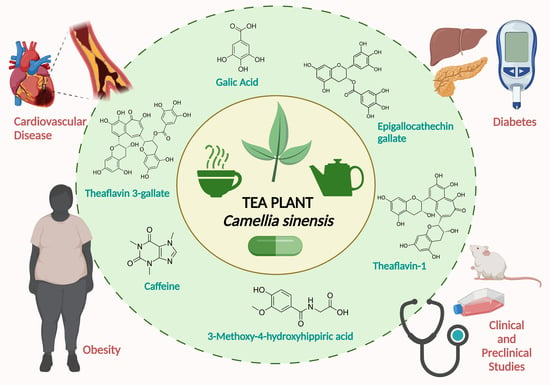
Tea Plant (Camellia sinensis): A Current Update on Use in Diabetes, Obesity, and Cardiovascular Disease
Abstract
1. Introduction
1.1. Tea Plant (Camellia sinensis) and Its Characteristics
1.2. Traditional and Modern Usage
1.2.1. Traditional Usage
1.2.2. Modern Usage
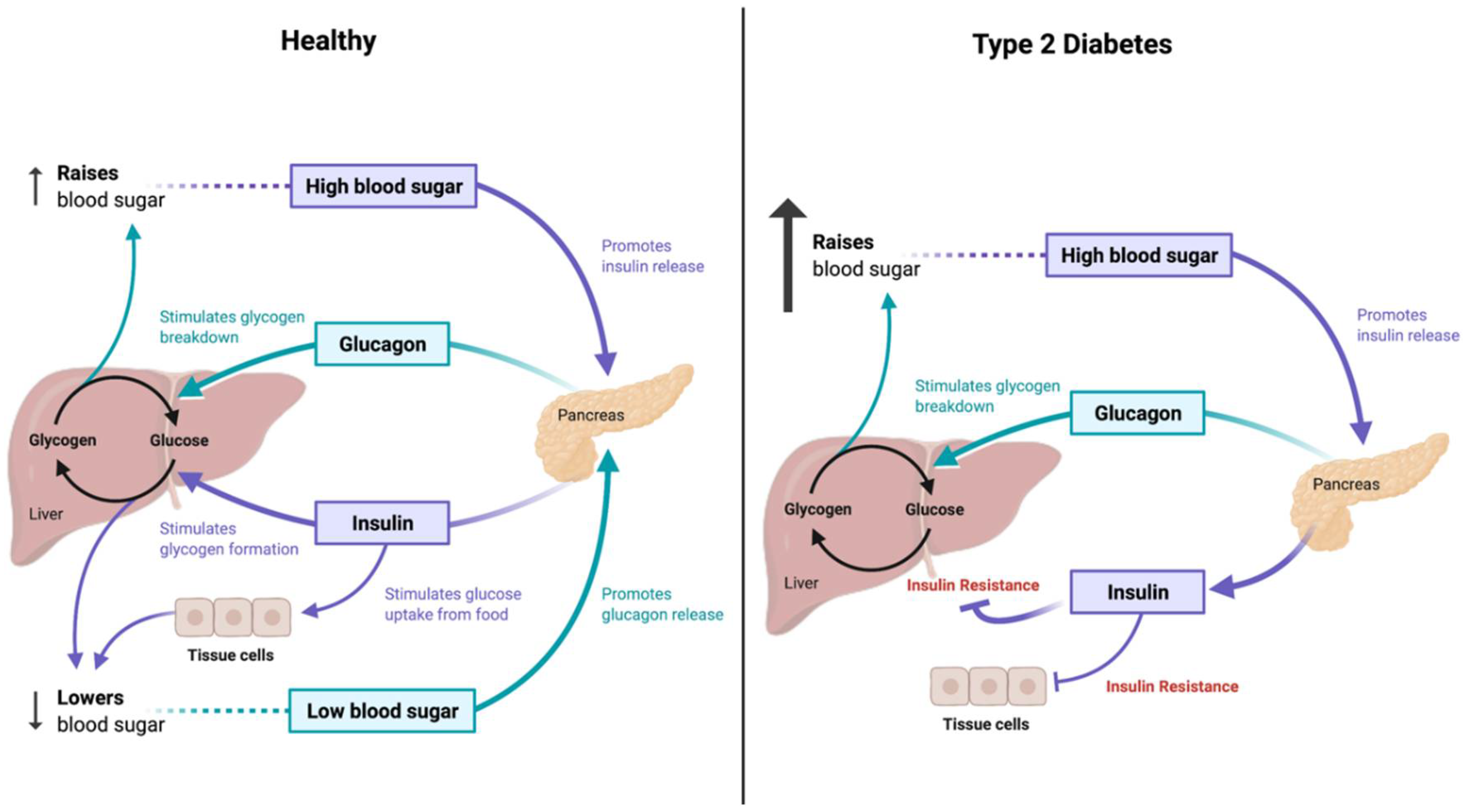
2. Role of Tea Plant and Related Compounds in Diabetes Mellitus
2.1. Tea Plant’s Anti-Diabetic Roles
2.2. Clinical Applications of Tea Plant for Treatment of Diabetes
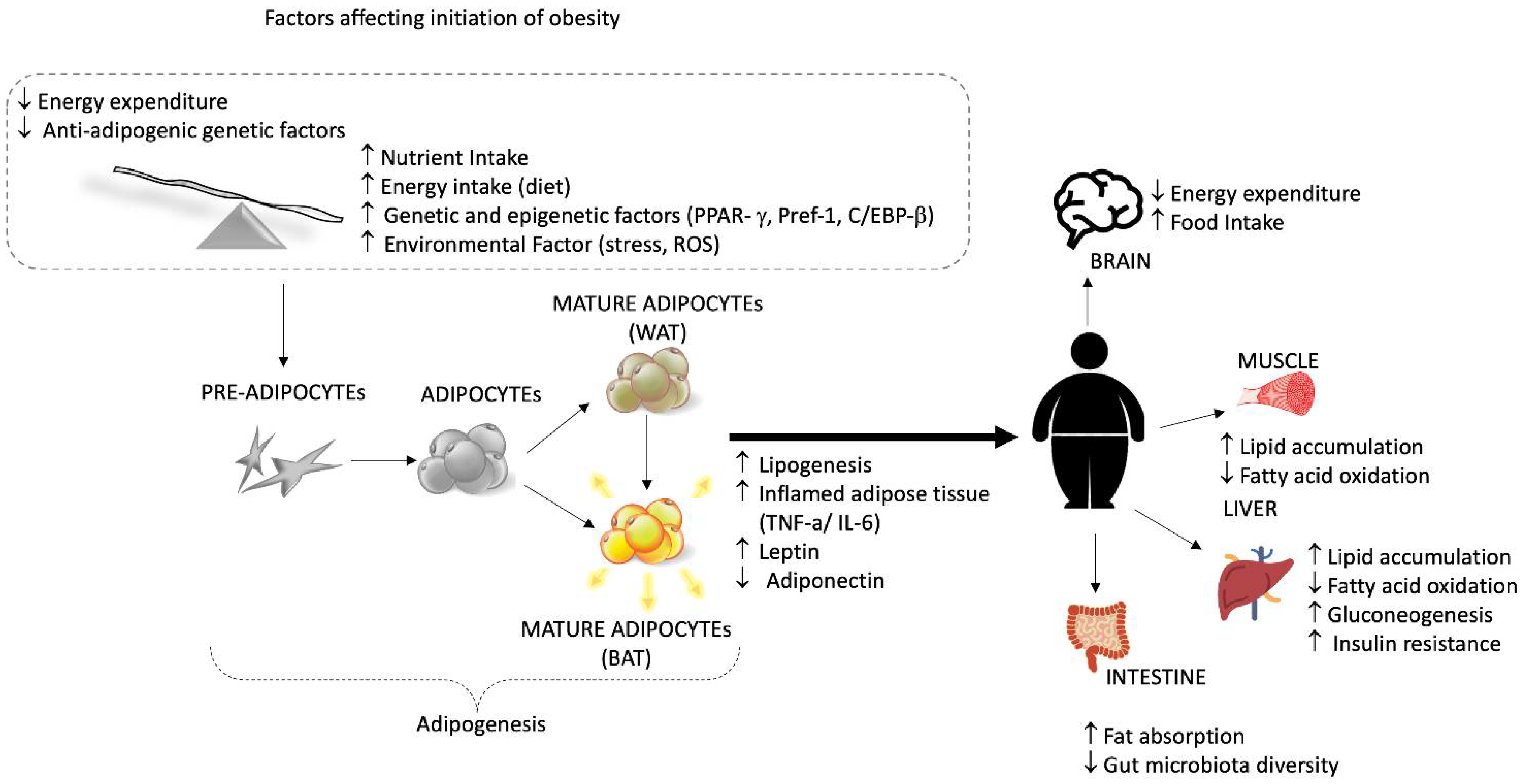
3. Role of Tea Plant and Related Compounds in Obesity
3.1. Anti-Obesity Mechanisms of Tea Plant
3.1.1. Action on Digestive Enzyme Inhibition
3.1.2. Action on Intestinal Microbiota
3.1.3. Action on Energy Regulation
3.1.4. Action on Adipose Tissue
3.2. Clinical Applications of Tea Plant for Treatment of Obesity
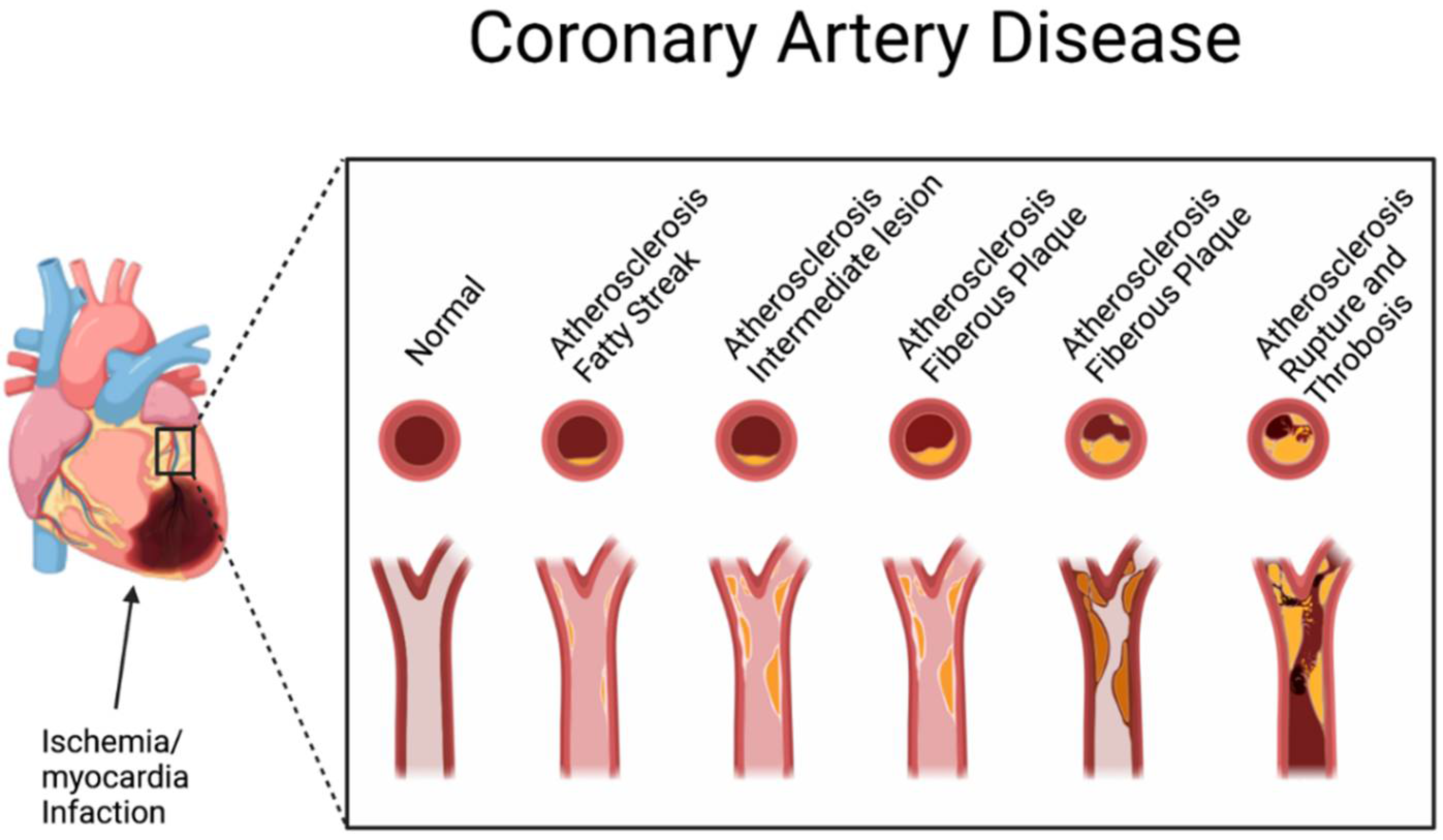
4. Role of Tea Plant and Related Compounds in Cardiovascular Disease
4.1. Anti-Cardiovascular Disease Mechanisms of Tea Plant
4.2. Clinical Applications of Tea Plant in Cardiovascular Disease
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Yang, R.; Li, Y.C.; Ni, X. The role of soil mineral multi-elements in improving the geographical origin discrimination of tea (Camellia sinensis). Biol. Trace Elem. Res. 2021, 199, 4330–4341. [Google Scholar] [CrossRef] [PubMed]
- Meegahakumbura, M.K.; Wambulwa, M.C.; Li, M.-M.; Thapa, K.K.; Sun, Y.-S.; Möller, M.; Xu, J.-C.; Yang, J.-B.; Liu, J.; Liu, B.-Y. Domestication origin and breeding history of the tea plant (Camellia sinensis) in China and India based on nuclear microsatellites and cpDNA sequence data. Front. Plant Sci. 2018, 8, 2270. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Aye, K.N. The legend of laphet: A Myanmar fermented tea leaf. J. Ethn. Foods 2015, 2, 173–178. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, M.; Wang, D.; Yu, F.; Zhang, N.; Song, C.; Granato, D. Analytical strategy coupled to chemometrics to differentiate Camellia sinensis tea types based on phenolic composition, alkaloids, and amino acids. J. Food Sci. 2020, 85, 3253–3263. [Google Scholar] [CrossRef]
- Wheeler, D.S.; Wheeler, W.J. The medicinal chemistry of tea. Drug Dev. Res. 2004, 61, 45–65. [Google Scholar] [CrossRef]
- Yang, C.S.; Chen, G.; Wu, Q. Recent scientific studies of a traditional Chinese medicine, tea, on prevention of chronic diseases. J. Tradit. Complement. Med. 2014, 4, 17–23. [Google Scholar] [CrossRef]
- Hayat, K.; Iqbal, H.; Malik, U.; Bilal, U.; Mushtaq, S. Tea and its consumption: Benefits and risks. Crit. Rev. Food Sci. Nutr. 2015, 55, 939–954. [Google Scholar] [CrossRef]
- Xia, X.; Wang, X.; Wang, H.; Lin, Z.; Shao, K.; Xu, J.; Zhao, Y. Ameliorative effect of white tea from 50-year-old tree of Camellia sinensis L. (Theaceae) on kidney damage in diabetic mice via SIRT1/AMPK pathway. J. Ethnopharmacol. 2021, 272, 113919. [Google Scholar] [CrossRef]
- Ferrara, L.; Montesano, D.; Senatore, A. The distribution of minerals and flavonoids in the tea plant (Camellia sinensis). Il Farm. 2001, 56, 397–401. [Google Scholar] [CrossRef]
- Chakravorty, S.; Bhattacharya, S.; Bhattacharya, D.; Sarkar, S.; Gachhui, R. 10—Kombucha: A promising functional beverage prepared from tea. In Non-Alcoholic Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 285–327. [Google Scholar]
