New Insights into the Relationship between Gut Microbiota and Radiotherapy for Cancer
Abstract
1. Introduction
2. The Relationship between the Gut Microbiota and Cancer
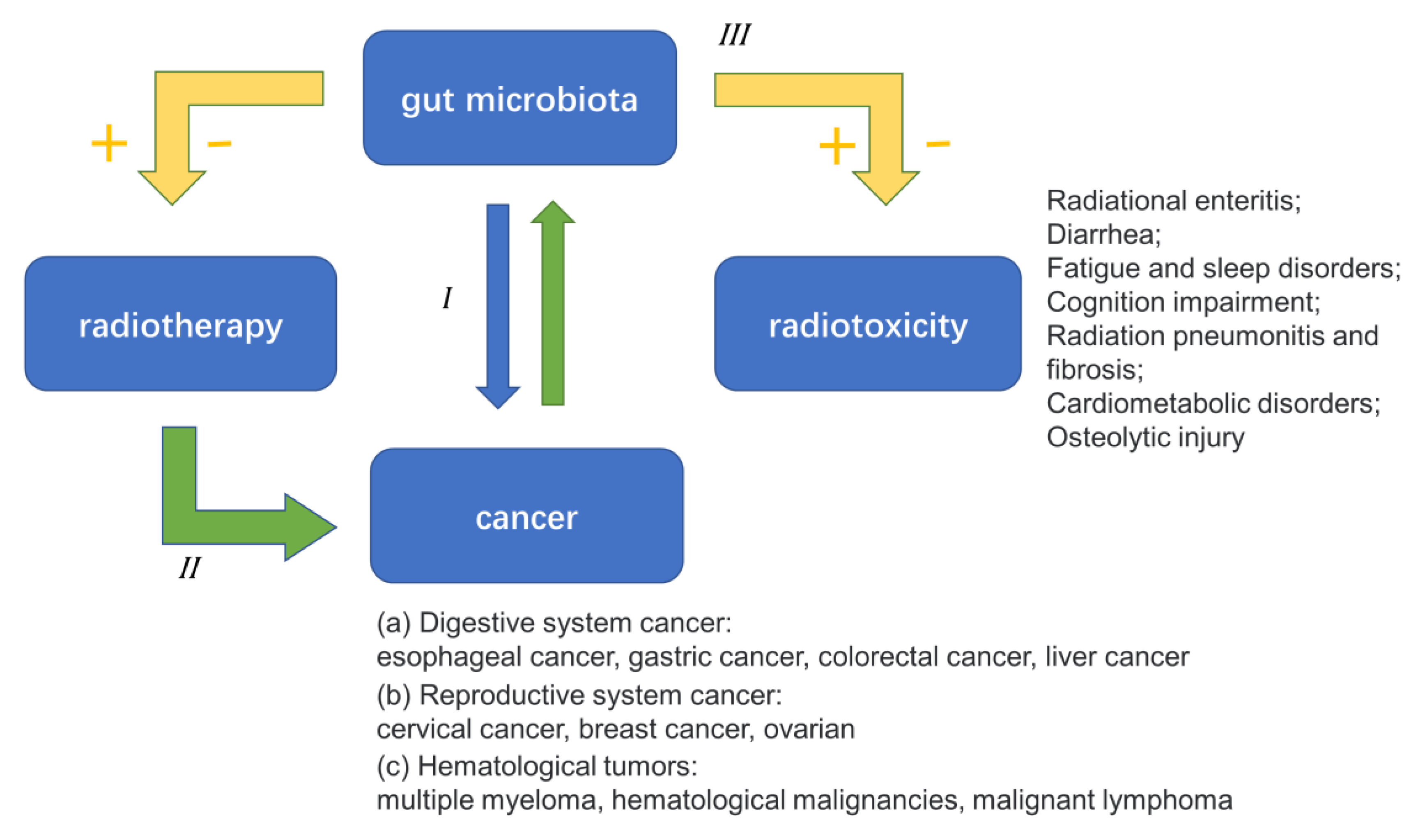
3. Interactions between Gut Microbiota and Radiotherapy
3.1. Gut Microbiota and the Side Effects of Radiotherapy
3.2. Gut-Organ Axis
3.3. Gut Microbiota and Radiotherapy Efficacy
4. Radiotherapy for Cancer Based on the Gut Microbiota
4.1. Treatment of Cancer and Metastasis
4.2. Prevention and Treatment of Radiation Injury
5. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kocarnik, J.M.; Compton, K.; Dean, F.E.; Fu, W.; Gaw, B.L.; Harvey, J.D.; Henrikson, H.J.; Lu, D.; Pennini, A.; Xu, R.; et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shah, K. The Potential of the Gut Microbiome to Reshape the Cancer Therapy Paradigm: A Review. JAMA Oncol. 2022, 8, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Konstantinov, S.R.; Smits, R.; Peppelenbosch, M.P. Bacterial Biofilms in Colorectal Cancer Initiation and Progression. Trends Mol. Med. 2017, 23, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Hekmatshoar, Y.; Rahbar Saadat, Y.; Hosseiniyan Khatibi, S.M.; Ozkan, T.; Zununi Vahed, F.; Nariman-Saleh-Fam, Z.; Pourghassem Gargari, B.; Sunguroglu, A.; Zununi Vahed, S. The impact of tumor and gut microbiotas on cancer therapy: Beneficial or detrimental? Life Sci. 2019, 233, 116680. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Barros, A.; Pita, I.; Miranda, I.; Conceição, G.; Borges-Canha, M.; Leite-Moreira, A.F.; Libânio, D.; Dinis-Ribeiro, M. Gastric microbiome profile throughout gastric carcinogenesis: Beyond helicobacter. Scand. J. Gastroenterol. 2021, 56, 708–716. [Google Scholar] [CrossRef]
- Yu, D.; Yang, J.; Jin, M.; Zhou, B.; Shi, L.; Zhao, L.; Zhang, J.; Lin, Z.; Ren, J.; Liu, L.; et al. Fecal Streptococcus Alteration Is Associated with Gastric Cancer Occurrence and Liver Metastasis. mBio 2021, 12, e0299421. [Google Scholar] [CrossRef]
- Ishaq, H.M.; Mohammad, I.S.; Sher Muhammad, K.; Li, H.; Abbas, R.Z.; Din Sindhu, Z.U.; Ullah, S.; Fan, Y.; Sadiq, A.; Raza, M.A.; et al. Gut microbial dysbiosis and its association with esophageal cancer. J. Appl. Biomed. 2021, 19, 1–13. [Google Scholar] [CrossRef]
- Zhao, F.; An, R.; Wang, L.; Shan, J.; Wang, X. Specific Gut Microbiome and Serum Metabolome Changes in Lung Cancer Patients. Front. Cell. Infect. Microbiol. 2021, 11, 725284. [Google Scholar] [CrossRef]
- Laborda-Illanes, A.; Sánchez-Alcoholado, L.; Boutriq, S.; Plaza-Andrades, I.; Peralta-Linero, J.; Alba, E.; González-González, A.; Queipo-Ortuño, M.I. A New Paradigm in the Relationship between Melatonin and Breast Cancer: Gut Microbiota Identified as a Potential Regulatory Agent. Cancers 2021, 13, 3141. [Google Scholar] [CrossRef]
- Wang, R.; Yang, X.; Liu, J.; Zhong, F.; Zhang, C.; Chen, Y.; Sun, T.; Ji, C.; Ma, D. Gut microbiota regulates acute myeloid leukaemia via alteration of intestinal barrier function mediated by butyrate. Nat. Commun. 2022, 13, 2522. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Yue, J. Radiotherapy and the gut microbiome: Facts and fiction. Radiat. Oncol. 2021, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Al-Qadami, G.; Van Sebille, Y.; Le, H.; Bowen, J. Gut microbiota: Implications for radiotherapy response and radiotherapy-induced mucositis. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.; Ali, S.A.; Singh, R.K. Emerging role of gut microbiota in modulation of neuroinflammation and neurodegeneration with emphasis on Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110112. [Google Scholar] [CrossRef]
- Joukar, F.; Mavaddati, S.; Mansour-Ghanaei, F.; Samadani, A.A. Gut Microbiota as a Positive Potential Therapeutic Factor in Carcinogenesis: An Overview of Microbiota-Targeted Therapy. J. Gastrointest. Cancer 2020, 51, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H. Gut microbiota modulation: A tool for the management of colorectal cancer. J. Transl. Med. 2022, 20, 178. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Helmink, B.A.; Khan, M.A.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The microbiome, cancer, and cancer therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef]
- Matson, V.; Chervin, C.S.; Gajewski, T.F. Cancer and the Microbiome-Influence of the Commensal Microbiota on Cancer, Immune Responses, and Immunotherapy. Gastroenterology 2021, 160, 600–613. [Google Scholar] [CrossRef]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef]
- Berbert, L.; Santos, A.; Magro, D.O.; Guadagnini, D.; Assalin, H.B.; Lourenço, L.H.; Martinez, C.A.R.; Saad, M.J.A.; Coy, C.S.R. Metagenomics analysis reveals universal signatures of the intestinal microbiota in colorectal cancer, regardless of regional differences. Braz. J. Med. Biol. Res. 2022, 55, e11832. [Google Scholar] [CrossRef]
- Zhong, M.; Xiong, Y.; Zhao, J.; Gao, Z.; Ma, J.; Wu, Z.; Song, Y.; Hong, X. Candida albicans disorder is associated with gastric carcinogenesis. Theranostics 2021, 11, 4945–4956. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Li, J.; He, B.; Chen, B.; Liu, F.; Chen, Z.; Zheng, J.; Shi, Z.; Zhang, T.; Deng, L.; et al. Gut microbiome alteration as a diagnostic tool and associated with inflammatory response marker in primary liver cancer. Hepatol. Int. 2022, 16, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Sims, T.T.; Colbert, L.E.; Zheng, J.; Delgado Medrano, A.Y.; Hoffman, K.L.; Ramondetta, L.; Jazaeri, A.; Jhingran, A.; Schmeler, K.M.; Daniel, C.R.; et al. Gut microbial diversity and genus-level differences identified in cervical cancer patients versus healthy controls. Gynecol. Oncol. 2019, 155, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Jian, X.; Zhu, Y.; Ouyang, J.; Wang, Y.; Lei, Q.; Xia, J.; Guan, Y.; Zhang, J.; Guo, J.; He, Y.; et al. Alterations of gut microbiome accelerate multiple myeloma progression by increasing the relative abundances of nitrogen-recycling bacteria. Microbiome 2020, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Privitera, G.; Rana, N.; Scaldaferri, F.; Armuzzi, A.; Pizarro, T.T. Novel Insights Into the Interactions Between the Gut Microbiome, Inflammasomes, and Gasdermins During Colorectal Cancer. Front. Cell. Infect. Microbiol. 2021, 11, 806680. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Fang, Y.; Zhang, W.; Zhang, H.; Tang, D.; Wang, D. Bacterial driver-passenger model in biofilms: A new mechanism in the development of colorectal cancer. Clin. Transl. Oncol. 2022, 24, 784–795. [Google Scholar] [CrossRef]
