Associations between Dietary Patterns, Anthropometric and Cardiometabolic Indices and the Number of MetS Components in Polish Adults with Metabolic Disorders
Abstract
1. Introduction
2. Materials and Methods
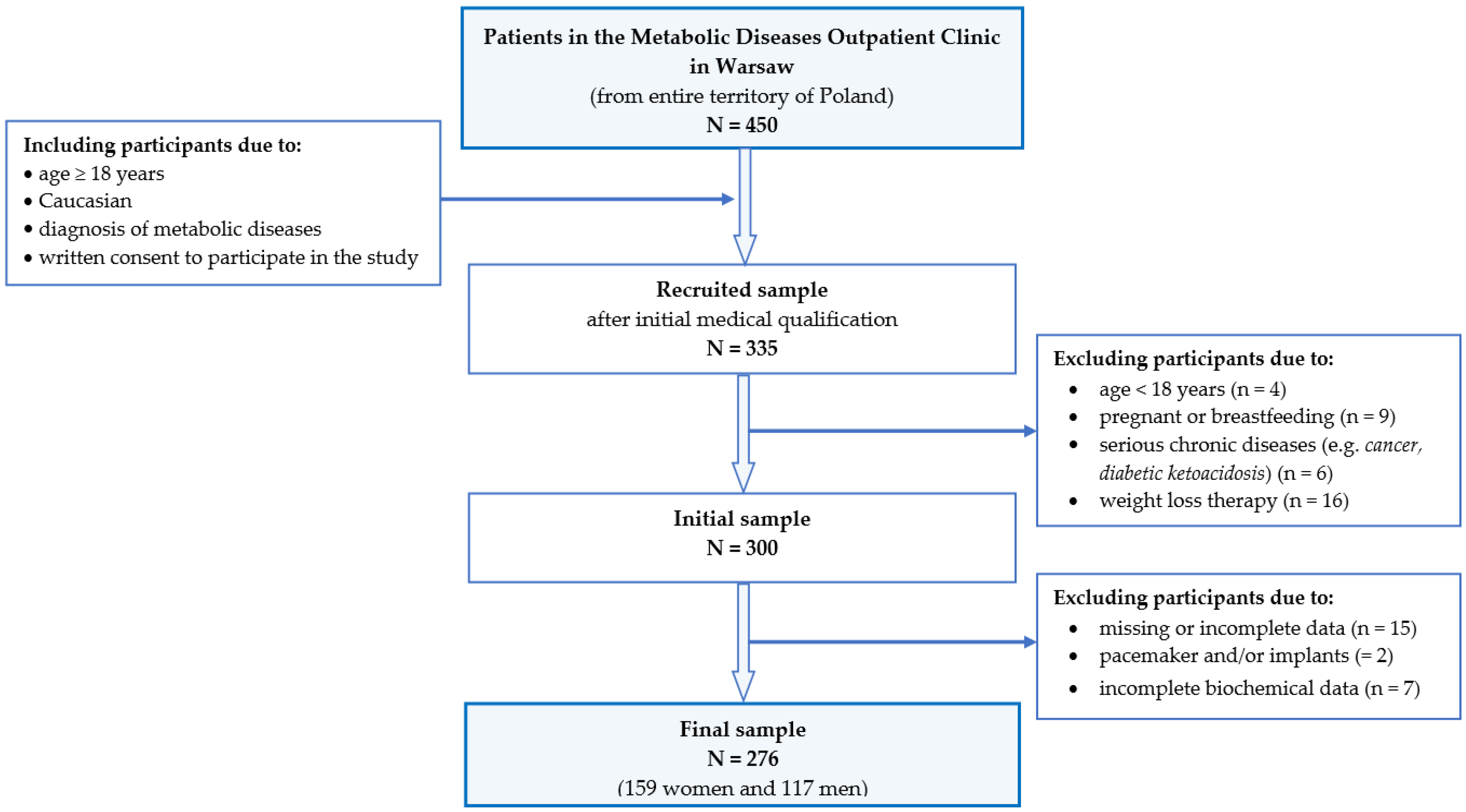
2.1. Study Design and Participants
2.2. Definition and Criteria of Metabolic Syndrome
2.3. Data Collection and Procedures
2.3.1. Anthropometrics
2.3.2. Blood Pressure Measurements
2.3.3. Biochemical Analysis
2.3.4. Metabolic Dysfunction Indices
2.3.5. Dietary Assessment
2.3.6. Dietary Patterns Identification
2.3.7. Sociodemographic and Lifestyle Data
2.3.8. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Dietary Patterns and Frequency of Consumption of Selected Group Products
3.3. Anthropometric Parameters, Cardiometabolic Indices and Number of MetS Components
3.4. Association between MetS Severity and Selected Nutritional Variables
4. Discussion
Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO European Regional Obesity Report 2022; WHO Regional Office for Europe: Copenhagen, Denmark, 2022; Available online: https://apps.who.int/iris/handle/10665/353747.pdf (accessed on 29 January 2023).
- The International Diabetes Federation IDF. Consensus Worldwide Definition of the Metabolic Syndrome. Available online: https://www.idf.org/e-library/consensus-statements/60-idfconsensus-worldwide-definitionof-the-metabolic-syndrome.html (accessed on 12 November 2022).