- de Miranda, J.F.; Ruiz, L.F.; Silva, C.B.; Uekane, T.M.; Silva, K.A.; Gonzalez, A.G.M.; Fernandes, F.F.; Lima, A.R. Kombucha: A review of substrates, regulations, composition, and biological properties. J. Food Sci. 2022, 87, 503–527. [Google Scholar] [CrossRef]
- Chen, I.J.; Liu, C.Y.; Chiu, J.P.; Hsu, C.H. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2016, 35, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Janssens, P.L.H.R.; Hursel, R.; Westerterp-Plantenga, M.S. Long-term green tea extract supplementation does not affect fat absorption, resting energy expenditure, and body composition in adults. J. Nutr. 2015, 145, 864–870. [Google Scholar] [CrossRef]
- Koch, W.; Zagórska, J.; Marzec, Z.; Kukula-Koch, W. Applications of tea (Camellia sinensis) and its active constituents in cosmetics. Molecules 2019, 24, 4277. [Google Scholar] [CrossRef] [PubMed]
- Nibir, Y.M.; Sumit, A.F.; Akhand, A.A.; Ahsan, N.; Hossain, M.S. Comparative assessment of total polyphenols, antioxidant and antimicrobial activity of different tea varieties of Bangladesh. Asian Pac. J. Trop. Biomed. 2017, 7, 352–357. [Google Scholar] [CrossRef]
- Shang, A.; Li, J.; Zhou, D.-D.; Gan, R.-Y.; Li, H.-B. Molecular mechanisms underlying health benefits of tea compounds. Free Radic. Biol. Med. 2021, 172, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, M.; Takemoto, H. Synthesis of theaflavins and their functions. Molecules 2018, 23, 918. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, M.; Wu, T.; Dai, S.; Xu, J.; Zhou, Z. The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet. Food Funct. 2015, 6, 297–304. [Google Scholar] [CrossRef]
- Ramadan, G.; El-Beih, N.M.; Abd El-Ghffar, E.A. Modulatory effects of black v. green tea aqueous extract on hyperglycaemia, hyperlipidaemia and liver dysfunction in diabetic and obese rat models. Br. J. Nutr. 2009, 102, 1611–1619. [Google Scholar] [CrossRef]
- Wolfram, S.; Raederstorff, D.; Preller, M.; Wang, Y.; Teixeira, S.R.; Riegger, C.; Weber, P. Epigallocatechin gallate supplementation alleviates diabetes in rodents. J. Nutr. 2006, 136, 2512–2518. [Google Scholar] [CrossRef]
- Chung, M.; Zhao, N.; Wang, D.; Shams-White, M.; Karlsen, M.; Cassidy, A.; Ferruzzi, M.; Jacques, P.F.; Johnson, E.J.; Wallace, T.C. Dose–response relation between tea consumption and risk of cardiovascular disease and all-cause mortality: A systematic review and meta-analysis of population-based studies. Adv. Nutr. 2020, 11, 790–814. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, B.; Mao, G.; Fang, X.; Liu, Y.; Huang, Y.; Wang, N. Epigallocatechin-3-O-gallate, a green tea polyphenol, induces expression of Pim-1 kinase via PPARγ in human vascular endothelial cells. Cardiovasc. Toxicol. 2013, 13, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.S.; Chung, K.H.; Chung, J.H.; Lee, J.Y.; Park, J.B.; Zhang, Y.H.; Yoo, H.S.; Yun, Y.P. Antiplatelet activity of green tea catechins is mediated by inhibition of cytoplasmic calcium increase. J Cardiovasc Pharm. 2001, 38, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Q.; Wang, X.-B.; Guan, R.-F.; Tu, J.; Gong, Z.-H.; Zheng, N.; Yang, J.-H.; Zhang, Y.-Y.; Ying, M.-M. Blood anticoagulation and antiplatelet activity of green tea (−)-epigallocatechin (EGC) in mice. Food Funct. 2013, 4, 1521–1525. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Wang, W.; Ke, J.-P.; Zhang, P.; Chu, G.-X.; Bao, G.-H. Discovery of Camellia sinensis catechins as SARS-CoV-2 3CL protease inhibitors through molecular docking, intra and extra cellular assays. Phytomedicine 2022, 96, 153853. [Google Scholar] [CrossRef]