- Nadler, N.; Kvich, L.; Bjarnsholt, T.; Jensen, J.B.; Gögenur, I.; Azawi, N. The discovery of bacterial biofilm in patients with muscle invasive bladder cancer. APMIS 2021, 129, 265–270. [Google Scholar] [CrossRef]
- Moghadam, M.T.; Chegini, Z.; Khoshbayan, A.; Farahani, I.; Shariati, A. Helicobacter pylori Biofilm and New Strategies to Combat it. Curr. Mol. Med. 2021, 21, 549–561. [Google Scholar] [CrossRef]
- Bennedsen, A.L.B.; Furbo, S.; Bjarnsholt, T.; Raskov, H.; Gögenur, I.; Kvich, L. The gut microbiota can orchestrate the signaling pathways in colorectal cancer. APMIS 2022, 130, 121–139. [Google Scholar] [CrossRef]
- Münch, N.S.; Fang, H.Y.; Ingermann, J.; Maurer, H.C.; Anand, A.; Kellner, V.; Sahm, V.; Wiethaler, M.; Baumeister, T.; Wein, F.; et al. High-Fat Diet Accelerates Carcinogenesis in a Mouse Model of Barrett’s Esophagus via Interleukin 8 and Alterations to the Gut Microbiome. Gastroenterology 2019, 157, 492–506.e2. [Google Scholar] [CrossRef]
- Clay, S.L.; Fonseca-Pereira, D.; Garrett, W.S. Colorectal cancer: The facts in the case of the microbiota. J. Clin. Investig. 2022, 132, e155101. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Nong, C.; Zhao, J.; Meng, L.; Song, J. An Integrative Bioinformatic Analysis of Microbiome and Transcriptome for Predicting the Risk of Colon Adenocarcinoma. Dis. Markers 2022, 2022, 7994074. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.; Sharma, P.; Are, A.C.; Vickers, S.M.; Dudeja, V. New Insights Into the Cancer-Microbiome-Immune Axis: Decrypting a Decade of Discoveries. Front. Immunol. 2021, 12, 622064. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, Y.R.; Saini, A.K.; Thakur, V.K.; Saini, R.V. New Insights into Molecular Links Between Microbiota and Gastrointestinal Cancers: A Literature Review. Int. J. Mol. Sci. 2020, 21, 3212. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, L.; Liang, X.; Li, S.; Ma, L.; Zheng, L.; Li, T.; Yu, H.; Chan, H.; Chen, C.; et al. Helicobacter pylori-induced YAP1 nuclear translocation promotes gastric carcinogenesis by enhancing IL-1β expression. Cancer Med. 2019, 8, 3965–3980. [Google Scholar] [CrossRef]
- Dalmasso, G.; Cougnoux, A.; Delmas, J.; Darfeuille-Michaud, A.; Bonnet, R. The bacterial genotoxin colibactin promotes colon tumor growth by modifying the tumor microenvironment. Gut Microbes 2014, 5, 675–680. [Google Scholar] [CrossRef]
- Buc, E.; Dubois, D.; Sauvanet, P.; Raisch, J.; Delmas, J.; Darfeuille-Michaud, A.; Pezet, D.; Bonnet, R. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS ONE 2013, 8, e56964. [Google Scholar] [CrossRef]
- Shmuely, H.; Passaro, D.; Figer, A.; Niv, Y.; Pitlik, S.; Samra, Z.; Koren, R.; Yahav, J. Relationship between Helicobacter pylori CagA status and colorectal cancer. Am. J. Gastroenterol. 2001, 96, 3406–3410. [Google Scholar] [CrossRef]
- Cheng, K.W.; Tseng, C.H.; Chen, I.J.; Huang, B.C.; Liu, H.J.; Ho, K.W.; Lin, W.W.; Chuang, C.H.; Huang, M.Y.; Leu, Y.L.; et al. Inhibition of gut microbial β-glucuronidase effectively prevents carcinogen-induced microbial dysbiosis and intestinal tumorigenesis. Pharmacol. Res. 2022, 177, 106115. [Google Scholar] [CrossRef]
- Pollet, R.M.; D’Agostino, E.H.; Walton, W.G.; Xu, Y.; Little, M.S.; Biernat, K.A.; Pellock, S.J.; Patterson, L.M.; Creekmore, B.C.; Isenberg, H.N.; et al. An Atlas of β-Glucuronidases in the Human Intestinal Microbiome. Structure 2017, 25, 967–977.e5. [Google Scholar] [CrossRef]
- Ternes, D.; Tsenkova, M.; Pozdeev, V.I.; Meyers, M.; Koncina, E.; Atatri, S.; Schmitz, M.; Karta, J.; Schmoetten, M.; Heinken, A.; et al. The gut microbial metabolite formate exacerbates colorectal cancer progression. Nat. Metab. 2022, 4, 458–475. [Google Scholar] [CrossRef]
- Hou, H.; Chen, D.; Zhang, K.; Zhang, W.; Liu, T.; Wang, S.; Dai, X.; Wang, B.; Zhong, W.; Cao, H. Gut microbiota-derived short-chain fatty acids and colorectal cancer: Ready for clinical translation? Cancer Lett. 2022, 526, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Siedlecka, K.; Daca, A.; Roviello, G.; Catalano, M.; Połom, K. Interdisciplinary insights into the link between gut microbiome and gastric carcinogenesis—what is currently known? Gastric Cancer 2022, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.; Barzi, A.; Brezden-Masley, C.; Puccini, A.; Berger, M.D.; Tokunaga, R.; Battaglin, F.; Soni, S.; McSkane, M.; Zhang, W.; et al. Outlooks on Epstein-Barr virus associated gastric cancer. Cancer Treat. Rev. 2018, 66, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Matthews, G.M.; Howarth, G.S.; Butler, R.N. Short-chain fatty acid modulation of apoptosis in the Kato III human gastric carcinoma cell line. Cancer Biol. Ther. 2007, 6, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lu, Y.; Yuan, S.; Cai, X.; He, Y.; Chen, J.; Wu, Q.; He, D.; Fang, A.; Bo, Y.; et al. Gut microbiota-derived metabolite trimethylamine-N-oxide and multiple health outcomes: An umbrella review and updated meta-analysis. Am. J. Clin. Nutr. 2022, 116, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.G.; Cowley, E.S.; Breister, A.; Matatov, S.; Lucio, L.; Polak, P.; Ridlon, J.M.; Gaskins, H.R.; Anantharaman, K. Diversity and distribution of sulfur metabolic genes in the human gut microbiome and their association with colorectal cancer. Microbiome 2022, 10, 64. [Google Scholar] [CrossRef]