- Ambroselli, D.; Masciulli, F.; Romano, E.; Catanzaro, G.; Besharat, Z.M.; Massari, M.C.; Ferretti, E.; Migliaccio, S.; Izzo, L.; Ritieni, A.; et al. New Advances in Metabolic Syndrome, from Prevention to Treatment: The Role of Diet and Food. Nutrients 2023, 15, 640. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Pluta, W.; Dudzińska, W.; Lubkowska, A. Metabolic Obesity in People with Normal Body Weight (MONW)—Review of Diagnostic Criteria. Int. J. Environ. Res. Public Health 2022, 19, 624. [Google Scholar] [CrossRef]
- Moore, J.X.; Chaudhary, N.; Akinyemiju, T. Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev. Chronic. Dis. 2017, 16, E24. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, A.; Laurent, S.; Cucca, F.; Cockcroft, J.; Cunha, P.G.; Mañas, L.R.; Raso, F.U.M.; Muiesan, M.L.; Ryliškyte, L.; Rietzschel, E.; et al. Metabolic Syndrome and Arteries Research (MARE) Consortium. Metabolic syndrome across Europe: Different clusters of risk factors. Eur. J. Prev. Cardiol. 2015, 22, 486–491. [Google Scholar] [CrossRef]
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Regufe, V.M.G.; Pinto, C.M.C.B.; Perez, P.M.V.H. Metabolic syndrome in type 2 diabetic patients: A review of current evidence. Porto Biomed. J. 2020, 5, e101. [Google Scholar] [CrossRef]
- de la Iglesia, R.; Loria-Kohen, V.; Zulet, M.A.; Martinez, J.A.; Reglero, G.; de Molina, A.R. Dietary Strategies Implicated in the Prevention and Treatment of Metabolic Syndrome. Int. J. Mol. Sci. 2016, 17, 1877. [Google Scholar] [CrossRef]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef]
- Agodi, A.; Maugeri, A.; Kunzova, S.; Sochor, O.; Bauerova, H.; Kiacova, N.; Barchitta, M.; Vinciguerra, M. Association of Dietary Patterns with Metabolic Syndrome: Results from the Kardiovize Brno 2030 Study. Nutrients 2018, 10, 898. [Google Scholar] [CrossRef] [PubMed]
- ISAK. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry: Potchefstroom. South Africa. 2001. Available online: http://www.ceap.br/material/MAT17032011184632.pdf (accessed on 2 April 2017).
- Stewart, A.; Marfell-Jones, M.J.; International Society for the Advancement of Kinanthropometry. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry: Lower Hutt, New Zealand, 2011. [Google Scholar]
- Holmes, C.J.; Racette, S.B. The Utility of Body Composition Assessment in Nutrition and Clinical Practice: An Overview of Current Methodology. Nutrients 2021, 13, 2493. [Google Scholar] [CrossRef]
- Górnicka, M.; Szewczyk, K.; Białkowska, A.; Jancichova, K.; Habanova, M.; Górnicki, K.; Hamulka, J. Anthropometric Indices as Predictive Screening Tools for Obesity in Adults; The Need to Define Sex-Specific Cut-Off Points for Anthropometric Indices. Appl. Sci. 2022, 12, 6165. [Google Scholar] [CrossRef]
- Lee, D.H.; Keum, N.; Hu, F.B.; Orav, E.; Rimm, E.; Sun, Q.; Willett, W.C.; Giovannucci, E. Development and validation of anthropometric prediction equations for lean body mass, fat mass and percent fat in adults using the National Health and Nutrition Examination Survey (NHANES) 1999–2006. Br. J. Nutr. 2017, 118, 858–866. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE) Hypertension in Adults: Diagnosis and Management. Available online: https://www.nice.org.uk/guidance/ng136 (accessed on 19 September 2022).
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrillo, P.L.; Aguirre-Tostado, P.I.; Macías-Cervantes, M.H.; Alegría-Torres, J.A.; Luevano-Contreras, C. Novel Adiposity and Biochemical-Anthropometric Indices to Identify Cardiometabolic Risk and Metabolic Syndrome in Mexican Adults. Healthcare 2021, 9, 1561. [Google Scholar] [CrossRef]
- Dietary Habits and Nutrition Beliefs Questionnaire and the Manual for Developing of Nutritional Data KomPAN [in Polish: Kwestionariusz do Badania Poglądów i Zwyczajów Żywieniowych Oraz Procedura Opracowania Danych (KomPAN®): Wersja Polskojęzyczna. Committee of Human Nutrition Science. Polish Academy of Science. Warsaw. 2014. Available online: http://www.knozc.pan.pl/ (accessed on 23 February 2017).