- Ishimoto, K.; Hatanaka, N.; Otani, S.; Maeda, S.; Xu, B.; Yasugi, M.; Moore, J.E.; Suzuki, M.; Nakagawa, S.; Yamasaki, S. Tea crude extracts effectively inactivate severe acute respiratory syndrome coronavirus 2. Lett. Appl. Microbiol. 2022, 74, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.-Q.; Tang, P.; Gao, T.-H.; Yang, C.-X.; Tan, L.; Yue, P.; Hua, Y.-N.; Liu, S.-J.; Guo, J.-L. Regulation of the intestinal flora: A potential mechanism of natural medicines in the treatment of type 2 diabetes mellitus. Biomed. Pharmacother. 2022, 151, 113091. [Google Scholar] [CrossRef]
- Patil, S.P. Atypical Diabetes and Management Considerations. Prim. Care Clin. Off. Pract. 2022, 49, 225–237. [Google Scholar] [CrossRef]
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997, 20, 1183–1197. [Google Scholar] [CrossRef]
- Knip, M.; Siljander, H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2016, 12, 154–167. [Google Scholar] [CrossRef]
- Zhao, L.; Lou, H.; Peng, Y.; Chen, S.; Fan, L.; Li, X. Elevated levels of circulating short-chain fatty acids and bile acids in type 2 diabetes are linked to gut barrier disruption and disordered gut microbiota. Diabetes Res. Clin. Pract. 2020, 169, 108418. [Google Scholar] [CrossRef]
- Singh, H.; Venkatesan, V. Safety and Performance of Continuous Glucose Monitoring: An Overview. Curr. Diabetes Rev. 2022. [Google Scholar] [CrossRef] [PubMed]
- Simmons, K.; Riddley, S. Diabetes and Technology. Prim. Care Clin. Off. Pract. 2022, 49, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Salimifar, M.; Fatehi-Hassanabad, Z.; Fatehi, M. A review on natural products for controlling type 2 diabetes with an emphasis on their mechanisms of actions. Curr. Diabetes Rev. 2013, 9, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Gushiken, L.F.; Beserra, F.P.; Rozza, A.L.; Bérgamo, P.L.; Bérgamo, D.A.; Pellizzon, C.H. Chemical and biological aspects of extracts from medicinal plants with antidiabetic effects. Rev. Diabet. Stud. RDS 2016, 13, 96. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, M.; Yang, J.; Xu, Q.; Liang, C.; Chen, B.; Zhang, J.; Yang, Y.; Wang, H.; Shang, Y. Response of gut microbiota in type 2 diabetes to hypoglycemic agents. Endocrine 2019, 66, 485–493. [Google Scholar] [CrossRef]
- Sundaram, R.; Naresh, R.; Shanthi, P.; Sachdanandam, P. Modulatory effect of green tea extract on hepatic key enzymes of glucose metabolism in streptozotocin and high fat diet induced diabetic rats. Phytomedicine 2013, 20, 577–584. [Google Scholar] [CrossRef]
- Banerjee, S.; Ghoshal, S.; Porter, T.D. Phosphorylation of hepatic AMP-activated protein kinase and liver kinase B1 is increased after a single oral dose of green tea extract to mice. Nutr. Res. 2012, 32, 985–990. [Google Scholar] [CrossRef]
- Wu, L.-Y.; Juan, C.-C.; Ho, L.-T.; Hsu, Y.-P.; Hwang, L.S. Effect of green tea supplementation on insulin sensitivity in Sprague− Dawley rats. J. Agric. Food Chem. 2004, 52, 643–648. [Google Scholar] [CrossRef]
- Ferreira, M.; Silva, D.; de Morais, A., Jr.; Mota, J.; Botelho, P. Therapeutic potential of green tea on risk factors for type 2 diabetes in obese adults–a review. Obes. Rev. 2016, 17, 1316–1328. [Google Scholar] [CrossRef]