- Li, X.; Khan, I.; Huang, G.; Lu, Y.; Wang, L.; Liu, Y.; Lu, L.; Hsiao, W.L.W.; Liu, Z. Kaempferol acts on bile acid signaling and gut microbiota to attenuate the tumor burden in ApcMin/+ mice. Eur. J. Pharmacol. 2022, 918, 174773. [Google Scholar] [CrossRef]
- Xing, J.; Liao, Y.; Zhang, H.; Zhang, W.; Zhang, Z.; Zhang, J.; Wang, D.; Tang, D. Impacts of MicroRNAs Induced by the Gut Microbiome on Regulating the Development of Colorectal Cancer. Front. Cell. Infect. Microbiol. 2022, 12, 804689. [Google Scholar] [CrossRef]
- Lu, G.; Yu, X.; Jiang, W.; Luo, Q.; Tong, J.; Fan, S.; Chai, L.; Gao, D.; Qiao, T.; Wang, R.; et al. Alterations of Gut Microbiome and Metabolite Profiles Associated With Anabatic Lipid Dysmetabolism in Thyroid Cancer. Front. Endocrinol. 2022, 13, 893164. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Yuan, X.; Cui, Y.Y.; Liu, J.; Shen, J.; Jin, B.Y.; Feng, B.C.; Zhai, Y.J.; Zheng, M.Q.; Kou, G.J.; et al. Melatonin Mitigates Oxazolone-Induced Colitis in Microbiota-Dependent Manner. Front. Immunol. 2021, 12, 783806. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Hupp, T.; Duchnowska, R.; Marek-Trzonkowska, N.; Połom, K. Next-generation probiotics—do they open new therapeutic strategies for cancer patients? Gut Microbes 2022, 14, 2035659. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Meng, X.; Liu, S.; Zhou, P.; Zeng, C.; Fu, C.; Dou, Q.; Wu, A.; Li, C. Gut Microbiota Composition Associated With Clostridium difficile-Positive Diarrhea and C. difficile Type in ICU Patients. Front. Cell. Infect. Microbiol. 2020, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Pang, X.; Zhu, X.; Meng, Z.; Chen, X.; Zhang, J.; Ding, Q.; Li, Q.; Dou, G.; Ma, B. Lycium barbarum mitigates radiation injury via regulation of the immune function, gut microbiota, and related metabolites. Biomed. Pharmacother. 2021, 139, 111654. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.Y.; Wang, J.; Ding, Q.Q.; Chen, W.; Xu, X.K.; Wei, X.T.; Lv, Y.H.; Wei, Y.P.; Feng, Y.; Zu, X.P. Potential role of gut microbiota and its metabolites in radiation-induced intestinal damage. Ecotoxicol. Environ. Saf. 2022, 248, 114341. [Google Scholar] [CrossRef]
- Wierzbicka, A.; Mańkowska-Wierzbicka, D.; Mardas, M.; Stelmach-Mardas, M. Role of Probiotics in Modulating Human Gut Microbiota Populations and Activities in Patients with Colorectal Cancer-A Systematic Review of Clinical Trials. Nutrients 2021, 13, 1160. [Google Scholar] [CrossRef]
- Fernandes, A.; Oliveira, A.; Soares, R.; Barata, P. The Effects of Ionizing Radiation on Gut Microbiota, a Systematic Review. Nutrients 2021, 13, 3025. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Wei, K.; He, J.; Ding, N.; Hua, J.; Zhou, T.; Niu, F.; Zhou, G.; Shi, T.; et al. Review: Effect of Gut Microbiota and Its Metabolite SCFAs on Radiation-Induced Intestinal Injury. Front. Cell. Infect. Microbiol. 2021, 11, 577236. [Google Scholar] [CrossRef]
- Lam, V.; Moulder, J.E.; Salzman, N.H.; Dubinsky, E.A.; Andersen, G.L.; Baker, J.E. Intestinal microbiota as novel biomarkers of prior radiation exposure. Radiat. Res. 2012, 177, 573–583. [Google Scholar] [CrossRef]
- El Alam, M.B.; Sims, T.T.; Kouzy, R.; Biegert, G.W.G.; Jaoude, J.; Karpinets, T.V.; Yoshida-Court, K.; Wu, X.; Delgado-Medrano, A.Y.; Mezzari, M.P.; et al. A prospective study of the adaptive changes in the gut microbiome during standard-of-care chemoradiotherapy for gynecologic cancers. PLoS ONE 2021, 16, e0247905. [Google Scholar] [CrossRef]
- Oliva, M.; Schneeberger, P.H.H.; Rey, V.; Cho, M.; Taylor, R.; Hansen, A.R.; Taylor, K.; Hosni, A.; Bayley, A.; Hope, A.J.; et al. Transitions in oral and gut microbiome of HPV+ oropharyngeal squamous cell carcinoma following definitive chemoradiotherapy (ROMA LA-OPSCC study). Br. J. Cancer 2021, 124, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, H.; Dong, J.; Luo, D.; Wang, H.; Zhang, S.; Zhu, T.; Zhu, C.; Cui, M.; Fan, S. Gut Microbiota Metabolite Fights Against Dietary Polysorbate 80-Aggravated Radiation Enteritis. Front. Microbiol. 2020, 11, 1450. [Google Scholar] [CrossRef] [PubMed]
- Gately, S. Human Microbiota and Personalized Cancer Treatments: Role of Commensal Microbes in Treatment Outcomes for Cancer Patients. Cancer Treat. Res. 2019, 178, 253–264. [Google Scholar] [CrossRef]
- Wang, A.; Ling, Z.; Yang, Z.; Kiela, P.R.; Wang, T.; Wang, C.; Cao, L.; Geng, F.; Shen, M.; Ran, X.; et al. Gut microbial dysbiosis may predict diarrhea and fatigue in patients undergoing pelvic cancer radiotherapy: A pilot study. PLoS ONE 2015, 10, e0126312. [Google Scholar] [CrossRef] [PubMed]
- Gerassy-Vainberg, S.; Blatt, A.; Danin-Poleg, Y.; Gershovich, K.; Sabo, E.; Nevelsky, A.; Daniel, S.; Dahan, A.; Ziv, O.; Dheer, R.; et al. Radiation induces proinflammatory dysbiosis: Transmission of inflammatory susceptibility by host cytokine induction. Gut 2018, 67, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chou, W.C.; Lai, Y.; Liang, K.; Tam, J.W.; Brickey, W.J.; Chen, L.; Montgomery, N.D.; Li, X.; Bohannon, L.M.; et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 2020, 370, eaay9097. [Google Scholar] [CrossRef] [PubMed]
- Segers, C.; Verslegers, M.; Baatout, S.; Leys, N.; Lebeer, S.; Mastroleo, F. Food Supplements to Mitigate Detrimental Effects of Pelvic Radiotherapy. Microorganisms 2019, 7, 97. [Google Scholar] [CrossRef]