- Kowalkowska, J.; Wadolowska, L.; Czarnocinska, J.; Czlapka-Matyasik, M.; Galinski, G.; Jezewska-Zychowicz, M.; Bronkowska, M.; Dlugosz, A.; Loboda, D.; Wyka, J. Reproducibility of a Questionnaire for Dietary Habits, Lifestyle and Nutrition Knowledge Assessment (KomPAN) in Polish Adolescents and Adults. Nutrients 2018, 10, 1845. [Google Scholar] [CrossRef]
- Osadnik, K.; Osadnik, T.; Lonnie, M.; Lejawa, M.; Reguła, R.; Fronczek, M.; Gawlita, M.; Wądołowska, L.; Gąsior, M.; Pawlas, N. Metabolically healthy obese and metabolic syndrome of the lean: The importance of diet quality. Analysis of MAGNETIC cohort. Nutr. J. 2020, 19, 19. [Google Scholar] [CrossRef]
- Fabiani, R.; Naldini, G.; Chiavarini, M. Dietary Patterns and Metabolic Syndrome in Adult Subjects: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2056. [Google Scholar] [CrossRef] [PubMed]
- Gierach, M.; Junik, R. Insulin resistance in metabolic syndrome depending on the occurrence of its components. Endokrynol. Pol. 2021, 72, 243–248. [Google Scholar] [CrossRef]
- Mata, J.; Kadel, P.; Frank, R.; Schüz, B. Education- and income-related differences in processed meat consumption across Europe: The role of food-related attitudes. Appetite 2023, 182, 106417. [Google Scholar] [CrossRef]
- Jezewska-Zychowicz, M.; Gębski, J.; Plichta, M.; Guzek, D.; Kosicka-Gębska, M. Diet-Related Factors, Physical Activity, and Weight Status in Polish Adults. Nutrients 2019, 11, 2532. [Google Scholar] [CrossRef]
- Al Thani, M.; Al Thani, A.A.; Al-Chetachi, W.; Al Malki, B.; Khalifa, S.A.H.; Bakri, A.H.; Hwalla, N.; Nasreddine, L.; Naja, F. A ‘High Risk’ Lifestyle Pattern Is Associated with Metabolic Syndrome among Qatari Women of Reproductive Age: A Cross-Sectional National Study. Int. J. Mol. Sci. 2016, 17, 698. [Google Scholar] [CrossRef]
- Honerlaw, J.P.; Ho, Y.L.; Nguyen, X.M.T. Fried food consumption and risk of coronary artery disease: The Million Veteran Program. Clin. Nutr. 2020, 39, 1203–1208. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, J. Association between fried food consumption and hypertension in Korean adults. Br. J. Nutr. 2016, 115, 87–94. [Google Scholar] [CrossRef]
- Pan, F.; Wang, Z.; Wang, H.; Zhang, J.; Su, C.; Jia, X.; Du, W.; Jiang, H.; Li, W.; Wang, L.; et al. Association between Ultra-Processed Food Consumption and Metabolic Syndrome among Adults in China—Results from the China Health and Nutrition Survey. Nutrients 2023, 15, 752. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Batal, M.; Johnson-Down, L.; Moubarac, J.C.; Ing, A.; Fediuk, K.; Sadik, T.; Tikhonov, C.; Chan, L.; Willows, N. Quantifying associations of the dietary share of ultra-processed foods with overall diet quality in First Nations peoples in the Canadian provinces of British Columbia, Alberta, Manitoba and Ontario. Public Health Nutr. 2018, 21, 103–113. [Google Scholar] [CrossRef]
- US Departament of Health and Human Services. Dietary Guidelines for Americans. Available online: https://health.gov/our-work/nutrition-physical-activity/dietary-guidelines (accessed on 2 February 2023).
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. (Eds.) Polish Dietary Reference Intakes—Revision; National Institute of Public Health—National Institute of Hygiene: Warsaw, Poland, 2020; ISBN 9788365870285. [Google Scholar]