- Park, J.-M.; Shin, Y.; Kim, S.H.; Jin, M.; Choi, J.J. Dietary epigallocatechin-3-gallate alters the gut microbiota of obese diabetic db/db mice: Lactobacillus is a putative target. J. Med. Food 2020, 23, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-Z.; Zhou, F.; Ouyang, J.; Wang, Q.-Y.; Li, Y.-L.; Wu, J.-L.; Huang, J.-A.; Liu, Z.-H. Combined use of epigallocatechin-3-gallate (EGCG) and caffeine in low doses exhibits marked anti-obesity synergy through regulation of gut microbiota and bile acid metabolism. Food Funct. 2021, 12, 4105–4116. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Hu, F.B.; Hu, G. Genes, environment, and interactions in prevention of type 2 diabetes: A focus on physical activity and lifestyle changes. Curr. Mol. Med. 2008, 8, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Wang, W.Y.; Fan, W.Y.; Deng, Q.; Wang, X. Tea consumption and risk of type 2 diabetes: A dose-response meta-analysis of cohort studies. Br. J. Nutr. 2014, 111, 1329–1339. [Google Scholar] [CrossRef]
- Asbaghi, O.; Fouladvand, F.; Gonzalez, M.J.; Aghamohammadi, V.; Choghakhori, R.; Abbasnezhad, A. Effect of Green Tea on Anthropometric Indices and Body Composition in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Complement. Ther. Med. 2021, 28, 244–251. [Google Scholar] [CrossRef]
- Asbaghi, O.; Fouladvand, F.; Moradi, S.; Ashtary-Larky, D.; Choghakhori, R.; Abbasnezhad, A. Effect of green tea extract on lipid profile in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2020, 14, 293–301. [Google Scholar] [CrossRef]
- Asbaghi, O.; Fouladvand, F.; Gonzalez, M.J.; Aghamohammadi, V.; Choghakhori, R.; Abbasnezhad, A. The effect of green tea on C-reactive protein and biomarkers of oxidative stress in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2019, 46, 210–216. [Google Scholar] [CrossRef]
- Silva, T.E.; Colombo, G.; Schiavon, L.L. Adiponectin: A multitasking player in the field of liver diseases. Diabetes Metab. 2014, 40, 95–107. [Google Scholar] [CrossRef]
- Yatagai, T.; Nagasaka, S.; Taniguchi, A.; Fukushima, M.; Nakamura, T.; Kuroe, A.; Nakai, Y.; Ishibashi, S. Hypoadiponectinemia is associated with visceral fat accumulation and insulin resistance in Japanese men with type 2 diabetes mellitus. Metabolism 2003, 52, 1274–1278. [Google Scholar] [CrossRef]
- Asbaghi, O.; Fouladvand, F.; Ashtary-Larky, D.; Bagheri, R.; Choghakhori, R.; Wong, A.; Baker, J.S.; Abbasnezhad, A. Effects of green tea supplementation on serum concentrations of adiponectin in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Arch. Physiol. Biochem. 2020, 1–8. [Google Scholar] [CrossRef]
- Liao, S.; Kao, Y.H.; Hiipakka, R.A. Green tea: Biochemical and biological basis for health benefits. Vitam. Horm. 2001, 62, 1–94. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Honda, M. The inhibition of α-amylase by tea polyphenols. Agric. Biol. Chem. 1990, 54, 1939–1945. [Google Scholar] [CrossRef]
- Honda, M.; Hara, Y. Inhibition of rat small intestinal sucrase and α-glucosidase activities by tea polyphenols. Biosci. Biotechnol. Biochem. 1993, 57, 123–124. [Google Scholar] [CrossRef]
