Are Signals Regulating Energy Homeostasis Related to Neuropsychological and Clinical Features of Gambling Disorder? A Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Hormonal Assays
2.2.2. Neuropsychological Variables
2.2.3. Clinical Variables
2.2.4. Other Variables
2.3. Procedure
2.4. Statistical Analysis
3. Results
3.1. Comparison of Endocrine Measures
3.2. Comparison of Neuropsychological and Clinical Measures
3.3. Associations between Endocrine Variables and Neuropsychological and Clinical Measures
3.4. Predictive Model for GD Presence
4. Discussion
Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- APA American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Potenza, M.N.; Balodis, I.M.; Derevensky, J.; Grant, J.E.; Petry, N.M.; Verdejo-Garcia, A.; Yip, S.W. Gambling disorder. Nat. Rev. Dis. Prim. 2019, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Solé-Morata, N.; Baenas, I.; Etxandi, M.; Granero, R.; Forcales, S.V.; Gené, M.; Barrot, C.; Gómez-Peña, M.; Menchón, J.M.; Ramoz, N.; et al. The role of neurotrophin genes involved in the vulnerability to gambling disorder. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Linnet, J. The anticipatory dopamine response in addiction: A common neurobiological underpinning of gambling disorder and substance use disorder? Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 98, 109802. [Google Scholar] [CrossRef] [PubMed]
- Shevchouk, O.T.; Tufvesson-Alm, M.; Jerlhag, E. An Overview of Appetite-Regulatory Peptides in Addiction Processes; From Bench to Bed Side. Front. Neurosci. 2021, 15, 774050. [Google Scholar] [CrossRef] [PubMed]
- Lüscher, C.; Robbins, T.W.; Everitt, B.J. The transition to compulsion in addiction. Nat. Rev. Neurosci. 2021, 21, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Geisel, O.; Panneck, P.; Hellweg, R.; Wiedemann, K.; Müller, K.A. Hypothalamic-pituitary-adrenal axis activity in patients with pathological gambling and internet use disorder. Psychiatry Res. 2015, 226, 97–102. [Google Scholar] [CrossRef]
- Pettorruso, M.; Zoratto, F.; Miuli, A.; De Risio, L.; Santorelli, M.; Pierotti, A.; Martinotti, G.; Adriani, W.; di Giannantonio, M. Exploring dopaminergic transmission in gambling addiction: A systematic translational review. Neurosci. Biobehav. Rev. 2020, 119, 481–511. [Google Scholar] [CrossRef]
- Farokhnia, M.; Grodin, E.N.; Lee, M.R.; Oot, E.N.; Balckburn, A.N.; Stangl, B.L.; Schwandt, M.L.; Farinelli, L.A.; Momenan, R.; Ramchandani, V.A.; et al. Exogenous ghrelin administration increases alcohol self-administration and modulates brain functional activity in heavy-drinking alcohol-dependent individuals. Mol. Psychiatry 2018, 23, 2029–2038. [Google Scholar] [CrossRef]
- Martinotti, G.; Montemitro, C.; Baroni, G.; Andreoli, S.; Alimonti, F.; Di Nicola, M.; Tonioni, F.; Leggio, L.; di Giannantonio, M.; Janiri, L. Relationship between craving and plasma leptin concentrations in patients with cocaine addiction. Psychoneuroendocrinology 2017, 85, 35–41. [Google Scholar] [CrossRef]
- Iovino, M.; Messana, T.; Lisco, G.; Mariano, F.; Giagulli, V.A.; Guastamacchia, E.; De Pergola, G.; Triggiani, V. Neuroendocrine modulation of food intake and eating behavior. Endocr. Metab. Immune Disord. Drug Targets 2022, 22. [Google Scholar] [CrossRef]
- Revitsky, A.R.; Klein, L.C. Role of ghrelin in drug abuse and reward-relevant behaviors: A burgeoning field and gaps in the literature. Curr. Drug Abuse Rev. 2013, 6, 231–244. [Google Scholar] [CrossRef]
- Micioni Di Bonaventura, E.; Botticelli, L.; Del Bello, F.; Giorgioni, G.; Piergentili, A.; Quaglia, W.; Cifani, C.; Micioni Di Bonaventura, M.V. Assessing the role of ghrelin and the enzyme ghrelin O-acyltransferase (GOAT) system in food reward, food motivation, and binge eating behavior. Pharmacol. Res. 2021, 172, 105847. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Fernandez, I.D.; Meng, Y.; Zhao, W.; Groth, S.W. Gut hormones, adipokines, and pro- and anti-inflammatory cytokines/markers in loss of control eating: A scoping review. Appetite 2021, 166, 105442. [Google Scholar] [CrossRef] [PubMed]