- Hauer-Jensen, M.; Denham, J.W.; Andreyev, H.J. Radiation enteropathy—pathogenesis, treatment and prevention. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 470–479. [Google Scholar] [CrossRef]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Li, Y. Radioactive Enteritis and Gut Microecology. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2020, 42, 405–409. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Q.; Wang, X.; Zhu, L.; Chen, J.; Zhang, B.; Chen, Y.; Yuan, Z. Gut microbial dysbiosis is associated with development and progression of radiation enteritis during pelvic radiotherapy. J. Cell. Mol. Med. 2019, 23, 3747–3756. [Google Scholar] [CrossRef]
- Reis Ferreira, M.; Andreyev, H.J.N.; Mohammed, K.; Truelove, L.; Gowan, S.M.; Li, J.; Gulliford, S.L.; Marchesi, J.R.; Dearnaley, D.P. Microbiota- and Radiotherapy-Induced Gastrointestinal Side-Effects (MARS) Study: A Large Pilot Study of the Microbiome in Acute and Late-Radiation Enteropathy. Clin. Cancer Res. 2019, 25, 6487–6500. [Google Scholar] [CrossRef]
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B.; et al. Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004, 100, 1995–2025. [Google Scholar] [CrossRef]
- van Vliet, M.J.; Harmsen, H.J.; de Bont, E.S.; Tissing, W.J. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog. 2010, 6, e1000879. [Google Scholar] [CrossRef]
- Li, Y.; Yan, H.; Zhang, Y.; Li, Q.; Yu, L.; Li, Q.; Liu, C.; Xie, Y.; Chen, K.; Ye, F.; et al. Alterations of the Gut Microbiome Composition and Lipid Metabolic Profile in Radiation Enteritis. Front. Cell. Infect. Microbiol. 2020, 10, 541178. [Google Scholar] [CrossRef]
- Cai, J.; Sun, L.; Gonzalez, F.J. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 2022, 30, 289–300. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. [Google Scholar] [CrossRef] [PubMed]
- da Silva Ferreira, A.R.; Wardill, H.R.; Tissing, W.J.E.; Harmsen, H.J.M. Pitfalls and novel experimental approaches to optimize microbial interventions for chemotherapy-induced gastrointestinal mucositis. Curr. Opin. Support. Palliat. Care 2020, 14, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.Z.; Zhang, D.; Cao, Y.F.; Xie, C.; Lu, D.; Sun, D.X.; Tanaka, N.; Jiang, C.; Chen, Q.; Chen, Y.; et al. Irinotecan (CPT-11)-induced elevation of bile acids potentiates suppression of IL-10 expression. Toxicol. Appl. Pharmacol. 2016, 291, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Zhang, G.; Ma, Y.; Zhang, Q.; Li, Z.; Ran, J.; Hou, X.; Geng, Y.; Yang, Z.; et al. The impact of pelvic radiotherapy on the gut microbiome and its role in radiation-induced diarrhoea: A systematic review. Radiat. Oncol. 2021, 16, 187. [Google Scholar] [CrossRef]
- Touchefeu, Y.; Montassier, E.; Nieman, K.; Gastinne, T.; Potel, G.; Bruley des Varannes, S.; Le Vacon, F.; de La Cochetière, M.F. Systematic review: The role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis—current evidence and potential clinical applications. Aliment. Pharmacol. Ther. 2014, 40, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Manichanh, C.; Varela, E.; Martinez, C.; Antolin, M.; Llopis, M.; Doré, J.; Giralt, J.; Guarner, F.; Malagelada, J.R. The gut microbiota predispose to the pathophysiology of acute postradiotherapy diarrhea. Am. J. Gastroenterol. 2008, 103, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.; Eade, T.; Lamoury, G.; Carroll, S.; Morgia, M.; Kneebone, A.; Hruby, G.; Stevens, M.; Boyle, F.; Clarke, S.; et al. The Gut Microbiome and Gastrointestinal Toxicities in Pelvic Radiation Therapy: A Clinical Review. Cancers 2021, 13, 2353. [Google Scholar] [CrossRef]
- Nam, Y.D.; Kim, H.J.; Seo, J.G.; Kang, S.W.; Bae, J.W. Impact of pelvic radiotherapy on gut microbiota of gynecological cancer patients revealed by massive pyrosequencing. PLoS ONE 2013, 8, e82659. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- González-Mercado, V.J.; Lim, J.; Marrero, S.; Pedro, E.; Saligan, L.N. Gut microbiota and fatigue in rectal cancer patients: A cross-sectional pilot study. Support. Care Cancer 2021, 29, 4615–4621. [Google Scholar] [CrossRef] [PubMed]
- González-Mercado, V.J.; Henderson, W.A.; Sarkar, A.; Lim, J.; Saligan, L.N.; Berk, L.; Dishaw, L.; McMillan, S.; Groer, M.; Sepehri, F.; et al. Changes in Gut Microbiome Associated With Co-Occurring Symptoms Development During Chemo-Radiation for Rectal Cancer: A Proof of Concept Study. Biol. Res. Nurs. 2021, 23, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mercado, V.J.; Marrero, S.; Pérez-Santiago, J.; Tirado-Gómez, M.; Marrero-Falcón, M.A.; Pedro, E.; Saligan, L.N. Association of Radiotherapy-Related Intestinal Injury and Cancer-related Fatigue: A Brief Review and Commentary. P. R. Health Sci. J. 2021, 40, 6–11. [Google Scholar]
- Xiao, C.; Fedirko, V.; Beitler, J.; Bai, J.; Peng, G.; Zhou, C.; Gu, J.; Zhao, H.; Lin, I.H.; Chico, C.E.; et al. The role of the gut microbiome in cancer-related fatigue: Pilot study on epigenetic mechanisms. Support. Care Cancer 2021, 29, 3173–3182. [Google Scholar] [CrossRef] [PubMed]
- González-Mercado, V.J.; Sarkar, A.; Penedo, F.J.; Pérez-Santiago, J.; McMillan, S.; Marrero, S.J.; Marrero-Falcón, M.A.; Munro, C.L. Gut microbiota perturbation is associated with acute sleep disturbance among rectal cancer patients. J. Sleep Res. 2020, 29, e12915. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.X.; Yang, C.; Zhan, G.F.; Li, S.; Hua, D.Y.; Luo, A.L.; Yuan, X.L. Whole brain radiotherapy induces cognitive dysfunction in mice: Key role of gut microbiota. Psychopharmacology 2020, 237, 2089–2101. [Google Scholar] [CrossRef]
- Yang, S.; Fu, Z.Z.; Zhang, Y.Q.; Fu, B.H.; Dong, L. The G to A transformation of rs4702 polymorphism in 3’UTR of FURIN reduced the risk of radiotherapy-induced cognitive impairment in glioma patients. J. Cell. Mol. Med. 2022, 26, 684–692. [Google Scholar] [CrossRef]
- Lensu, S.; Waselius, T.; Mäkinen, E.; Kettunen, H.; Virtanen, A.; Tiirola, M.; Penttonen, M.; Pekkala, S.; Nokia, M.S. Irradiation of the head reduces adult hippocampal neurogenesis and impairs spatial memory, but leaves overall health intact in rats. Eur. J. Neurosci. 2021, 53, 1885–1904. [Google Scholar] [CrossRef]