- Murakami, S.; Muramatsu, M.; Otomo, S. Gastric H+, K+-ATPase inhibition by catechins. J. Pharm. Pharmacol. 1992, 44, 926–928. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Suzuki, M.; Satsu, H.; Arai, S.; Hara, Y.; Suzuki, K.; Miyamoto, Y.; Shimizu, M. Green tea polyphenols inhibit the sodium-dependent glucose transporter of intestinal epithelial cells by a competitive mechanism. J. Agric. Food Chem. 2000, 48, 5618–5623. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Fouladvand, F.; Gonzalez, M.J.; Ashtary-Larky, D.; Choghakhori, R.; Abbasnezhad, A. Effect of green tea on glycemic control in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2021, 15, 23–31. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Obesity Collaborators, Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017, 377, 13–27. [CrossRef]
- LeRoith, D.; Novosyadlyy, R.; Gallagher, E.J.; Lann, D.; Vijayakumar, A.; Yakar, S. Obesity and type 2 diabetes are associated with an increased risk of developing cancer and a worse prognosis; epidemiological and mechanistic evidence. Exp. Clin. Endocrinol. Diabetes 2008, 116 (Suppl. S1), S4–S6. [Google Scholar] [CrossRef]
- Blüher, M. Adipose tissue dysfunction in obesity. Exp. Clin. Endocrinol. Diabetes 2009, 117, 241–250. [Google Scholar] [CrossRef]
- Kannel, W.B.; Cupples, L.A.; Ramaswami, R.; Stokes, J., 3rd; Kreger, B.E.; Higgins, M. Regional obesity and risk of cardiovascular disease; the Framingham Study. J. Clin. Epidemiol. 1991, 44, 183–190. [Google Scholar] [CrossRef]
- Pedersen, S.D. Metabolic complications of obesity. Best. Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Clinical relevance of adipokines. Diabetes Metab. J. 2012, 36, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Dahlman, I.; Elsen, M.; Tennagels, N.; Korn, M.; Brockmann, B.; Sell, H.; Eckel, J.; Arner, P. Functional annotation of the human fat cell secretome. Arch. Physiol. Biochem. 2012, 118, 84–91. [Google Scholar] [CrossRef]
- Sell, H.; Habich, C.; Eckel, J. Adaptive immunity in obesity and insulin resistance. Nat. Rev. Endocrinol. 2012, 8, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.; Tan, T.; Bloom, S. Gut hormones and obesity: Physiology and therapies. Vitam. Horm. 2013, 91, 143–194. [Google Scholar] [CrossRef] [PubMed]
- Misra, M. Obesity pharmacotherapy: Current perspectives and future directions. Curr. Cardiol. Rev. 2013, 9, 33–54. [Google Scholar] [CrossRef] [PubMed]
- Pilitsi, E.; Farr, O.M.; Polyzos, S.A.; Perakakis, N.; Nolen-Doerr, E.; Papathanasiou, A.E.; Mantzoros, C.S. Pharmacotherapy of obesity: Available medications and drugs under investigation. Metabolism 2019, 92, 170–192. [Google Scholar] [CrossRef]
- Daneschvar, H.L.; Aronson, M.D.; Smetana, G.W. FDA-Approved Anti-Obesity Drugs in the United States. Am. J. Med. 2016, 129, 879.e871–876. [Google Scholar] [CrossRef]
- Li, M.F.; Cheung, B.M. Rise and fall of anti-obesity drugs. World J. Diabetes 2011, 2, 19–23. [Google Scholar] [CrossRef]
- Kang, J.G.; Park, C.Y. Anti-Obesity Drugs: A Review about Their Effects and Safety. Diabetes Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P. Anti-obesity drugs: A review about their effects and their safety. Expert Opin. Drug Saf. 2012, 11, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Vernarelli, J.A.; Lambert, J.D. Tea consumption is inversely associated with weight status and other markers for metabolic syndrome in US adults. Eur. J. Nutr. 2013, 52, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Glisan, S.L.; Grove, K.A.; Yennawar, N.H.; Lambert, J.D. Inhibition of pancreatic lipase by black tea theaflavins: Comparative enzymology and in silico modeling studies. Food Chem. 2017, 216, 296–300. [Google Scholar] [CrossRef] [PubMed]