- Sustkova-Fiserova, M.; Charalambous, C.; Khryakova, A.; Certilina, A.; Lapka, M.; Šlamberová, R. The Role of Ghrelin/GHS-R1A Signaling in Nonalcohol Drug Addictions. Int. J. Mol. Sci. 2022, 23, 761. [Google Scholar] [CrossRef]
- Koopmann, A.; Schuster, R.; Kiefer, F. The impact of the appetite-regulating, orexigenic peptide ghrelin on alcohol use disorders: A systematic review of preclinical and clinical data. Biol Psychol. 2018, 131, 13–40. [Google Scholar] [CrossRef]
- Leggio, L.; Ferrulli, A.; Cardone, S.; Nesci, A.; Micelo, A.; Malandrino, N.; Capristo, E.; Canestrelli, B.; Monteleone, P.; Kenna, G.A.; et al. Ghrelin system in alcohol-dependent subjects: Role of plasma ghrelin levels in alcohol drinking and craving. Addict. Biol. 2012, 17, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Addolorato, G.; Capristo, E.; Leggio, L.; Ferulli, A.; Abenavoli, L.; Malandrino, N.; Farnetti, S.; Domenicali, M.; D’Angelo, C.; Vonghia, L.; et al. Relationship between ghrelin levels, alcohol craving, and nutritional status in current alcoholic patients. Alcohol. Clin. Exp. Res. 2006, 30, 1933–1937. [Google Scholar] [CrossRef]
- Tessari, M.; Catalano, A.; Pellitteri, M.; Di Francesco, C.; Marini, F.; Gerrard, P.A.; Heidbreder, C.A.; Melotto, S. Correlation between serum ghrelin levels and cocaine-seeking behaviour triggered by cocaine-associated conditioned stimuli in rats. Addict Biol. 2007, 12, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Edvardsson, C.E.; Vestlund, J.; Jerlhag, E. A ghrelin receptor antagonist reduces the ability of ghrelin, alcohol, or amphetamine to induce a dopamine release in the ventral tegmental area and in nucleus accumbens shell in rats. Eur. J. Pharmacol. 2021, 899, 174039. [Google Scholar] [CrossRef]
- Suchankova, P.; Steensland, P.; Fredriksson, I.; Engel, J.A.; Jerlhag, E. Ghrelin Receptor (GHS-R1A) Antagonism Suppresses Both Alcohol Consumption and the Alcohol Deprivation Effect in Rats following Long-Term Voluntary Alcohol Consumption. PLoS ONE 2013, 8, e71284. [Google Scholar] [CrossRef]
- Ge, X.; Yang, H.; Bednarek, M.A.; Galon-Tilleman, H.; Chen, P.; Chen, M.; Lichtman, J.S.; Wang, L.; Dalmas, O.; Yin, Y.; et al. LEAP2 Is an Endogenous Antagonist of the Ghrelin Receptor. Cell Metab. 2018, 27, 461–469.e6. [Google Scholar] [CrossRef] [PubMed]
- Lugilde, J.; Casado, S.; Beiroa, D.; Cuñarro, J.; García-Lavandeira, M.; Álvarez, C.V.; Nogueiras, R.; Tovar, S.; Diéguez, C. LEAP-2 Counteracts Ghrelin-Induced Food Intake in a NutrientGrowth Hormone and Age Independent Manner. Cells 2022, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Voigt, K.; Giddens, E.; Stark, R.; Frisch, E.; Moskovsky, N.; Kakoschke, N.; Stout, J.C.; Bellgrove, M.A.; Andrews, Z.B.; Verdejo García, A. The hunger games: Homeostatic state-dependent fluctuations in disinhibition measured with a novel gamified test battery. Nutrients 2021, 13, 2001. [Google Scholar] [CrossRef] [PubMed]
- Zallar, L.J.; Beurmann, S.; Tunstall, B.J.; Fraser, C.M.; Koob, G.F.; Vendruscolo, L.F.; Leggio, L. Ghrelin receptor deletion reduces binge-like alcohol drinking in rats. J. Neuroendocrinol. 2019, 31, e12663. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.A.; Jerlhag, E. Role of appetite-regulating peptides in the pathophysiology of addiction: Implications for pharmacotherapy. CNS Drugs 2014, 28, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Farokhnia, M.; Lee, M.R.; Farinelli, L.A.; Ramchandani, V.; Akhlaghi, F.; Leggio, L. Pharmacological manipulation of the ghrelin system and alcohol hangover symptoms in heavy drinking individuals: Is there a link? Pharmacol. Biochem. Behav. 2018, 172, 39–49. [Google Scholar] [CrossRef]