- Li, W.; Lu, L.; Liu, B.; Qin, S. Effects of phycocyanin on pulmonary and gut microbiota in a radiation-induced pulmonary fibrosis model. Biomed. Pharmacother. 2020, 132, 110826. [Google Scholar] [CrossRef]
- Maier, I.; Ruegger, P.M.; Deutschmann, J.; Helbich, T.H.; Pietschmann, P.; Schiestl, R.H.; Borneman, J. Particle Radiation Side-Effects: Intestinal Microbiota Composition Shapes Interferon-γ-Induced Osteo-Immunogenicity. Radiat. Res. 2022, 197, 184–192. [Google Scholar] [CrossRef]
- Group, N.H.W.; Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; et al. The NIH Human Microbiome Project. Genome Res. 2009, 19, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Mainali, R.; Ahmadi, S.; Wang, S.; Singh, R.; Kavanagh, K.; Kitzman, D.W.; Kushugulova, A.; Marotta, F.; Yadav, H. Gut microbiome and aging: Physiological and mechanistic insights. Nutr. Healthy Aging 2018, 4, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Imhann, F.; Bonder, M.J.; Vich Vila, A.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, M.; Beck, R.; Caccio, S.M.; Duim, B.; Fraaij, P.; Le Guyader, F.S.; Lecuit, M.; Le Pendu, J.; de Wit, E.; Schultsz, C. Sustained fecal-oral human-to-human transmission following a zoonotic event. Curr. Opin. Virol. 2017, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, M.; Lozupone, C. Prevalence and Source of Fecal and Oral Bacteria on Infant, Child, and Adult Hands. mSystems 2018, 3, e00192-17. [Google Scholar] [CrossRef] [PubMed]
- Gaetti-Jardim, E.; Jardim, E.C.G.; Schweitzer, C.M.; da Silva, J.C.L.; Oliveira, M.M.; Masocatto, D.C.; Dos Santos, C.M. Supragingival and subgingival microbiota from patients with poor oral hygiene submitted to radiotherapy for head and neck cancer treatment. Arch. Oral Biol. 2018, 90, 45–52. [Google Scholar] [CrossRef]
- Kobayashi, R.; Ogawa, Y.; Hashizume-Takizawa, T.; Kurita-Ochiai, T. Oral bacteria affect the gut microbiome and intestinal immunity. Pathog. Dis. 2020, 78, ftaa024. [Google Scholar] [CrossRef]
- Mo, S.; Ru, H.; Huang, M.; Cheng, L.; Mo, X.; Yan, L. Oral-Intestinal Microbiota in Colorectal Cancer: Inflammation and Immunosuppression. J. Inflamm. Res. 2022, 15, 747–759. [Google Scholar] [CrossRef]
- Flemer, B.; Warren, R.D.; Barrett, M.P.; Cisek, K.; Das, A.; Jeffery, I.B.; Hurley, E.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. The oral microbiota in colorectal cancer is distinctive and predictive. Gut 2018, 67, 1454–1463. [Google Scholar] [CrossRef]
- Dong, J.; Li, Y.; Xiao, H.; Zhang, S.; Wang, B.; Wang, H.; Li, Y.; Fan, S.; Cui, M. Oral microbiota affects the efficacy and prognosis of radiotherapy for colorectal cancer in mouse models. Cell Rep. 2021, 37, 109886. [Google Scholar] [CrossRef]
- Spielman, L.J.; Gibson, D.L.; Klegeris, A. Unhealthy gut, unhealthy brain: The role of the intestinal microbiota in neurodegenerative diseases. Neurochem. Int. 2018, 120, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Boeri, L.; Perottoni, S.; Izzo, L.; Giordano, C.; Albani, D. Microbiota-Host Immunity Communication in Neurodegenerative Disorders: Bioengineering Challenges for In Vitro Modeling. Adv. Healthc. Mater. 2021, 10, e2002043. [Google Scholar] [CrossRef] [PubMed]
- Leblhuber, F.; Ehrlich, D.; Steiner, K.; Geisler, S.; Fuchs, D.; Lanser, L.; Kurz, K. The Immunopathogenesis of Alzheimer’s Disease Is Related to the Composition of Gut Microbiota. Nutrients 2021, 13, 361. [Google Scholar] [CrossRef] [PubMed]
- Grasset, E.; Burcelin, R. The gut microbiota to the brain axis in the metabolic control. Rev. Endocr. Metab. Disord. 2019, 20, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Hutchison, K.E.; Portillo, S.; Vegara, V.; Ellingson, J.M.; Liu, J.; Krauter, K.S.; Carroll-Portillo, A.; Calhoun, V.D. Association between the oral microbiome and brain resting state connectivity in smokers. NeuroImage 2019, 200, 121–131. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, X.; Liang, S.; Shao, X.; Wang, Q.; Chen, R.; Zhu, W.; Shao, C.; Jin, F.; Jia, C. A dynamic mouse peptidome landscape reveals probiotic modulation of the gut-brain axis. Sci. Signal. 2020, 13, eabb0443. [Google Scholar] [CrossRef]
- Boonchooduang, N.; Louthrenoo, O.; Chattipakorn, N.; Chattipakorn, S.C. Possible links between gut-microbiota and attention-deficit/hyperactivity disorders in children and adolescents. Eur. J. Nutr. 2020, 59, 3391–3403. [Google Scholar] [CrossRef]
- Dodiya, H.B.; Forsyth, C.B.; Voigt, R.M.; Engen, P.A.; Patel, J.; Shaikh, M.; Green, S.J.; Naqib, A.; Roy, A.; Kordower, J.H.; et al. Chronic stress-induced gut dysfunction exacerbates Parkinson’s disease phenotype and pathology in a rotenone-induced mouse model of Parkinson’s disease. Neurobiol. Dis. 2020, 135, 104352. [Google Scholar] [CrossRef]
- Malan-Muller, S.; Valles-Colomer, M.; Foxx, C.L.; Vieira-Silva, S.; van den Heuvel, L.L.; Raes, J.; Seedat, S.; Lowry, C.A.; Hemmings, S.M.J. Exploring the relationship between the gut microbiome and mental health outcomes in a posttraumatic stress disorder cohort relative to trauma-exposed controls. Eur. Neuropsychopharmacol. 2022, 56, 24–38. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, B.; Mu, L.; Wang, H.; Luo, J.; Yang, Y.; Yang, H.; Li, M.; Zhou, L.; Tao, C. Long-Term Exposure to Ceftriaxone Sodium Induces Alteration of Gut Microbiota Accompanied by Abnormal Behaviors in Mice. Front. Cell. Infect. Microbiol. 2020, 10, 258. [Google Scholar] [CrossRef]