- Khan, S.U.; Lone, A.N.; Khan, M.S.; Virani, S.S.; Blumenthal, R.; Nasir, K.; Miller, M.; Michos, E.D.; Ballantyne, C.M.; Boden, W.E.; et al. Effect of omega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. E Clin. Med. 2021, 8, 100997. [Google Scholar] [CrossRef]
- Albracht-Schulte, K.; Kalupahana, N.S.; Ramalingam, L.; Wang, S.; Rahman, S.M.; Robert-McComb, J. Omega-3 fatty acids in obesity and metabolic syndrome: A mechanistic update. J. Nutr. Biochem. 2018, 58, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Pedley, A.; Hoffmann, U.; Massaro, J.M.; Fox, C.S. Association of Changes in Abdominal Fat Quantity and Quality With Incident Cardiovascular Disease Risk Factors. J. Am. Coll. Cardiol. 2016, 68, 1509–1521. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhang, X.; Xu, Y.; Dong, H. Feasibility of body roundness index for identifying a clustering of cardiometabolic abnormalities compared to BMI, waist circumference and other anthropometric indices: The China Health and Nutrition Survey, 2008 to 2009. Medicine 2016, 95, e4642. [Google Scholar] [CrossRef]
- Li, G.; Wu, H.K.; Wu, X.W.; Cao, Z.; Tu, Y.C.; Ma, Y.; Li, B.N.; Peng, Q.Y.; Cheng, J.; Wu, B.; et al. The feasibility of two anthropometric indices to identify metabolic syndrome, insulin resistance and inflammatory factors in obese and overweight adults. Nutrition 2019, 57, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Rico-Martín, S.; Calderón-García, J.F.; Sánchez-Rey, P.; Franco-Antonio, C.; Martínez Alvarez, M.; Sánchez Muñoz-Torrero, J.F. Effectiveness of body roundness index in predicting metabolic syndrome: A systematic review and meta-analysis. Obes. Rev. 2020, 21, e13023. [Google Scholar] [CrossRef]
- Anto, E.O.; Frimpong, J.; Ivy, W.; Boadu, O. Prevalence of Cardiometabolic Syndrome and its Association with Body Shape Index and A Body Roundness Index Among Type 2 Diabetes Mellitus Patients: A Hospital-Based Cross-Sectional Study in a Ghanaian Population. Front. Clin. Diabetes Healthc. 2022, 2, 807201. [Google Scholar] [CrossRef]
- Andersen, C.J.; Fernandez, M.L. Dietary strategies to reduce metabolic syndrome. Rev. Endocr. Metab. Disord. 2013, 14, 241–254. [Google Scholar] [CrossRef]
- Pérez-Martínez, P.; Mikhailidis, D.P.; Athyros, V.G.; Bullo, M.; Couture, P.; Covas, M.I.; de Koning, L.; Delgado-Lista, J.; Díaz-López, A.; Drevon, C.A.; et al. Lifestyle recommendations for the prevention and management of metabolic syndrome: An international panel recommendation. Nutr. Rev. 2017, 75, 307–326. [Google Scholar] [CrossRef]
| Parameter | Cut-Off Point for Men | Cut-Off Point for Women |
|---|---|---|
| WC | ≥94 cm | ≥80 cm |
| Glucose | ≥100 mg/dL (≥5.56 mmol/L) | ≥100 mg/dL (≥5.56 mmol/L) |
| Triglycerides | ≥150 mg/dL (≥1.69 mmol/L) | ≥150 mg/dL (≥1.69 mmol/L) |
| HDL-C | <40 mg/dL (<1.03 mmol/L) | <50 mg/dL (<1.29 mmol/L) |
| Blood pressure | SBP ≥ 130 or DBP ≥ 85 mmHg | SBP ≥ 130 or DBP ≥ 85 mmHg |
| Variable | Number of Metabolic Syndrome Criteria | |||
|---|---|---|---|---|
| 3 MetS (n = 150) | 4 MetS (n = 70) | 5 MetS (n = 56) | p-Value | |
| Age (years) | 51.6 ± 13.2 | 54.9 ± 12.2 | 58.2 ± 9.6 | 0.004 |
| Sex (%) | ||||
| men | 22 | 69 | 64 | <0.0001 |
| women | 78 | 31 | 36 | |
| Education (%) | ||||
| primary and vocational | 19 | 23 | 36 | 0.03 |
| secondary | 44 | 50 | 32 | |
| university | 37 | 27 | 32 | |
| Physical activity (%) | ||||