- Sztainert, T.; Hay, R.; Wohl, M.J.A.; Abizaid, A. Hungry to gamble? Ghrelin as a predictor of persistent gambling in the face of loss. Biol. Psychol. 2018, 139, 115–123. [Google Scholar] [CrossRef]
- Sutin, A.R.; Zonderman, A.B.; Uda, M.; Deiana, B.; Taub, D.D.; Longo, D.L.; Ferrucci, L.; Schlessinger, D.; Cucca, F.; Terracciano, A. Personality traits and leptin. Psychosom. Med. 2013, 75, 505–509. [Google Scholar] [CrossRef]
- Bach, P.; Koopmann, A.; Kiefer, F. The Impact of Appetite-Regulating Neuropeptide Leptin on Alcohol Use, Alcohol Craving and Addictive Behavior: A Systematic Review of Preclinical and Clinical Data. Alcohol Alcohol. 2021, 56, 149–165. [Google Scholar] [CrossRef]
- Peters, T.; Antel, J.; Föcker, M.; Esber, S.; Hinney, A.; Schéle, E.; Dickson, S.L.; Albayrak, O.; Hebebrand, J. The association of serum leptin levels with food addiction is moderated by weight status in adolescent psychiatric inpatients. Eur. Eat Disord. Rev. 2018, 26, 618–628. [Google Scholar] [CrossRef]
- Bach, P.; Bumb, J.M.; Schuster, R.; Vollstädt-Klein, S.; Reinhard, I.; Rietschel, M.; Witt, S.H.; Wiedemann, K.; Kiefer, F.; Koopmann, A. Effects of leptin and ghrelin on neural cue-reactivity in alcohol addiction: Two streams merge to one river? Psychoneuroendocrinology 2019, 100, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Escobar, M.; Scherer, J.N.; Ornell, F.; Bristot, G.; Medino-Soares, C.; Pinto-Guimarães, L.S.; Von Diemen, L.; Pechansky, F. Leptin levels and its correlation with crack-cocaine use severity: A preliminary study. Neurosci. Lett. 2018, 671, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Baruah, A.; Das, S.; Avinash, P.; Chetia, D.; Gupta, D. Leptin levels in alcohol dependent patients and their relationship with withdrawal and craving. Asian J. Psychiatr. 2020, 51, 101967. [Google Scholar] [CrossRef] [PubMed]
- Geisel, O.; Hellweg, R.; Wiedemann, K.; Müller, C.A. Plasma levels of leptin in patients with pathological gambling, internet gaming disorder and alcohol use disorder. Psychiatry Res. 2018, 268, 193–197. [Google Scholar] [CrossRef]
- Shahouzehi, B.; Shokoohi, M.; Najafipour, H. The effect of opium addiction on serum adiponectin and leptin levels in male subjects: A case control study from Kerman Coronary Artery disease risk factors study (KERCADRS). EXCLI J. 2013, 12, 916. [Google Scholar]
- Hillemacher, T.; Weinland, C.; Heberlein, A.; Gröschl, M.; Schanze, A.; Frieling, H.; Wilhelm, J.; Kornhuber, J.; Bleich, S. Increased levels of adiponectin and resistin in alcohol dependence--possible link to craving. Drug Alcohol. Depend. 2009, 99, 333–337. [Google Scholar] [CrossRef]
- Lozano-Madrid, M.; Bryan, D.C.; Granero, R.; Sánchez, I.; Riesco, N.; Mallorquí-Bagué, N.; Jiménez-Murcia, S.; Treasure, J.; Fernández-Aranda, F. Impulsivity, emotional dysregulation, and executive function deficits could be associated with alcohol and drug abuse in eating disorders. J. Clin. Med. 2020, 9, 1936. [Google Scholar] [CrossRef]
- van Timmeren, T.; Daams, J.G.; van Holst, R.J.; Goudriaan, A.E. Compulsivity-related neurocognitive performance deficits in gambling disorder: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018, 84, 204–217. [Google Scholar] [CrossRef]
- Hinson, J.M.; Jameson, T.L.; Whitney, P. Impulsive decision making and working memory. J. Exp. Psychology. Learn. Mem. Cogn. 2003, 29, 298–306. [Google Scholar] [CrossRef]
- Mallorquí-Bagué, N.; Tolosa-Sola, I.; Fernández-Aranda, F.; Granero, R.; Fagundo, A.B.; Lozano-Madrid, M.; Mestre-Bach, G.; Gómez-Peña, M.; Aymamí, N.; Borrás-González, I.; et al. Cognitive Deficits in Executive Functions and Decision-Making Impairments Cluster Gambling Disorder Sub-types. J. Gambl. Stud. 2018, 34, 209–223. [Google Scholar] [CrossRef]