- Chen, B.; Huang, H.; Pan, C.Q. The role of gut microbiota in hepatitis B disease progression and treatment. J. Viral Hepat. 2022, 29, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Li, J.; Kang, J.H.; Eto, H.; Zai, K.; Kishimura, A.; Hyodo, F.; Mori, T.; Katayama, Y. A Lipid-Based Nanocarrier Containing Active Vitamin D3 Ameliorates NASH in Mice via Direct and Intestine-Mediated Effects on Liver Inflammation. Biol. Pharm. Bull. 2020, 43, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Hyogo, H.; Ono, A.; Nagaoki, Y.; Kawaoka, T.; Miki, D.; Tsuge, M.; Hiraga, N.; Hayes, C.N.; Hiramatsu, A.; et al. Involvement of Porphyromonas gingivalis in the progression of non-alcoholic fatty liver disease. J. Gastroenterol. 2018, 53, 269–280. [Google Scholar] [CrossRef]
- Nagasaki, A.; Sakamoto, S.; Chea, C.; Ishida, E.; Furusho, H.; Fujii, M.; Takata, T.; Miyauchi, M. Odontogenic infection by Porphyromonas gingivalis exacerbates fibrosis in NASH via hepatic stellate cell activation. Sci. Rep. 2020, 10, 4134. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Funatsu, Y.; Ohnishi, M.; Isogawa, M.; Kawashima, K.; Tanaka, M.; Moriya, K.; Kawaratani, H.; Momoda, R.; Iio, E.; et al. Bile acid dysmetabolism in the gut-microbiota-liver axis under hepatitis C virus infection. Liver Int 2022, 42, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Garajová, I.; Balsano, R.; Wang, H.; Leonardi, F.; Giovannetti, E.; Deng, D.; Peters, G.J. The role of the microbiome in drug resistance in gastrointestinal cancers. Expert Rev. Anticancer Ther. 2021, 21, 165–176. [Google Scholar] [CrossRef]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Q.; Zhou, C.; Chen, K.; Chang, H.; Xiao, W.; Gao, Y. Colorectal cancer, radiotherapy and gut microbiota. Chin. J. Cancer Res. 2019, 31, 212–222. [Google Scholar] [CrossRef]
- Li, W.; Deng, X.; Chen, T. Exploring the Modulatory Effects of Gut Microbiota in Anti-Cancer Therapy. Front. Oncol. 2021, 11, 644454. [Google Scholar] [CrossRef]
- Dong, J.; Li, Y.; Xiao, H.; Cui, M.; Fan, S. Commensal microbiota in the digestive tract: A review of its roles in carcinogenesis and radiotherapy. Cancer Biol. Med. 2021, 19, 43–55. [Google Scholar] [CrossRef]
- Crawford, P.A.; Gordon, J.I. Microbial regulation of intestinal radiosensitivity. Proc. Natl. Acad. Sci. USA 2005, 102, 13254–13259. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Xiao, H.; Luo, D.; Zhang, X.; Zhao, S.; Zheng, Q.; Li, Y.; Zhao, Y.; Dong, J.; Li, H.; et al. Circadian Rhythm Shapes the Gut Microbiota Affecting Host Radiosensitivity. Int. J. Mol. Sci. 2016, 17, 1786. [Google Scholar] [CrossRef] [PubMed]
- Paulos, C.M.; Wrzesinski, C.; Kaiser, A.; Hinrichs, C.S.; Chieppa, M.; Cassard, L.; Palmer, D.C.; Boni, A.; Muranski, P.; Yu, Z.; et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J. Clin. Investig. 2007, 117, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
- Nenclares, P.; Bhide, S.A.; Sandoval-Insausti, H.; Pialat, P.; Gunn, L.; Melcher, A.; Newbold, K.; Nutting, C.M.; Harrington, K.J. Impact of antibiotic use during curative treatment of locally advanced head and neck cancers with chemotherapy and radiotherapy. Eur. J. Cancer 2020, 131, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, Q.; Dai, M.; Peng, R.; Li, X.; Zuo, W.; Gou, J.; Zhou, F.; Yu, S.; Liu, H.; et al. FOXQ1-mediated SIRT1 upregulation enhances stemness and radio-resistance of colorectal cancer cells and restores intestinal microbiota function by promoting β-catenin nuclear translocation. J. Exp. Clin. Cancer Res. 2022, 41, 70. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.B.; Zhu, D.Q.; Shao, M.; Li, A.W.; Liu, Z.R.; Gao, R.J.; Liu, S.Y.; Lou, D.D.; Lv, Y.; Fan, Q. Effects of Shengmai Jianghuang San on intestinal flora in nude mice with radio resistant cells of nasopharyngeal carcinoma. Chin. J. Chin. Mater. Med. 2019, 44, 553–558. (In Chinese) [Google Scholar] [CrossRef]
- Akash, M.S.H.; Fiayyaz, F.; Rehman, K.; Sabir, S.; Rasool, M.H. Gut Microbiota and Metabolic Disorders: Advances in Therapeutic Interventions. Crit. Rev. Immunol. 2019, 39, 223–237. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Oikawa, T.; Ochiai, Y.; Roudkenar, M.H.; Fukumoto, M.; Shimura, T.; Ohtake, Y.; Ohkubo, Y.; Mori, S.; Uchiyama, Y.; et al. Enhancement of autophagy is a potential modality for tumors refractory to radiotherapy. Cell Death Dis. 2011, 2, e177. [Google Scholar] [CrossRef]
- Huang, R.; Xiang, J.; Zhou, P. Vitamin D, gut microbiota, and radiation-related resistance: A love-hate triangle. J. Exp. Clin. Cancer Res. 2019, 38, 493. [Google Scholar] [CrossRef]
- Uribe-Herranz, M.; Rafail, S.; Beghi, S.; Gil-de-Gómez, L.; Verginadis, I.; Bittinger, K.; Pustylnikov, S.; Pierini, S.; Perales-Linares, R.; Blair, I.A.; et al. Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J. Clin. Investig. 2020, 130, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Jarosz-Biej, M.; Smolarczyk, R.; Cichoń, T.; Kułach, N. Tumor Microenvironment as A “Game Changer” in Cancer Radiotherapy. Int. J. Mol. Sci. 2019, 20, 3212. [Google Scholar] [CrossRef] [PubMed]
- Golden, E.B.; Frances, D.; Pellicciotta, I.; Demaria, S.; Helen Barcellos-Hoff, M.; Formenti, S.C. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology 2014, 3, e28518. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, H.; Pitt, J.M.; Kroemer, G.; Zitvogel, L. Therapy-induced microenvironmental changes in cancer. J. Mol. Med. 2016, 94, 497–508. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, X.; Wang, M.; He, Z.; Li, H.; Wang, J.; Li, Q. Gut microbiota influence immunotherapy responses: Mechanisms and therapeutic strategies. J. Hematol. Oncol. 2022, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Q.; Liao, Q.; Zhao, Y. Pancreatic Cancer, Gut Microbiota, and Therapeutic Efficacy. J. Cancer 2020, 11, 2749–2758. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Y.; Chen, C.; Shao, B.; Zhao, L.; Zhou, Q.; Liu, J.; Wang, G.; Yuan, W.; Sun, Z. Interaction between intestinal microbiota and tumour immunity in the tumour microenvironment. Immunology 2021, 164, 476–493. [Google Scholar] [CrossRef]