| low | 71 | 80 | 78 | ns |
| moderate | 25 | 19 | 20 | |
| vigorous | 4 | 1 | 2 | |
| Smoking (%) | 23 | 34 | 18 | 0.004 |
| Anthropometric indices: | ||||
| BMI (%) | ||||
| <18.5 | 3.3 | 1.4 | 0 | 0.00015 |
| 18.5–24.99 | 31.3 | 8.6 | 3.6 | |
| 25.0–29.99 | 22.0 | 32.9 | 32.1 | |
| 30.0–34.99 | 24.0 | 27.1 | 25.0 | |
| 35.0–39.99 | 11.3 | 14.3 | 21.4 | |
| >40 | 8.0 | 15.7 | 17.9 | |
| BMI (kg/m2) | 28.91 ± 6.91 28.15 a | 32.19 ± 6.41 31.41 b | 33.89 ± 6.71 33.09 b | <0.0001 |
| BRI | 5.32 ± 2.20 5.24 a | 6.57 ± 1.98 6.21 b | 7.43 ± 2.16 7.35 b | <0.0001 |
| WC (cm) | 97.24 ± 15.77 98.0 a | 111 ± 15.17 111.0 b | 115 ± 13.66 116.5 b | <0.0001 |
| WHtR | 0.59 ± 0.10 0.59 a | 0.64 ± 0.08 0.63 b | 0.68 ± 0.08 0.68 b | <0.0001 |
| Fat mass (FM) (%) | 33.92 ± 10.67 34.70 | 32.02 ± 9.43 30.75 | 33.81 ± 9.99 33.70 | ns |
| Blood pressure: | ||||
| SBP (mmHg) | 131 ± 18.07 126.00 a | 139 ± 16.84 139.50 b | 144 ± 14.99 141.00 b | <0.0001 |
| DBP (mmHg) | 78.14 ± 12.28 80.00 a | 84.60 ± 11.24 85.00 | 84.82 ± 9.89 85.00 | <0.0001 |
| FPG (mmol/L) | 6.62 ± 2.93 5.72 a | 7.68 ± 3.38 6.36 b | 8.31 ± 3.72 6.86 b | <0.0001 |
| Lipid profile: | ||||
| CHOL (mmol/L) | 5.04 ± 0.86 5.22 | 5.01 ± 0.98 5.20 | 5.09 ± 0.99 5.22 | ns |
| TG (mmol/L) | 1.73 ± 0.53 1.73 a | 2.07 ± 0.85 1.81 b | 2.43 ± 0.73 2.13 c | <0.0001 |
| HDL-C (mmol/L) | 1.27 ± 0.28 1.25 a | 1.01 ± 0.27 0.95 b | 0.86 ± 0.15 0.49 c | <0.0001 |
| LDL-C (mmol/L) | 2.79 ± 0.77 2.72 | 2.72 ± 0.89 2.70 | 2.53 ± 0.78 2.50 | ns |
| Cardiometabolic indices: | ||||
| AIP | 1.12 ± 0.37 1.10 a | 1.50 ± 0.44 1.44 b | 1.83 ± 0.32 1.81 c | <0.0001 |
| CMI (mmol/L) | 0.85 ± 0.40 0.74 a | 1.42 ± 0.81 1.19 b | 1.97 ± 0.78 1.77 c | <0.0001 |
| LAP (mmol/L) | 66.24 ± 38.77 60.41 a | 101 ± 51.97 91.59 b | 130 ± 56.29 121.53 c | <0.0001 |
| TG/HDL-C ratio (mmol/L) | 1.45 ± 0.61 1.33 a | 2.21 ± 1.27 1.86 b | 2.89 ± 1.00 2.70 c | <0.0001 |
| TyG | 9.00 ± 0.44 8.89 a | 9.29 ± 0.47 9.15 ab | 9.56 ± 0.52 9.41 b | <0.0001 |
| TyG-BMI | 260 ± 62.91 248 a | 299 ± 60.96 298 b | 324 ± 66.57 316 b | <0.0001 |
| TyG-WC | 874 ± 146 878 a | 1035± 153 1062 b | 1105 ± 154 1093 b | <0.0001 |
| VAI (mmol/L) | 2.64 ± 1.15 2.43 a | 3.58 ± 2.07 2.94 b | 4.80 ± 1.68 4.50 c | <0.0001 |
| Dietary patterns: | ||||
| Western | 27 | 37 | 29 | ns |
| Prudent | 45 | 39 | 32 | |
| Low Food | 28 | 24 | 39 | |
| Variable | Men (n = 117) | p-Value | Women (n = 159) | p-Value | p-Value Men vs. Women | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 MetS (n = 33) | 4 MetS (n = 48) | 5 MetS (n = 36) | 3 MetS (n = 117) | 4 MetS (n = 20) | 5 MetS (n = 22) | 3 MetS | 4 MetS | 5 MetS | |||
| Dietary patterns (%) | |||||||||||
| Western | 36 | 31 | 28 | ns | 24 | 50 | 30 | 0.077 | ns | ||
| Prudent | 39 | 40 | 33 | 46 | 36 | 30 | ns | ||||
| Low Food | 25 | 29 | 39 | 30 | 14 | 40 | ns | ||||
| Food groups # (Mean ± SD) | |||||||||||
| Vegetable | 5.5 ± 1.1 | 5.3 ± 1.4 | 4.9 ± 1.5 | ns | 5.6 ± 1.0 | 5.5 ± 1.0 | 5.5 ± 0.9 | ns | ns | ns | ns |
| Fruit | 6.0 ± 0.8 | 5.7 ± 1.2 | 5.9 ± 0.8 | ns | 5.9 ± 0.9 | 5.9 ± 0.8 | 6.3 ± 0.9 | ns | ns | ns | 0.095 |
| Milk, fermented milk beverages, cottage cheese | 5.1 ± 1.0 | 4.4 ± 1.4 | 4.1 ± 1.4 | 0.007 | 4.8 ± 1.3 | 5.1 ± 0.9 | 4.6 ± 1.7 | ns | ns | 0.081 | ns |
| Cheese | 4.1 ± 1.2 | 4.0 ± 1.3 | 3.9 ± 1.1 | ns | 3.7 ± 1.2 | 3.6 ± 1.2 | 3.7 ± 1.2 | ns | 0.073 | ns | ns |