- Mestre-Bach, G.; Fernández-Aranda, F.; Jiménez-Murcia, S.; Potenza, M.N. Decision-Making in Gambling Disorder, Problematic Pornography Use, and Binge Eating Disorder: Similarities and Differences. Curr. Behav. Neurosci. Rep. 2020, 7, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Granero, R.; Penelo, E.; Stinchfield, R.; Fernández-Aranda, F.; Savvidou, L.G.; Fröberg, F.; Aymamí, N.; Gómez-Peña, M.; Pérez-Serrano, M.; del Pino-Gutiérrez, A.; et al. Is Pathological Gambling Moderated by Age? J. Gambl. Stud. 2014, 30, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Murcia, S.; Granero, R.; Fernández-Aranda, F.; Menchón, J.M. Comparison of gambling profiles based on strategic versus non-strategic preferences. Curr. Opin. Behav. Sci. 2020, 31, 13–20. [Google Scholar] [CrossRef]
- Álvarez-Moya, E.M.; Ochoa, C.; Jiménez-Murcia, S.; Aymamí, M.N.; Gómez-Peña, M.; Fernández-Aranda, F.; Santamaría, J.; Moragas, L.; Bove, F.; Menchón, J.M. Effect of executive functioning, decision-making and self-reported impulsivity on the treatment outcome of pathologic gambling. J. Psychiatry Neurosci. 2011, 36, 165–175. [Google Scholar] [CrossRef]
- Del Pino-Gutiérrez, A.; Jiménez-Murcia, S.; Fernández-Aranda, F.; Agüera, Z.; Granero, R.; Hakansson, A.; Fagundo, A.B.; Bolao, F.; Valdepérez, A.; Mestre-Bach, G.; et al. The relevance of personality traits in impulsivity-related disorders: From substance use disorders and gambling disorder to bulimia nervosa. J. Behav. Addict. 2017, 6, 396–405. [Google Scholar] [CrossRef]
- Lara-Huallipe, M.L.; Granero, R.; Fernández-Aranda, F.; Gónez-Peña, M.; Moragas, L.; del Pino-Gutiérrez, A.; Valenciano-Mendoza, E.; Mora-Maltas, B.; Baenas, I.; Etxandi, M.; et al. Clustering Treatment Outcomes in Women with Gambling Disorder. J. Gambl. Stud. 2021, 38, 1469–1491. [Google Scholar] [CrossRef] [PubMed]
- Dash, G.F.; Slutske, W.S.; Martin, N.G.; Statham, D.J.; Agrawal, A.; Lynskey, M.T. Big Five personality traits and alcohol, nicotine, cannabis, and gambling disorder comorbidity. Psychol. Addict. Behav. 2019, 33, 420. [Google Scholar] [CrossRef]
- Zilberman, N.; Yadid, G.; Efrati, Y.; Neumark, Y.; Rassovsky, I. Personality profiles of substance and behavioral addictions. Addict. Behav. 2018, 82, 174–181. [Google Scholar] [CrossRef]
- Vintró-Alcaraz, C.; Mestre-Bach, G.; Granero, R.; Vázquez-Cobela, R.; Seoane, M.L.; Diéguez, C.; Leis, R.; Tovar, S. Do emotion regulation and impulsivity differ according to gambling preferences in clinical samples of gamblers? Addict. Behav. 2022, 126, 107176. [Google Scholar] [CrossRef]
- Barja-Fernández, S.; Lugilde, J.; Castelao, C.; Vázquez-Cobela, R.; Seoane, L.M.; Diéguez, C.; Leis, R.; Tovar, S. Circulating LEAP-2 is associated with puberty in girls. Int. J. Obes. 2021, 45, 502–514. [Google Scholar] [CrossRef]
- Mani, B.K.; Puzziferri, N.; He, Z.; Rodríguez, J.; Osborne-Lawrence, S.; Metzger, N.P.; Chhina, N.; Gaylinn, B.; Thorner, M.O.; Thomas, E.L.; et al. LEAP2 changes with body mass and food intake in humans and mice. J. Clin. Investig. 2019, 129, 3909–3923. [Google Scholar] [CrossRef] [PubMed]
- Pena-Bello, L.; Pertega-Diaz, S.; Outeiriño-Blanco, E.; García-Buela, J.; Tovar, S.; Sangiao-Alvarellos, S.; Diéguez, C.; Cordido, F. Effect of oral glucose administration on rebound growth hormone release in normal and obese women: The role of adiposity, insulin sensitivity and ghrelin. PLoS ONE. 2015, 10, e121087. [Google Scholar] [CrossRef] [PubMed]
- Bechara, A.; Damasio, A.R.; Damasio, H.; Anderson, S.W. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 1994, 50, 7–15. [Google Scholar] [CrossRef]
- Grant, D.A.; Berg, E. A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. J. Exp. Psychol. 1948, 38, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Golden, C.J. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses; Stoelting: Chicago, IL, USA, 1978. [Google Scholar]