- Formenti, S.C.; Demaria, S. Combining radiotherapy and cancer immunotherapy: A paradigm shift. J. Natl. Cancer Inst. 2013, 105, 256–265. [Google Scholar] [CrossRef]
- Russell, J.S.; Brown, J.M. The irradiated tumor microenvironment: Role of tumor-associated macrophages in vascular recovery. Front. Physiol. 2013, 4, 157. [Google Scholar] [CrossRef]
- Golden, E.B.; Demaria, S.; Schiff, P.B.; Chachoua, A.; Formenti, S.C. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol. Res. 2013, 1, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef]
- Demaria, S.; Coleman, C.N.; Formenti, S.C. Radiotherapy: Changing the Game in Immunotherapy. Trends Cancer 2016, 2, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.Y.; Wu, C.Y.; Yu, J. The role of gut microbiota in cancer treatment: Friend or foe? Gut 2020, 69, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Makaranka, S.; Scutt, F.; Frixou, M.; Wensley, K.E.; Sharma, R.; Greenhowe, J. The gut microbiome and melanoma: A review. Exp. Dermatol. 2022, 31, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, Z.; Zhang, X.; Fan, M.; Hong, Y.; Feng, Y.; Dong, Q.; Diao, H.; Wang, G. Sodium butyrate modulates gut microbiota and immune response in colorectal cancer liver metastatic mice. Cell Biol. Toxicol. 2020, 36, 509–515. [Google Scholar] [CrossRef]
- Gheorghe, A.S.; Negru, Ș.M.; Preda, M.; Mihăilă, R.I.; Komporaly, I.A.; Dumitrescu, E.A.; Lungulescu, C.V.; Kajanto, L.A.; Georgescu, B.; Radu, E.A.; et al. Biochemical and Metabolical Pathways Associated with Microbiota-Derived Butyrate in Colorectal Cancer and Omega-3 Fatty Acids Implications: A Narrative Review. Nutrients 2022, 14, 1152. [Google Scholar] [CrossRef]
- Chang, Z.Y.; Liu, H.M.; Leu, Y.L.; Hsu, C.H.; Lee, T.Y. Modulation of Gut Microbiota Combined with Upregulation of Intestinal Tight Junction Explains Anti-Inflammatory Effect of Corylin on Colitis-Associated Cancer in Mice. Int. J. Mol. Sci. 2022, 23, 2667. [Google Scholar] [CrossRef]
- Wu, R.; Shen, Q.; Li, P.; Shang, N. Sturgeon Chondroitin Sulfate Restores the Balance of Gut Microbiota in Colorectal Cancer Bearing Mice. Int. J. Mol. Sci. 2022, 23, 3723. [Google Scholar] [CrossRef]
- Liang, J.; Tang, M.; Wang, L.; Huang, R.; Fu, A.; Zhou, J. Design and development of novel fasudil derivatives as potent antibreast cancer agent that improves intestinal flora and intestinal barrier function in rats. Chem. Biol. Drug Des. 2021, 98, 1065–1078. [Google Scholar] [CrossRef]
- Taghinezhad, S.S.; Mohseni, A.H.; Fu, X. Intervention on gut microbiota may change the strategy for management of colorectal cancer. J. Gastroenterol. Hepatol. 2021, 36, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Chadha, J.; Nandi, D.; Atri, Y.; Nag, A. Significance of human microbiome in breast cancer: Tale of an invisible and an invincible. Semin. Cancer Biol. 2021, 70, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yan, Y.; Chen, D.; Yang, Y. Quxie Capsule Modulating Gut Microbiome and Its Association With T cell Regulation in Patients With Metastatic Colorectal Cancer: Result From a Randomized Controlled Clinical Trial. Integr. Cancer Ther. 2020, 19, 1534735420969820. [Google Scholar] [CrossRef]
- Pan, L.J.; Ma, S.Y.; Wen, J.; Zhang, X.Q.; Xing, H.J.; Jia, C.S. Direct contact moxibustion promotes apoptosis of gastric cancer cells in rats by regulating intestinal flora. J. Tradit. Chin. Med. 2021, 41, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Han, Y.; Zhang, H.; Tu, W.; Zhang, S. Radiotherapy-Induced Digestive Injury: Diagnosis, Treatment and Mechanisms. Front. Oncol. 2021, 11, 757973. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak-Siedlecka, K.; Daca, A.; Fic, M.; van de Wetering, T.; Folwarski, M.; Makarewicz, W. Therapeutic methods of gut microbiota modification in colorectal cancer management—fecal microbiota transplantation, prebiotics, probiotics, and synbiotics. Gut Microbes 2020, 11, 1518–1530. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, H.; Xia, C.; Dong, Q.; Chen, E.; Qiu, Y.; Su, Y.; Xie, H.; Zeng, L.; Kuang, J.; et al. A randomized, double-blind, placebo-controlled trial of probiotics to reduce the severity of oral mucositis induced by chemoradiotherapy for patients with nasopharyngeal carcinoma. Cancer 2019, 125, 1081–1090. [Google Scholar] [CrossRef]
- Al-Qadami, G.; Verma, G.; Van Sebille, Y.; Le, H.; Hewson, I.; Bateman, E.; Wardill, H.; Bowen, J. Antibiotic-Induced Gut Microbiota Depletion Accelerates the Recovery of Radiation-Induced Oral Mucositis in Rats. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 845–858. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, B.; Dong, J.; Li, Y.; Zhang, S.; Zeng, X.; Xiao, H.; Fan, S.; Cui, M. Gut Microbiota-Derived l-Histidine/Imidazole Propionate Axis Fights against the Radiation-Induced Cardiopulmonary Injury. Int. J. Mol. Sci. 2021, 22, 11436. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Xiao, H.W.; Dong, J.L.; Li, Y.; Wang, B.; Fan, S.J.; Cui, M. Gut Microbiota-Derived PGF2α Fights against Radiation-Induced Lung Toxicity through the MAPK/NF-κB Pathway. Antioxidants 2021, 11, 65. [Google Scholar] [CrossRef]
- Jian, Y.; Zhang, D.; Liu, M.; Wang, Y.; Xu, Z.X. The Impact of Gut Microbiota on Radiation-Induced Enteritis. Front. Cell. Infect. Microbiol. 2021, 11, 586392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, Y.; Lu, P.; Wang, X.; Li, W.; Dong, H.; Fan, S.; Li, D. Gut metabolite Urolithin A mitigates ionizing radiation-induced intestinal damage. J. Cell. Mol. Med. 2021, 25, 10306–10312. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Xie, L.W.; Xu, J.Y.; Zhou, H.; Yang, C.; Tang, L.F.; Tian, Y.; Li, M. (−)-Epigallocatechin-3-Gallate (EGCG) Modulates the Composition of the Gut Microbiota to Protect Against Radiation-Induced Intestinal Injury in Mice. Front. Oncol. 2022, 12, 848107. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jin, Y.X.; Dong, J.L.; Xiao, H.W.; Zhang, S.Q.; Li, Y.; Chen, Z.Y.; Yang, X.D.; Fan, S.J.; Cui, M. Low-Intensity Exercise Modulates Gut Microbiota to Fight Against Radiation-Induced Gut Toxicity in Mouse Models. Front. Cell Dev. Biol. 2021, 9, 706755. [Google Scholar] [CrossRef]