| Fish | 3.0 ± 0.8 | 2.6 ± 0.8 | 2.7 ± 0.7 | ns | 2.7 ± 0.7 | 2.6 ± 0.7 | 2.6 ± 0.7 | ns | ns | ns | ns |
| Red meat | 3.9 ± 0.7 | 4.1 ± 0.8 | 4.2 ± 0.7 | ns | 3.9 ± 0.8 | 3.8 ± 0.8 | 3.7 ± 0.8 | ns | ns | ns | ns |
| White meat | 3.9 ± 0.7 | 3.9 ± 0.9 | 3.9 ± 0.8 | ns | 4.1 ± 0.8 | 3.9 ± 0.6 | 3.9 ± 1.1 | ns | ns | ns | ns |
| Processed meat | 5.9 ± 1.0 | 5.9 ± 1.1 | 5.9 ± 1.1 | ns | 5.7 ± 1.0 | 5.7 ± 1.1 | 5.9 ± 1.0 | ns | ns | ns | ns |
| Sweets | 4.0 ± 1.8 | 3.7 ± 1.6 | 3.5 ± 1.9 | ns | 3.9 ± 1.6 | 4.1 ± 1.5 | 2.9 ± 1.3 | 0.007 | ns | ns | ns |
| Whole grains | 3.9 ± 1.9 | 3.9 ± 1.8 | 4.3 ± 1.8 | ns | 4.2 ± 1.9 | 4.2 ± 1.7 | 3.9 ± 2.1 | ns | ns | ns | ns |
| Non-whole grains | 6.0 ± 1.3 | 5.9 ± 1.5 | 6.0 ± 1.4 | ns | 5.8 ± 1.3 | 6.44 ± 0.7 | 6.2 ± 1.2 | 0.045 | ns | ns | ns |
| Fried foods | 4.1 ± 1.4 | 4.2 ± 1.2 | 4.1 ± 1.3 | ns | 3.5 ± 1.3 | 3.8 ± 1.2 | 4.3 ± 1.6 | ns | 0.048 | ns | 0.074 |
| Fast food | 2.4 ± 1.1 | 2.0 ± 0.8 | 2.1 ± 1.0 | ns | 1.8 ± 0.7 | 1.9 ± 0.9 | 1.9 ± 0.7 | ns | 0.008 | ns | ns |
| Water | 5.6 ± 0.7 | 5.4 ± 1.2 | 5.7 ± 0.8 | ns | 5.6 ± 0.8 | 5.6 ± 0.7 | 5.9 ± 0.5 | ns | ns | ns | ns |
| Juices | 3.7 ± 1.5 | 3.9 ± 1.6 | 4.1 ± 1.6 | ns | 4.1 ± 1.5 | 4.1 ± 1.4 | 4.4 ± 1.7 | ns | ns | ns | ns |
| Sweet beverages | 3.7 ± 1.8 | 3.5 ± 1.8 | 4.1 ± 1.6 | ns | 3.0 ± 1.5 | 2.8 ± 1.3 | 2.6 ± 1.4 | ns | ns | ns | 0.001 |
| Coffee and tea | 6.4 ± 1.6 | 6.1 ± 1.9 | 6.3 ± 1.7 | ns | 6.5 ± 1.5 | 6.7 ± 1.3 | 6.8 ± 0.7 | ns | ns | ns | ns |
| Energy drinks | 1.4 ± 0.9 | 1.3 ± 0.7 | 1.2 ± 0.5 | ns | 1.2 ± 0.5 | 1.2 ± 0.5 | 1.1 ± 0.2 | ns | ns | ns | ns |
| Variable | Men (n = 117) | p-Value | Women (n = 159) | p-Value | p-Value Men vs. Women | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 MetS (n = 33) | 4 MetS (n = 48) | 5 MetS (n = 36) | 3 MetS (n = 117) | 4 MetS (n = 20) | 5 MetS (n = 22) | 3 MetS | 4 MetS | 5 MetS | |||
| Anthropometric indices: | |||||||||||
| BMI (kg/m2) | 26.9 ± 5.2 26.5 a | 31.70 ± 5.6 31.4 b | 32.7 ± 5.1 31.1 b | <0.0001 | 29.5 ± 7.2 29.3 a | 33.3 ± 7.9 32.1 ab | 36.0 ± 8.7 36.3 b | 0.002 | ns | ns | ns |
| BRI | 4.7 ± 1.7 4.4 a | 6.5 ± 1.9 6.0 b | 7.0 ± 1.7 6.7 b | <0.0001 | 5.5 ± 2.3 5.4 a | 6.8 ± 2.2 7.2 ab | 8.3 ± 2.7 8.6 b | <0.0001 | ns | ns | 0.042 |
| WC (cm) | 99.4 ± 14.8 97.0 a | 113.8 ± 14.5 112.5 b | 116.6 ± 12.8 116.5 b | <0.0001 | 96.6 ± 16.0 98.5 a | 106 ± 15.6 107.5 b | 114 ± 15.2 116.0 b | <0.0001 | ns | ns | ns |
| WHtR | 0.56 ± 0.08 0.55 a | 0.64 ± 0.08 0.63 b | 0.66 ± 0.07 0.65 b | <0.0001 | 0.59 ± 0.10 0.60 a | 0.65 ± 0.09 0.67 ab | 0.71 ± 0.10 0.73 b | <0.0001 | ns | ns | 0.042 |
| FM (%) | 23.2 ± 9.2 24.2 a | 28.1 ± 7.0 28.3 b | 29.2 ± 7.4 29.0 b | 0.007 | 36.9 ± 9.0 38.2 a | 40.5 ± 8.6 41.1 ab | 42.1 ± 8.8 45.1 b | 0.019 | 0.000 | 0.000 | 0.000 |
| Blood pressure: | |||||||||||
| SBP (mmHg) | 129 ± 16.2 128 a | 138 ± 17.5 140 b | 141 ± 14.6 140 b | 0.005 | 132 ± 18.6 126 a | 140 ± 15.7 139 b | 148 ± 14.8 145 b | <0.0001 | ns | ns | ns |
| DBP (mmHg) | 76.5 ± 12.1 78.0 a | 85.5 ± 11.9 85.0 b | 86.1 ± 8.6 85.0 b | 0.001 | 78.6 ± 12.3 80.0 | 82.5 ±9.7 83.5 | 82.5 ± 11.8 85.0 | ns | ns | ns | ns |
| FPG (mmol/L) | 6.43 ± 2.84 5.4 a | 8.03 ± 3.71 6.4 b | 8.52 ±3.93 6.9 b | 0.0006 | 6.67 ± 2.97 5.75 a | 6.92 ± 2.44 6.36 ab | 7.92 ± 3.34 6.78 b | 0.006 | ns | ns | ns |
| Lipid profile: | |||||||||||