- He, R.R.; Chen, L.; Lin, B.H.; Matsui, Y.; Yao, X.S.; Kurihara, H. Beneficial effects of oolong tea consumption on diet-induced overweight and obese subjects. Chin. J. Integr. Med. 2009, 15, 34–41. [Google Scholar] [CrossRef]
- Li, H.; Kek, H.C.; Lim, J.; Gelling, R.W.; Han, W. Green tea (-)-epigallocatechin-3-gallate counteracts daytime overeating induced by high-fat diet in mice. Mol. Nutr. Food Res. 2016, 60, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.; Gao, Y.; Zhang, X.; Sun, Y.; Hu, B.; Zhou, L.; Jabbar, S.; Zeng, X. Effects of Oolong tea polyphenols, EGCG, and EGCG3″Me on pancreatic α-amylase activity in vitro. J. Agric. Food Chem. 2014, 62, 9507–9514. [Google Scholar] [CrossRef]
- Satoh, T.; Igarashi, M.; Yamada, S.; Takahashi, N.; Watanabe, K. Inhibitory effect of black tea and its combination with acarbose on small intestinal α-glucosidase activity. J. Ethnopharmacol. 2015, 161, 147–155. [Google Scholar] [CrossRef]
- Nishiumi, S.; Bessyo, H.; Kubo, M.; Aoki, Y.; Tanaka, A.; Yoshida, K.; Ashida, H. Green and black tea suppress hyperglycemia and insulin resistance by retaining the expression of glucose transporter 4 in muscle of high-fat diet-fed C57BL/6J mice. J. Agric. Food Chem. 2010, 58, 12916–12923. [Google Scholar] [CrossRef]
- Ding, Y.; Zou, X.; Jiang, X.; Wu, J.; Zhang, Y.; Chen, D.; Liang, B. Pu-erh tea down-regulates sterol regulatory element-binding protein and stearyol-CoA desaturase to reduce fat storage in Caenorhaditis elegans. PLoS ONE 2015, 10, e0113815. [Google Scholar] [CrossRef]
- Seo, D.B.; Jeong, H.W.; Cho, D.; Lee, B.J.; Lee, J.H.; Choi, J.Y.; Bae, I.H.; Lee, S.J. Fermented green tea extract alleviates obesity and related complications and alters gut microbiota composition in diet-induced obese mice. J. Med. Food 2015, 18, 549–556. [Google Scholar] [CrossRef]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Dai, Z.; Ye, H.; Hu, B.; Zeng, X.; Liu, Z. Fuzhuan Brick Tea Polysaccharides Attenuate Metabolic Syndrome in High-Fat Diet Induced Mice in Association with Modulation in the Gut Microbiota. J. Agric. Food Chem. 2018, 66, 2783–2795. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cheng, M.; Zhang, X.; Cao, J.; Wu, Z.; Weng, P. Green tea polyphenols reduce obesity in high-fat diet-induced mice by modulating intestinal microbiota composition. Int. J. Food Sci. Technol. 2017, 52, 1723–1730. [Google Scholar] [CrossRef]
- Henning, S.M.; Yang, J.; Hsu, M.; Lee, R.P.; Grojean, E.M.; Ly, A.; Tseng, C.H.; Heber, D.; Li, Z. Decaffeinated green and black tea polyphenols decrease weight gain and alter microbiome populations and function in diet-induced obese mice. Eur. J. Nutr. 2018, 57, 2759–2769. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Lu, H.F.; Chen, J.C.; Huang, H.C.; Chen, Y.H.; Su, Y.S.; Tung, C.Y.; Huang, C. Purple-leaf tea (Camellia sinensis L.) ameliorates high-fat diet induced obesity and metabolic disorder through the modulation of the gut microbiota in mice. BMC Complement. Med. Ther. 2020, 20, 376. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, J.; Zhang, N.; Fu, Y.; Zhang, Z.; Li, Y.; Wang, W.; Li, Y.; Shen, P.; Cao, Y. Ripened Pu-erh Tea Extract Protects Mice from Obesity by Modulating Gut Microbiota Composition. J. Agric. Food Chem. 2019, 67, 6978–6994. [Google Scholar] [CrossRef]