- Reitan, R.M. Validity of the Trail Making Test as an Indicator of Organic Brain Damage Perceptual and Motor Skills. Percept. Mot. Ski. 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Memory Scale, 3rd ed.; The Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Wechsler, D. KA WAIS-III: Wechsler Adult Intelligence Scale, 3rd ed.; [Book in Spanish]; TEA Ediciones SA: Madrid, Spain, 1999. [Google Scholar]
- De Oliveira, M.O.; Nitrini, R.; Yassuda, M.S.; Brucki, S.M.D. Vocabulary is an appropriate measure of premorbid intelligence in a sample with heterogeneous educational level in Brazil. Behav. Neurol. 2014, 2014, 875960. [Google Scholar] [CrossRef]
- Lesieur, H.R.; Blume, S.B. The South Oaks Gambling Screen (SOGS): A new instrument for the identification of pathological gamblers. Am. J. Psychiatry 1987, 144, 1184–1188. [Google Scholar] [CrossRef]
- Echeburúa, E.; Baez, C.; Fernández-Montalvo, J.; Páez, D. Cuestionario de Juego Patológico de South Oaks (SOGS): Validación española. Análisis Modif Conduct. 1994, 20, 769–791. [Google Scholar]
- Stinchfield, R. Reliability, validity, and classification accuracy of a measure of DSM-IV diagnostic criteria for pathological gambling. Am. J. Psychiatry 2003, 160, 180–182. [Google Scholar] [CrossRef]
- Jiménez-Murcia, S.; Stinchfield, R.; Álvarez-Moya, E.; Jaurrieta, N.; Bueno, B.; Granero, R.; Aymamí, M.N.; Gómez-Peña, M.; Martínez-Giménez, R.; Fernández-Aranda, F.; et al. Reliability, validity, and classification accuracy of a Spanish translation of a measure of DSM-IV diagnostic criteria for pathological gambling. J. Gambl. Stud. 2009, 25, 93–104. [Google Scholar] [CrossRef]
- Derogatis, L.R. SCL-90-R: Symptom Checklist-90-R. Administration, Scoring and Procedures Manuall—II for the Revised Version; Clinical Psychometric Research: Towson, MD, USA, 1994. [Google Scholar]
- Derogatis, L.R. SCL-90-R. Cuestionario de 90 Síntomas-Manual; TEA Editorial: Madrid, Spain, 2002. [Google Scholar]
- Cloninger, C.R. The Temperament and Character Inventory—Revised. Center for Psychobiology of Personality; Washington University: Washington, DC, USA, 1999. [Google Scholar]
- Gutiérrez-Zotes, J.A.; Bayón, C.; Montserrat, C.; Valero, J.; Labad, A.; Cloninger, C.R.; Fernández-Aranda, F. Temperament and Character Inventory Revised (TCI-R). Standardization and normative data in a general population sample. Actas Esp Psiquiatr. 2004, 32, 8–15. [Google Scholar] [PubMed]
- Whiteside, S.P.; Lynam, D.R.; Miller, J.D.; Reynolds, S.K. Validation of the UPPS impulsive behaviour scale: A four-factor model of impulsivity. Eur. J. Pers. 2005, 19, 559–574. [Google Scholar] [CrossRef]
- Verdejo-García, A.; Lozano, Ó.; Moya, M.; Alcázar, M.A.; Pérez-García, M. Psychometric properties of a Spanish version of the UPPS-P impulsive behavior scale: Reliability, validity and association with trait and cognitive impulsivity. J. Pers. Assess. 2010, 92, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Murcia, S.; Aymamí-Sanromà, M.; Gómez-Pena, M.; Álvarez-Moya, E.; Vallejo, J. Protocols de Tractament Cognitivoconductual pel joc Patològic i D’altres Addiccions no Tòxiques [Protocols of Cognitive-Behaviour Therapy for Pathological Gambling and Other Behavioural Addictions]; Hospital Universitari de Bellvitge, Departament de Salut, Generalitat de Catalunya: Barcelona, Spain, 2006. [Google Scholar]
- Stata-Corp. Stata Statistical Software: Release 17; Stata Press Publication (StataCorp LLC): College Station, TX, USA, 2021. [Google Scholar]
- Kelley, K.; Preacher, K.J. On effect size. Psychol. Methods 2012, 17, 137–152. [Google Scholar] [CrossRef]