| CHOL (mmol/L) | 5.1 ± 0.9 5.2 | 4.9 ± 1.0 5.1 | 5.2 ± 1.1 5.2 | ns | 5.0 ± 0.9 5.2 | 5.2 ± 0.8 5.3 | 4.9 ± 0.8 5.2 | ns | ns | ns | ns |
| TG (mmol/L) | 1.7 ± 0.5 1.7 a | 2.1 ±1.0 1.8 a | 2.5 ± 0.8 2.1 b | <0.0001 | 1.7 ± 0.5 1.7 a | 2.0 ± 0.5 2.0 b | 2.2 ± 0.5 2.1 b | <0.0001 | ns | ns | ns |
| HDL-C (mmol/L) | 1.2 ± 0.3 1.1 a | 1.0 ± 0.3 0.9 b | 0.9 ± 0.2 0.9 b | <0.0001 | 1.3 ± 0.3 1.3 a | 1.1 ± 0.3 1.1 b | 0.9 ± 0.1 0.9 c | <0.0001 | 0.014 | 0.019 | ns |
| LDL-C (mmol/L) | 2.8 ± 0.7 2.8 | 2.7 ± 0.9 2.6 | 2.5 ± 0.8 2.4 | ns | 2.8 ± 0.8 2.7 | 2.8 ± 0.8 2.8 | 2.5 ± 0.7 2.7 c | ns | ns | ns | ns |
| Cardiometabolic indices: | |||||||||||
| AIP | 1.2 ± 0.4 1.2 a | 1.5 ± 0.5 1.5 b | 1.9 ± 0.3 1.8 c | <0.0001 | 1.1 ± 0.4 1.1 a | 1.4 ± 0.4 1.4 b | 1.8 ± 0.3 1.7 c | <0.0001 | ns | ns | ns |
| CMI (mmol/L) | 0.9 ± 0.5 0.8 a | 1.5 ± 0.9 1.2 b | 2.0 ± 0.9 1.8 c | <0.0001 | 0.8 ± 0.4 0.7 a | 1.3 ± 0.5 1.1 b | 1.9 ± 0.6 1.7 c | <0.0001 | ns | ns | ns |
| LAP (mmol/L) | 59.3 ± 40.2 48.6 a | 102 ± 55.1 89.5 b | 134 ± 62.2 127.4 c | <0.0001 | 68.2 ± 38.3 64.2 a | 99.4 ± 45.6 96.9 b | 123 ± 44.4 119 b | <0.0001 | ns | ns | ns |
| TG/HDL-C ratio (mmol/L) | 1.6 ± 0.7 1.4 a | 2.3 ± 1.4 1.9 b | 3.0 ± 1.1 2.8 c | <0.0001 | 1.4 ± 0.6 1.3 a | 1.9 ± 0.8 1.8 b | 2.7 ± 0.7 2.4 c | <0.0001 | ns | ns | ns |
| TyG | 8.9 ± 0.4 8.8 a | 9.3 ± 0.5 9.2 ab | 9.6 ± 0.5 9.4 b | <0.0001 | 9.0 ± 0.4 8.9 a | 9.2 ± 0.4 9.1 b | 9.5 ± 0.5 9.3 b | <0.0001 | ns | ns | ns |
| TyG-BMI | 240 ± 47.7 225 a | 295 ± 53.7 294 b | 315.± 55.7 295 b | <0.0001 | 266 ± 65.7 261 a | 308 ± 75.0 303 ab | 340 ± 81.9 344 b | <0.0001 | ns | ns | ns |
| TyG-WC | 888 ± 135 866 a | 1061 ± 146 1069 b | 1123 ± 156 1105 b | <0.0001 | 871 ± 150 880 a | 982 ± 159 992 b | 1072 ± 148 1091 b | <0.0001 | ns | ns | ns |
| VAI (mmol/L) | 2.2 ± 1.0 2.0 a | 3.4 ± 2.2 2.8 b | 4.4 ± 1.7 4.2 c | <0.0001 | 2.8 ± 1.2 2.6 a | 3.9 ± 1.7 3.4 b | 5.4 ± 1.5 5.1 c | <0.0001 | 0.003 | 0.049 | 0.013 |
| Variables | WC | WHR | WHtR | BMI | FM (%) | BRI | TyG | CHOL | HDL-C | TG | LDL-C | MetS Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anthropometric indices: | ||||||||||||
| BMI (kg/m2) | 0.889 *** | 0.375 ** | 0.900 *** | - | 0.830 *** | 0.899 *** | 0.110 | 0.117 | −0.135 * | 0.181 * | 0.098 | 0.324 ** |
| BRI | 0.958 *** | 0.600 ** | 0.995 *** | 0.899 *** | 0.789 *** | - | 0.174 * | 0.079 | −0.183 * | 0.215 * | 0.032 | 0.376 ** |
| FM (%) | 0.804 *** | 0.380 ** | 0.802 *** | 0.830 *** | - | 0.788 *** | 0.064 | 0.132 * | −0.029 | 0.182 * | 0.094 | 0.214 * |
| WC | - | 0.629 *** | 0.964 *** | 0.889 *** | 0.804 *** | 0.985 *** | 0.165 * | 0.105 | −0.166 * | 0.236 * | 0.080 | 0.363 ** |
| WHR | 0.629 *** | - | 0.608 *** | 0.375 ** | 0.380 ** | 0.598 ** | 0.178 * | 0.107 | −0.106 | 0.162 * | 0.085 | 0.214 * |
| WHtR | 0.964 *** | 0.608 *** | - | 0.900 *** | 0.802 *** | 0.995 *** | 0.164 * | 0.090 | −0.168 * | 0.218 * | 0.044 | 0.366 ** |
| Lipid profile: | ||||||||||||
| CHOL (mmol/L) | 0.105 | 0.107 | 0.090 | 0.117 | 0.132 * | 0.080 | 0.269 * | - | 0.241 * | 0.369 ** | 0.690 *** | 0.025 |
| HDL-C (mmol/l) | −0.166 * | −0.106 | −0.168 * | −0.135 * | −0.029 | −0.182 * | −0.213 * | 0.241 * | - | −0.201 * | 0.108 | −0.458 ** |
| TG (mmol/L) | 0.236 * | 0.162 * | 0.218 * | 0.181 * | 0.182 * | 0.217 * | 0.703 *** | 0.369 ** | −0.201 * | - | 0.145 * | 0.364 ** |
| LDL-C (mmol/L) | 0.080 | 0.085 | 0.044 | 0.098 | 0.094 | 0.084 | 0.084 | 0.690 *** | 0.108 | 0.145 * | - | −0.088 |