- Yuan, X.; Long, Y.; Ji, Z.; Gao, J.; Fu, T.; Yan, M.; Zhang, L.; Su, H.; Zhang, W.; Wen, X.; et al. Green Tea Liquid Consumption Alters the Human Intestinal and Oral Microbiome. Mol. Nutr. Food Res. 2018, 62, e1800178. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.S.; Touyama, M.; Hisada, T.; Benno, Y. Effects of green tea consumption on human fecal microbiota with special reference to Bifidobacterium species. Microbiol. Immunol. 2012, 56, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zeng, B.; Liu, Z.; Liao, Z.; Zhong, Q.; Gu, L.; Wei, H.; Fang, X. Green Tea Polyphenols Modulate Colonic Microbiota Diversity and Lipid Metabolism in High-Fat Diet Treated HFA Mice. J. Food Sci. 2018, 83, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Marlatt, K.L.; Ravussin, E. Brown Adipose Tissue: An Update on Recent Findings. Curr. Obes. Rep. 2017, 6, 389–396. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006, 444, 847–853. [Google Scholar] [CrossRef]
- Choo, J.J. Green tea reduces body fat accretion caused by high-fat diet in rats through beta-adrenoceptor activation of thermogenesis in brown adipose tissue. J. Nutr. Biochem. 2003, 14, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, A.; Kiefer, F.W. Brown adipose tissue and thermogenesis. Horm. Mol. Biol. Clin. Investig. 2014, 19, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-Activated Protein Kinase (AMPK) Regulates Energy Metabolism through Modulating Thermogenesis in Adipose Tissue. Front. Physiol. 2018, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Wang, L.; Wang, L.; Tanaka, Y.; Zhang, T.; Ashida, H. Oolong, black and pu-erh tea suppresses adiposity in mice via activation of AMP-activated protein kinase. Food Funct. 2014, 5, 2420–2429. [Google Scholar] [CrossRef] [PubMed]
- Yuan, E.; Duan, X.; Xiang, L.; Ren, J.; Lai, X.; Li, Q.; Sun, L.; Sun, S. Aged Oolong Tea Reduces High-Fat Diet-Induced Fat Accumulation and Dyslipidemia by Regulating the AMPK/ACC Signaling Pathway. Nutrients 2018, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, M.; Matsuda, M.; Maeda, N.; Funahashi, T.; Matsuzawa, Y.; Makishima, M.; Shimomura, I. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 2003, 52, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Ye, X.; Zhang, R.; Long, J.; Ren, W.; Ding, S.; Liao, D.; Jin, X.; Wu, H.; Xu, S.; et al. Green tea polyphenols reduced fat deposits in high fat-fed rats via erk1/2-PPARgamma-adiponectin pathway. PLoS ONE 2013, 8, e53796. [Google Scholar] [CrossRef]
- Chen, N.; Bezzina, R.; Hinch, E.; Lewandowski, P.A.; Cameron-Smith, D.; Mathai, M.L.; Jois, M.; Sinclair, A.J.; Begg, D.P.; Wark, J.D.; et al. Green tea, black tea, and epigallocatechin modify body composition, improve glucose tolerance, and differentially alter metabolic gene expression in rats fed a high-fat diet. Nutr. Res. 2009, 29, 784–793. [Google Scholar] [CrossRef]
- Chen, Y.-K.; Cheung, C.; Reuhl, K.R.; Liu, A.B.; Lee, M.-J.; Lu, Y.-P.; Yang, C.S. Effects of Green Tea Polyphenol (−)-Epigallocatechin-3-gallate on Newly Developed High-Fat/Western-Style Diet-Induced Obesity and Metabolic Syndrome in Mice. J. Agric. Food Chem. 2011, 59, 11862–11871. [Google Scholar] [CrossRef]
- Yoshitomi, R.; Yamamoto, M.; Kumazoe, M.; Fujimura, Y.; Yonekura, M.; Shimamoto, Y.; Nakasone, A.; Kondo, S.; Hattori, H.; Haseda, A.; et al. The combined effect of green tea and alpha-glucosyl hesperidin in preventing obesity: A randomized placebo-controlled clinical trial. Sci. Rep. 2021, 11, 19067. [Google Scholar] [CrossRef]