- Rosnow, R.L.; Rosenthal, R. Computing contrasts, effect sizes and counternulls on other people’s published data: General procedures for research consumers. Psychol. Methods 1996, 1, 331–340. [Google Scholar] [CrossRef]
- Finner, H.; Roters, M. On the false discovery rate and expecte type I errors. J. Am. Stat. Assoc. 2001, 88, 920–923. [Google Scholar] [CrossRef]
- Vengeliene, V. The role of ghrelin in drug and natural reward. Addict Biol. 2013, 18, 897–900. [Google Scholar] [CrossRef]
- Benchebra, L.; Alexandre, J.M.; Dubernet, J.; Fatséas, M.; Auriacombe, M. Gambling and Gaming disorders and physical health of players: A critical review of the literature. Presse Med. 2019, 48, 1551–1568. [Google Scholar] [CrossRef]
- Pilver, C.E.; Potenza, M.N. Increased incidence of cardiovascular conditions among older adults with pathological gambling features in a prospective study. J. Addict. Med. 2013, 7, 387–393. [Google Scholar] [CrossRef]
- Firouzabadi, N.; Haghnegahdar, M.; Khalvati, B.; Dehshahri, A.; Bahramali, E. Overexpression of adiponectin receptors in opium users with and without cancer. Clin. Pharmacol. Adv. Appl. 2020, 12, 59. [Google Scholar] [CrossRef]
- Álvarez-Moya, E.M.; Jiménez-Murcia, S.; Aymamí, M.N.; Gómez-Peña, M.; Granero, R.; Santamaría, J.; Menchón, J.M.; Fernández-Aranda, F. Subtyping study of a pathological gamblers sample. Can. J. Psychiatry 2010, 55, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Balodis, I.M.; Linnet, J.; Arshad, F.; Worhunsky, P.D.; Stevens, M.C.; Pearlson, G.D.; Potenza, M.N. Relating neural processing of reward and loss prospect to risky decision-making in individuals with and without Gambling Disorder. Int. Gambl. Stud. 2018, 18, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Bexkens, A.; Jansen, B.R.J.; Van der Molen, M.W.; Huizenga, H.M. Cool Decision-Making in Adolescents with Behavior Disorder and/or Mild-to-Borderline Intellectual Disability. J. Abnorm. Child Psychol. 2016, 44, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.J.; Corr, P.J. A Feedback-Response Pause Normalises Response Perseveration Deficits in Pathological Gamblers. Int. J. Ment. Health Addict. 2013, 11, 601–610. [Google Scholar] [CrossRef]
- Novelle, M.G.; Diéguez, C. Unravelling the role and mechanism of adipokine and gastrointestinal signals in animal models in the nonhomeostatic control of energy homeostasis: Implications for binge eating disorder. Eur. Eat. Disord. Rev. 2018, 26, 551–568. [Google Scholar] [CrossRef]
- Jiménez-Murcia, S.; Granero, R.; Fernández-Aranda, F.; Arcelus, J.; Aymamí, M.N.; Gómez-Peña, M.; Tárrega, S.; Moragas, L.; del Pino-Gutiérrez, A.; Sauchelli, S.; et al. Predictors of outcome among pathological gamblers receiving cognitive behavioral group therapy. Eur. Addict. Res. 2015, 21, 169–178. [Google Scholar] [CrossRef]
- Jiménez-Murcia, S.; Granero, R.; Tarrega, S.; Angulo, A.; Fernández-Aranda, F.; Arcelus, J.; Fagundo, A.B.; Aymamí, N.; Moragas, L.; Sauvaget, A.; et al. Mediational Role of Age of Onset in Gambling Disorder, a Path Modeling Analysis. J. Gambl. Stud. 2016, 32, 327–340. [Google Scholar] [CrossRef]
- Castrén, S.; Basnet, S.; Salonen, A.H.; Pankakoski, M.; Ronkainen, J.E.; Alho, H.; Lahti, T. Factors associated with disordered gambling in Finland. Subst. Abus. Treat. Prev. Policy 2013, 8, 1–10. [Google Scholar] [CrossRef]
- Martinotti, G.; Andreoli, S.; Giametta, E.; Poli, V.; Bria, P.; Janiri, L. The dimensional assessment of personality in pathologic and social gamblers: The role of novelty seeking and self-transcendence. Compr. Psychiatry 2006, 37, 350–356. [Google Scholar] [CrossRef]
- Valero-Solís, S.; Granero, R.; Fernández-Aranda, F.; Steward, T.; Mestre-Bach, G.; Mallorquí-Bagué, N.; Martín-Romera, V.; Aymamí, N.; Gómez-Peña, M.; del Pino-Gutiérrez, A.; et al. The contribution of sex, personality traits, age of onset and disorder duration to behavioral addictions. Front. Psychiatry 2018, 9, 497. [Google Scholar] [CrossRef]