- Rosli, D.; Shahar, S.; Manaf, Z.A.; Lau, H.J.; Yusof, N.Y.M.; Haron, M.R.; Majid, H.A. Randomized Controlled Trial on the Effect of Partially Hydrolyzed Guar Gum Supplementation on Diarrhea Frequency and Gut Microbiome Count Among Pelvic Radiation Patients. JPEN J. Parenter. Enter. Nutr. 2021, 45, 277–286. [Google Scholar] [CrossRef]
- Courtois, E.; Bouleftour, W.; Guy, J.B.; Louati, S.; Bensadoun, R.J.; Rodriguez-Lafrasse, C.; Magné, N. Mechanisms of PhotoBioModulation (PBM) focused on oral mucositis prevention and treatment: A scoping review. BMC Oral Health 2021, 21, 220. [Google Scholar] [CrossRef]
- Bicknell, B.; Laakso, E.L.; Liebert, A.; Kiat, H. Modifying the Microbiome as a Potential Mechanism of Photobiomodulation: A Case Report. Photobiomodul. Photomed. Laser Surg. 2022, 40, 88–97. [Google Scholar] [CrossRef]
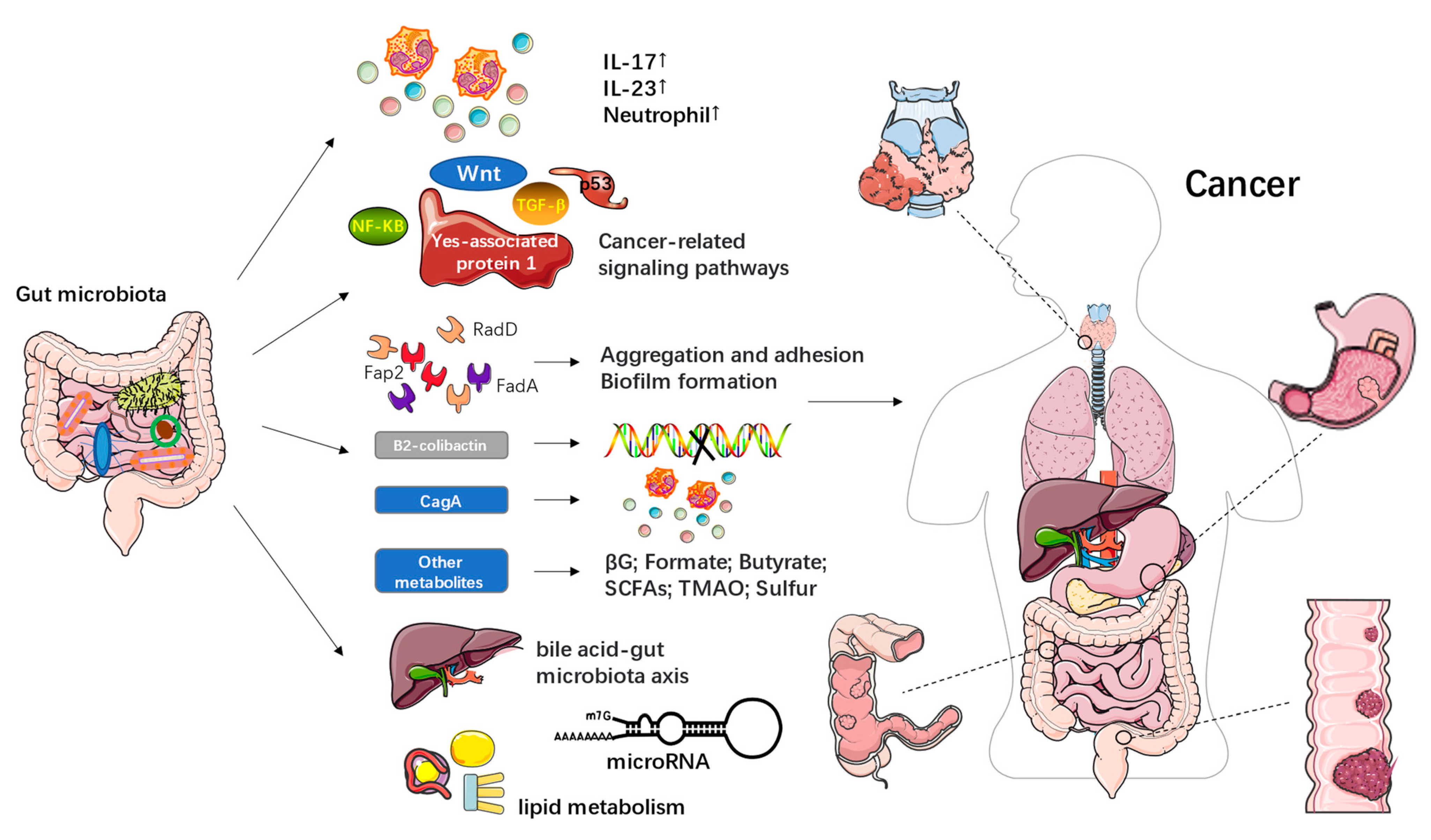

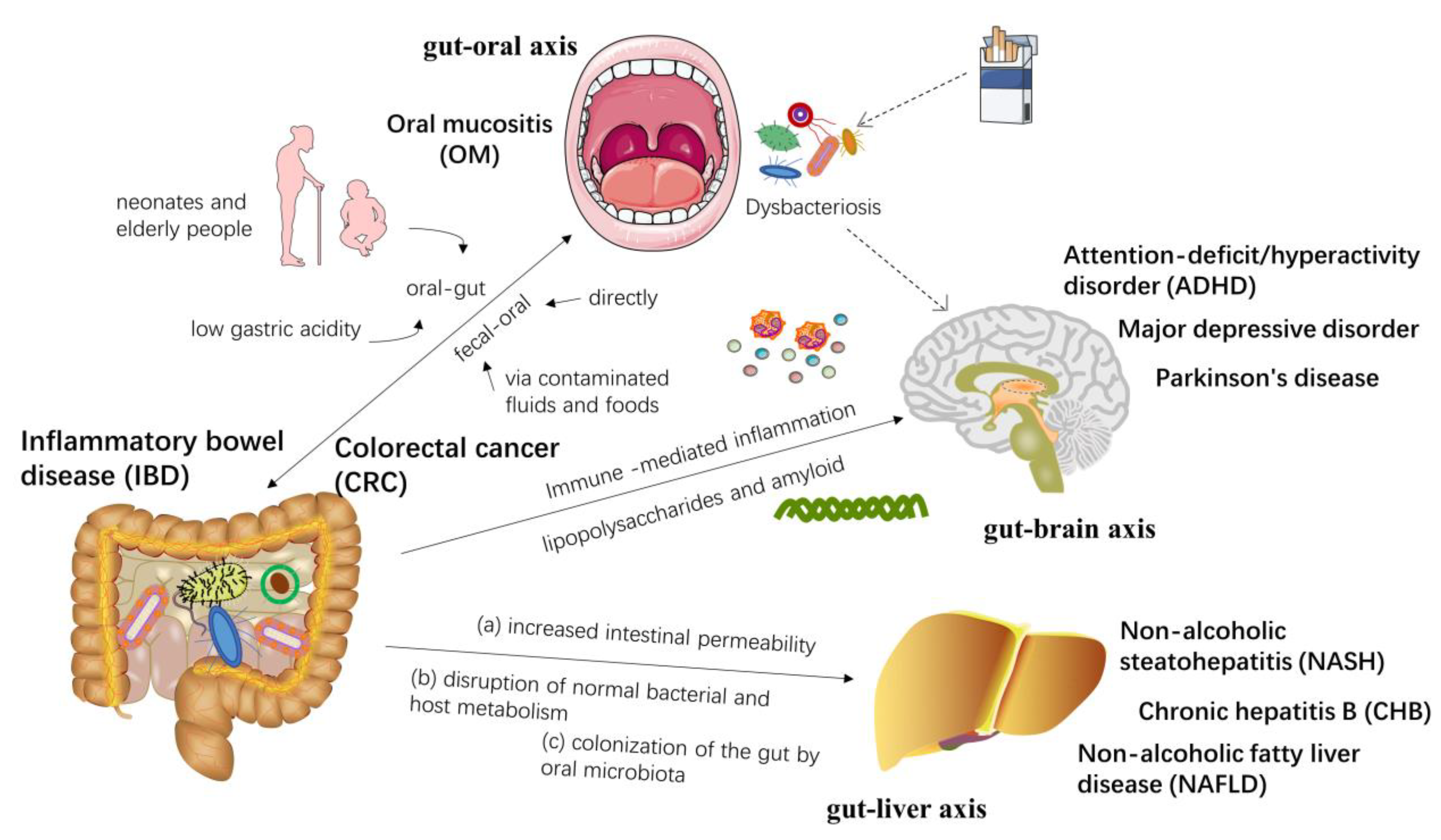
| Cancer | Object of Study | Sample Type | Method | Gut Microbiota Dysbacteriosis in Cancer Patients | Reference |
|---|---|---|---|---|---|
| colorectal cancer (CRC) | Brazilian CRC patients and a local control population | fecal samples | 16S rRNA gene Amplicon sequencing | The α-diversity increased significantly; Prevotella increased and Megamonas and Ruminococcus decreased. | [20] |
| gastric cancer (GC) | 45 GC cases from Shenyang, China. | cancer lesions and adjacent noncancerous tissues | ITS rDNA gene analysis | A significant increase of C. Albicans in GC; the abundance of Fusicolla acetilerea, Arcopilus aureus, and Fusicolla aquaeductuum were increased, while Candida glabrata, Aspergillus montevidensis, Saitozyma podzolica, and Penicillium arenicola were obviously decreased. | [21] |
| hepatocellular carcinoma (HCC) | 40 healthy volunteers and 143 HCC patients | fecal samples | 16S rRNA sequencing | Decreased α-diversity; a relatively lower average abundance of Bacteroidetes and a higher average abundance of Actinobacteria in the HCC group. | [22] |
| cervical cancer | 42 cervical cancer patients and 46 healthy female controls | stool samples | 16S rRNA gene sequencing | Higher alpha diversity in older women with cervical cancer; Prevotella, Porphyromonas, and Dialister were significantly enriched. | [23] |
| multiple myeloma (MM) | newly diagnosed patients with MM and healthy controls | fecal samples | deep metagenomic sequencing | Bacterial diversity was higher in MM; significantly enriched nitrogen-recycling bacteria in MM, such as Klebsiella and Streptococcus. | [24] |
| lung cancer (LC) | 41 LC patients and 40 healthy volunteers | Stool and serum samples | 16S rRNA gene sequencing and LC-MS analysis of serum samples | Halanaerobiaeota, Actinomyces, Veillonella, Megasphaera, Enterococcus, and Clostridioides were more abundant in the LC group. | [8] |
| Metabolites of Gut Microbiota | Effect in Cancer | Reference |
|---|---|---|
| Fusobacterial adhesins, including Fap2, RadD, and FadA | Promote Fn aggregation, adhesion to dysplastic tissues, and biofilm formation | [31] |
| B2-colicin produced by Escherichia coli | Induces DNA damage, and toxin-induced dsDNA breaks | [36,37] |
| CagA produced by Helicobacter pylori | Induces inflammatory pathways in gastric cancer; Involves in the tumorigenesis of CRC | [38] |
| Gut microbial β-glucuronidase (βG) | Promotes azoxymethane (AOM)-induced gut microbial dysbiosis and intestinal tumorigenesis | [39,40] |
| Formate | Drives CRC tumor invasion by triggering AhR signaling pathway; Increases cancer stem cell potency and promotes CRC development | [41] |
| Short-chain fatty acids (SCFAs), including butyrate, propionate and acetate | Link dietary patterns to gut microbiota; Butyrate has anticancer activity; Butyrate may promote carcinogenesis by increasing abnormal epithelial cells proliferation | [42,43,44,45] |
| Trimethylamine N-oxide (TMAO) | Associated with a variety of health outcomes, including pancreatic cancer, primary liver cancer, and prostate cancer | [46] |
| Microbial sulfur production | May be associated with colorectal cancer; Organic sulfur metabolism genes may be the most important contributors of H2S in the human gut | [47] |
| Beneficial Bacteria | Harmful Bacteria |
|---|---|
| Acidophilus Akkermansia muciniphila Bifidobacterium Bacteroides fragilis Bacteroides thetaiotaomicron Christensenella minuta Clostridium casei Faecalibacterium prausnitzii Lactobacillus acidophilus Prevotella copri Parabacteroides goldsteinii Saccharomycetes Turicibacter etc. | Acetothermia Cryptomycota Desulfovibrio Deferribacteres Enterococcus Fusobacterium Lachnospiraceae Porhyromonas Pseudomonas Peptococcaceae Proteobacteria Rikenellaceae_RC9_gut_group etc. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Ke, X.; Zuo, D.; Wang, Z.; Fang, F.; Li, B. New Insights into the Relationship between Gut Microbiota and Radiotherapy for Cancer. Nutrients 2023, 15, 48. https://doi.org/10.3390/nu15010048
Li Z, Ke X, Zuo D, Wang Z, Fang F, Li B. New Insights into the Relationship between Gut Microbiota and Radiotherapy for Cancer. Nutrients. 2023; 15(1):48. https://doi.org/10.3390/nu15010048
Chicago/Turabian StyleLi, Zhipeng, Xiyang Ke, Dan Zuo, Zhicheng Wang, Fang Fang, and Bo Li. 2023. "New Insights into the Relationship between Gut Microbiota and Radiotherapy for Cancer" Nutrients 15, no. 1: 48. https://doi.org/10.3390/nu15010048
APA StyleLi, Z., Ke, X., Zuo, D., Wang, Z., Fang, F., & Li, B. (2023). New Insights into the Relationship between Gut Microbiota and Radiotherapy for Cancer. Nutrients, 15(1), 48. https://doi.org/10.3390/nu15010048