| TyG | 0.165 * | 0.178 * | 0.164 * | 0.110 | 0.064 | 0.174 * | - | 0.269 | −0.213 * | 0.703 *** | 0.084 | 0.402 ** |
| MetS score | 0.363 ** | 0.214 * | 0.366 ** | 0.324 ** | 0.214 * | 0.376 ** | 0.402 ** | 0.025 | −0.458 ** | 0.364 ** | −0.088 | - |
| Cardiometabolic indices: | ||||||||||||
| AIP | 0.273 * | 0.180 * | 0.264 * | 0.212 * | 0.145 * | 0.274 * | 0.641 *** | 0.143 * | −0.701 *** | 0.817 *** | 0.039 | 0.548 ** |
| CMI (mmol/L) | 0.472 ** | 0.307 ** | 0.464 ** | 0.393 ** | 0.335 ** | 0.473 ** | 0.597 ** | 0.192 * | −0.570 ** | 0.814 *** | 0.064 | 0.540 ** |
| LAP (mmol/L) | 0.753 *** | 0.473 ** | 0.716 *** | 0.651 *** | 0.593 ** | 0.716 *** | 0.548 ** | 0.317 ** | −0.216 * | 0.792 *** | 0.143 * | 0.460 ** |
| TG/HDL (mmol/L) | 0.251 * | 0.178 * | 0.237 * | 0.184 * | 0.145 * | 0.244 * | 0.625 *** | 0.183 * | −0.595 ** | 0.847 *** | 0.066 | 0.484 ** |
| TyG-BMI | 0.884 *** | 0.401 ** | 0.892 *** | 0.974 *** | 0.805 *** | 0.894 *** | 0.329 * | 0.178 * | −0.173 * | 0.333 ** | 0.114 | 0.403 ** |
| TyG-WC | 0.949 *** | 0.624 *** | 0.915 *** | 0.829 *** | 0.743 *** | 0.913 *** | 0.465 ** | 0.188 * | −0.214 ** | 0.438 ** | 0.103 | 0.457 ** |
| VAI (mmol/L) | 0.312 ** | 0.258 * | 0.288 * | 0.190 * | 0.161 * | 0.293 * | 0.618 *** | 0.173 * | −0.612 *** | 0.829 *** | 0.069 | 0.504 ** |
| Variable | Univariate Model 1 | Multivariate Model 2 | Multivariate Model 3 |
|---|---|---|---|
| OR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| Fried foods: | |||
| Never or almost never | Ref. | Ref. | Ref. |
| At least one time per week | 2.13 (1.00–4.54) * | 1.43 (0.60–3.39) | 1.36 (0.56–3.32) |
| At least one time per day | 3.37 (1.30–8.74) ** | 1.92 (0.64–5.77) | 1.75 (0.55–5.56) |
| Fish intake: | |||
| Never or almost never | 1.35 (0.82–2.22) * | 2.03 (1.10–3.74) * | 1.98 (1.07–3.69) * |
| At least one time per week | Ref. | Ref. | Ref. |
| Dietary patterns: | |||
| Western | 1.56 (0.88–2.78) | 1.51 (0.76–3.01) | |
| Prudent | Ref. | Ref. | - |
| Low Food | 1.35 (0.76–2.40) | 1.30 (0.63–2.71) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Białkowska, A.; Górnicka, M.; Zielinska-Pukos, M.A.; Hamulka, J. Associations between Dietary Patterns, Anthropometric and Cardiometabolic Indices and the Number of MetS Components in Polish Adults with Metabolic Disorders. Nutrients 2023, 15, 2237. https://doi.org/10.3390/nu15102237
Białkowska A, Górnicka M, Zielinska-Pukos MA, Hamulka J. Associations between Dietary Patterns, Anthropometric and Cardiometabolic Indices and the Number of MetS Components in Polish Adults with Metabolic Disorders. Nutrients. 2023; 15(10):2237. https://doi.org/10.3390/nu15102237
Chicago/Turabian StyleBiałkowska, Agnieszka, Magdalena Górnicka, Monika A. Zielinska-Pukos, and Jadwiga Hamulka. 2023. "Associations between Dietary Patterns, Anthropometric and Cardiometabolic Indices and the Number of MetS Components in Polish Adults with Metabolic Disorders" Nutrients 15, no. 10: 2237. https://doi.org/10.3390/nu15102237
APA StyleBiałkowska, A., Górnicka, M., Zielinska-Pukos, M. A., & Hamulka, J. (2023). Associations between Dietary Patterns, Anthropometric and Cardiometabolic Indices and the Number of MetS Components in Polish Adults with Metabolic Disorders. Nutrients, 15(10), 2237. https://doi.org/10.3390/nu15102237