- Dodig, D. Assessment challenges and determinants of adolescents’ adverse psychosocial consequences of gambling. Kriminol. Soc. Integr. 2013, 21, 1–29. [Google Scholar]
- Misiak, B.; Kowalski, K.; Stańczykiewicz, B.; Bartoli, F.; Carrà, G.; Samochowiec, J.; Frydecka, D. Frontiers in Neuroendocrinology Appetite-regulating hormones in bipolar disorder: A systematic review and meta-analysis. Front. Neuroendocrinol. 2022, 67, 101013. [Google Scholar] [CrossRef] [PubMed]

| Control (N = 79) | GD (N = 297) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | |d| | |
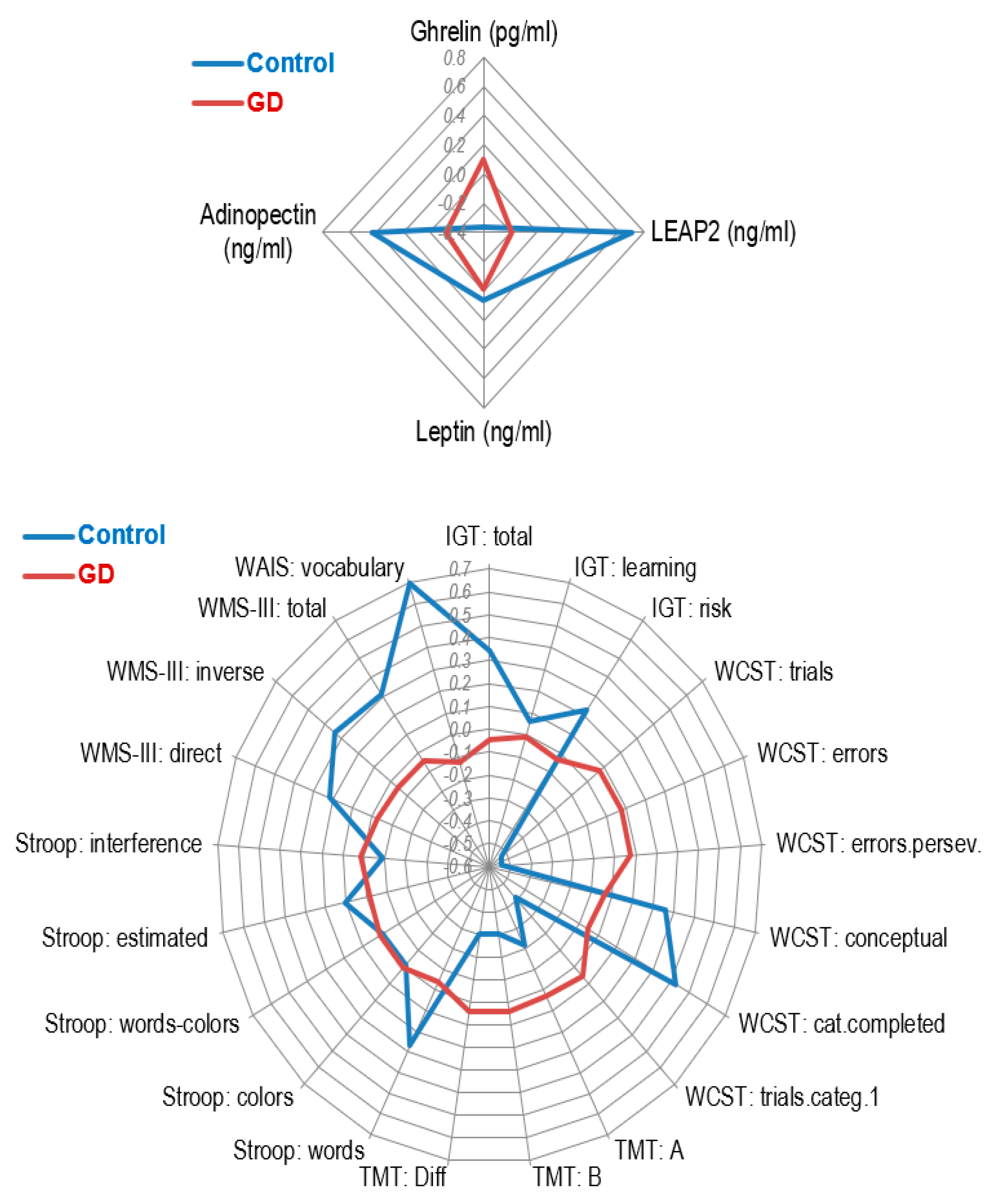
| 1 Ghrelin (pg/mL) | 544.92 | 673.59 | 958.48 | 753.26 | <0.001 * | 0.58 † |
| 1 LEAP2 (ng/mL) | 8.41 | 3.99 | 5.28 | 2.88 | <0.001 * | 0.90 † |
| 1 Leptin (ng/mL) | 9.00 | 8.13 | 8.18 | 7.85 | 0.402 | 0.10 |
| 1 Adiponectin (ng/mL) | 12784.98 | 14084.20 | 8381.47 | 4374.29 | <0.001 * | 0.42 |
| 2 BMI (kg/m2) | 24.99 | 2.36 | 26.57 | 5.04 | 0.005 * | 0.40 |
| Control (N = 41) | GD (N = 297) | |||||
|---|---|---|---|---|---|---|
| 1 Neuropsychological Measures | Mean | SD | Mean | SD | p | |d| |
| IGT: block-1 | −0.39 | 4.87 | −1.87 | 5.16 | 0.115 | 0.30 |
| IGT: block-2 | 0.65 | 5.87 | 0.09 | 5.52 | 0.583 | 0.10 |
| IGT: block-3 | 3.24 | 9.50 | 1.19 | 6.97 | 0.126 | 0.25 |
| IGT: block-4 | 2.34 | 9.85 | 1.25 | 7.46 | 0.446 | 0.12 |
| IGT: block-5 | 2.99 | 8.33 | 1.18 | 8.56 | 0.249 | 0.21 |
| IGT: total | 8.88 | 28.10 | 2.10 | 21.94 | 0.104 | 0.27 |
| IGT: learning | 5.07 | 15.12 | 4.22 | 13.77 | 0.739 | 0.06 |
| IGT: risk | 5.33 | 15.78 | 2.44 | 13.64 | 0.257 | 0.20 |
| WCST: trials | 91.61 | 19.76 | 102.98 | 19.58 | 0.001 * | 0.58 † |
| WCST: errors | 20.04 | 16.28 | 33.14 | 21.77 | <0.001 * | 0.68 † |
| WCST: errors perseverative | 9.13 | 6.27 | 15.09 | 10.06 | <0.001 * | 0.71 † |
| WCST: conceptual | 65.77 | 8.66 | 60.60 | 16.25 | 0.063 | 0.40 |
| WCST: categories completed | 5.59 | 1.07 | 4.76 | 1.80 | 0.006 * | 0.56 † |
| WCST: trials to complete 1-cat | 17.64 | 6.52 | 26.79 | 28.09 | 0.053 | 0.45 |
| TMT: A | 28.81 | 8.10 | 31.77 | 10.51 | 0.088 | 0.32 |
| TMT: B | 70.67 | 22.07 | 78.41 | 36.38 | 0.202 | 0.26 |
| TMT: Diff | 41.74 | 18.48 | 47.75 | 32.35 | 0.271 | 0.23 |
| Stroop: words | 101.50 | 13.27 | 98.13 | 13.91 | 0.173 | 0.25 |
| Stroop: colors | 67.82 | 9.97 | 68.28 | 11.00 | 0.812 | 0.04 |
| Stroop: words-colors | 43.07 | 10.25 | 42.97 | 10.55 | 0.955 | 0.01 |
| Stroop: estimated | 40.48 | 5.20 | 40.11 | 5.53 | 0.700 | 0.07 |
| Stroop: interference | 2.59 | 7.71 | 2.86 | 7.75 | 0.838 | 0.04 |
| WMS-III: direct | 8.91 | 1.93 | 8.96 | 2.02 | 0.902 | 0.02 |
| WMS-III: direct-span | 5.99 | 1.12 | 6.01 | 1.16 | 0.947 | 0.01 |
| WMS-III: inverse | 6.55 | 1.89 | 6.18 | 1.99 | 0.304 | 0.19 |
| WMS-III: inverse-span | 4.80 | 0.98 | 4.64 | 1.15 | 0.427 | 0.15 |
| WMS-III: total | 15.46 | 3.36 | 15.14 | 3.62 | 0.617 | 0.09 |
| WAIS: vocabulary | 45.30 | 5.29 | 38.50 | 8.52 | <0.001 * | 0.96 † |
| 2 Psychological measures | Mean | SD | Mean | SD | p | |d| |
| SCL-90R Somatization | 0.43 | 0.35 | 0.99 | 0.78 | <0.001 * | 0.92 † |
| SCL-90R Obsessive/compul. | 0.68 | 0.52 | 1.19 | 0.84 | <0.001 * | 0.74 † |
| SCL-90R Interp.sensitivity | 0.40 | 0.38 | 0.99 | 0.80 | <0.001 * | 0.95 † |
| SCL-90R Depressive | 0.51 | 0.59 | 1.54 | 0.93 | <0.001 * | 1.32 † |
| SCL-90R Anxiety | 0.36 | 0.34 | 1.00 | 0.80 | <0.001 * | 1.05 † |
| SCL-90R Hostility | 0.43 | 0.50 | 0.96 | 0.87 | <0.001 * | 0.76 † |
| SCL-90R Phobic anxiety | 0.06 | 0.16 | 0.41 | 0.61 | <0.001 * | 0.77 † |
| SCL-90R Paranoid Ideation | 0.46 | 0.47 | 0.95 | 0.79 | <0.001 * | 0.75 † |
| SCL-90R Psychotic | 0.22 | 0.26 | 0.90 | 0.75 | <0.001 * | 1.20 † |
| SCL-90R GSI score | 0.43 | 0.34 | 1.08 | 0.70 | <0.001 * | 1.18 † |
| SCL-90R PST score | 26.37 | 16.45 | 47.53 | 20.77 | <0.001 * | 1.13 † |
