Curcumin Consumption on Inflammation and Oxidative Stress in Patients on Hemodialysis: A Literature Review" Nutrients 15, no. 10: 2239. https://doi.org/10.3390/nu15102239
APA StyleD’andurain, J., López, V., Arazo-Rusindo, M., Tiscornia, C., Aicardi, V., Simón, L., & Mariotti-Celis, M. S. (2023). Effect of Curcumin Consumption on Inflammation and Oxidative Stress in Patients on Hemodialysis: A Literature Review. Nutrients, 15(10), 2239. https://doi.org/10.3390/nu15102239