| SCL-90R PSDI score | 1.41 | 0.33 | 1.86 | 0.59 | <0.001 * | 0.95 † |
| UPPS-P Lack premeditation | 20.98 | 4.02 | 24.32 | 5.51 | <0.001 * | 0.69 † |
| UPPS-P Lack perseverance | 19.26 | 4.13 | 21.97 | 4.83 | 0.001 | 0.60 † |
| UPPS-P Sensation seeking | 28.13 | 7.41 | 28.51 | 7.89 | 0.770 | 0.05 |
| UPPS-P Positive urgency | 20.70 | 5.85 | 31.92 | 9.22 | <0.001 * | 1.45 † |
| UPPS-P Negative urgency | 23.02 | 5.55 | 32.25 | 6.44 | <0.001 * | 1.54 † |
| UPPS-P Total | 112.13 | 18.36 | 138.83 | 22.37 | <0.001 * | 1.30 † |
| TCI-R Novelty seeking | 99.34 | 10.63 | 110.82 | 13.13 | <0.001 * | 0.96 † |
| TCI-R Harm avoidance | 88.04 | 17.86 | 98.79 | 16.83 | <0.001 * | 0.62 † |
| TCI-R Reward dependence | 103.95 | 13.99 | 97.97 | 13.50 | 0.009 * | 0.43 |
| TCI-R Persistence | 112.65 | 18.18 | 109.02 | 18.90 | 0.259 | 0.20 |
| TCI-R Self-directedness | 148.17 | 19.03 | 130.13 | 20.52 | <0.001 * | 0.91 † |
| TCI-R Cooperativeness | 136.98 | 15.25 | 130.18 | 15.42 | 0.010 * | 0.44 |
| TCI-R Self-transcendence | 66.73 | 15.93 | 61.38 | 13.83 | 0.025 * | 0.36 |
| Dependent Variable: 1 = GD vs. 0 = HC | B | SE | p | OR | 95% CI OR | |
|---|---|---|---|---|---|---|
| Covariates Sex (0 = women; 1 = men) | −0.781 | 1.498 | 0.602 | 0.458 | 0.024 | 8.623 |
| Age (years-old) | −0.100 | 0.033 | 0.002 | 0.905 | 0.848 | 0.965 |
| BMI (kg/m2) | 0.508 | 0.149 | 0.001 | 1.662 | 1.241 | 2.226 |
| Education (low levels) | 2.875 | 0.754 | 0.001 | 17.724 | 4.045 | 77.665 |
| Socioeconomic status (low levels) | 1.099 | 0.543 | 0.043 | 3.000 | 1.035 | 8.696 |
| Psychopathology distress (SCL-90R GSI) | 2.483 | 0.896 | 0.006 | 11.973 | 2.069 | 69.290 |
| Impulsivity (UPPS-P total) | 0.082 | 0.023 | 0.001 | 1.086 | 1.038 | 1.135 |
| Personality: TCI-R self-transcendence | −0.071 | 0.031 | 0.023 | 0.932 | 0.877 | 0.990 |
| WCST Perseverative errors | 0.180 | 0.068 | 0.008 | 1.198 | 1.048 | 1.368 |
| Stroop Color | 0.093 | 0.047 | 0.046 | 1.098 | 1.002 | 1.203 |
| WAIS Vocabulary | −0.175 | 0.064 | 0.007 | 0.840 | 0.740 | 0.953 |
| LEAP2 (ng/mL) | −0.326 | 0.126 | 0.009 | 0.722 | 0.564 | 0.923 |
| Fit statistics | H-L = 0.985; R2 = 0.427; AUC = 0.986 (95% CI: 0.973 to 0.998) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Etxandi, M.; Baenas, I.; Mora-Maltas, B.; Granero, R.; Fernández-Aranda, F.; Tovar, S.; Solé-Morata, N.; Lucas, I.; Casado, S.; Gómez-Peña, M.; et al. Are Signals Regulating Energy Homeostasis Related to Neuropsychological and Clinical Features of Gambling Disorder? A Case–Control Study. Nutrients 2022, 14, 5084. https://doi.org/10.3390/nu14235084
Etxandi M, Baenas I, Mora-Maltas B, Granero R, Fernández-Aranda F, Tovar S, Solé-Morata N, Lucas I, Casado S, Gómez-Peña M, et al. Are Signals Regulating Energy Homeostasis Related to Neuropsychological and Clinical Features of Gambling Disorder? A Case–Control Study. Nutrients. 2022; 14(23):5084. https://doi.org/10.3390/nu14235084
Chicago/Turabian StyleEtxandi, Mikel, Isabel Baenas, Bernat Mora-Maltas, Roser Granero, Fernando Fernández-Aranda, Sulay Tovar, Neus Solé-Morata, Ignacio Lucas, Sabela Casado, Mónica Gómez-Peña, and et al. 2022. "Are Signals Regulating Energy Homeostasis Related to Neuropsychological and Clinical Features of Gambling Disorder? A Case–Control Study" Nutrients 14, no. 23: 5084. https://doi.org/10.3390/nu14235084
APA StyleEtxandi, M., Baenas, I., Mora-Maltas, B., Granero, R., Fernández-Aranda, F., Tovar, S., Solé-Morata, N., Lucas, I., Casado, S., Gómez-Peña, M., Moragas, L., Pino-Gutiérrez, A. d., Codina, E., Valenciano-Mendoza, E., Potenza, M. N., Diéguez, C., & Jiménez-Murcia, S. (2022). Are Signals Regulating Energy Homeostasis Related to Neuropsychological and Clinical Features of Gambling Disorder? A Case–Control Study. Nutrients, 14(23), 5084. https://doi.org/10.3390/nu14235084