- Dostal, A.M.; Samavat, H.; Espejo, L.; Arikawa, A.Y.; Stendell-Hollis, N.R.; Kurzer, M.S. Green Tea Extract and Catechol-O-Methyltransferase Genotype Modify Fasting Serum Insulin and Plasma Adiponectin Concentrations in a Randomized Controlled Trial of Overweight and Obese Postmenopausal Women. J. Nutr. 2016, 146, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Auvichayapat, P.; Prapochanung, M.; Tunkamnerdthai, O.; Sripanidkulchai, B.O.; Auvichayapat, N.; Thinkhamrop, B.; Kunhasura, S.; Wongpratoom, S.; Sinawat, S.; Hongprapas, P. Effectiveness of green tea on weight reduction in obese Thais: A randomized, controlled trial. Physiol. Behav. 2008, 93, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Tsai, T.H.; Kao, Y.H.; Hwang, K.C.; Tseng, T.Y.; Chou, P. Effect of green tea extract on obese women: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2008, 27, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Rashidlamir, A.; Ashtary-Larky, D.; Wong, A.; Grubbs, B.; Motevalli, M.S.; Baker, J.S.; Laher, I.; Zouhal, H. Effects of green tea extract supplementation and endurance training on irisin, pro-inflammatory cytokines, and adiponectin concentrations in overweight middle-aged men. Eur. J. Appl. Physiol. 2020, 120, 915–923. [Google Scholar] [CrossRef]
- Brown, A.L.; Lane, J.; Holyoak, C.; Nicol, B.; Mayes, A.E.; Dadd, T. Health effects of green tea catechins in overweight and obese men: A randomised controlled cross-over trial. Br. J. Nutr. 2011, 106, 1880–1889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, Y.; Li, X.; Meguro, S.; Hayashi, S.; Katashima, M.; Yasumasu, T.; Wang, J.; Li, K. Effects of catechin-enriched green tea beverage on visceral fat loss in adults with a high proportion of visceral fat: A double-blind, placebo-controlled, randomized trial. J. Funct. Foods 2012, 4, 315–322. [Google Scholar] [CrossRef]
- Mielgo-Ayuso, J.; Barrenechea, L.; Alcorta, P.; Larrarte, E.; Margareto, J.; Labayen, I. Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: Randomised, double-blind, placebo-controlled clinical trial. Br. J. Nutr. 2014, 111, 1263–1271. [Google Scholar] [CrossRef]
- Roberts, J.D.; Willmott, A.G.B.; Beasley, L.; Boal, M.; Davies, R.; Martin, L.; Chichger, H.; Gautam, L.; Del Coso, J. The Impact of Decaffeinated Green Tea Extract on Fat Oxidation, Body Composition and Cardio-Metabolic Health in Overweight, Recreationally Active Individuals. Nutrients 2021, 13, 764. [Google Scholar] [CrossRef]
- Huang, L.H.; Liu, C.Y.; Wang, L.Y.; Huang, C.J.; Hsu, C.H. Effects of green tea extract on overweight and obese women with high levels of low density-lipoprotein-cholesterol (LDL-C): A randomised, double-blind, and cross-over placebo-controlled clinical trial. BMC Complement. Altern Med. 2018, 18, 294. [Google Scholar] [CrossRef]
- Chatree, S.; Sitticharoon, C.; Maikaew, P.; Pongwattanapakin, K.; Keadkraichaiwat, I.; Churintaraphan, M.; Sripong, C.; Sririwichitchai, R.; Tapechum, S. Epigallocatechin gallate decreases plasma triglyceride, blood pressure, and serum kisspeptin in obese human subjects. Exp. Biol. Med. 2021, 246, 163–176. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Ferdinand, K.C. Coronary artery disease in minority racial and ethnic groups in the United States. Am. J. Cardiol. 2006, 97, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Opoku-Acheampong, A.A.; Rosenkranz, R.R.; Adhikari, K.; Muturi, N.; Logan, C.; Kidd, T. Tools for Assessing Cardiovascular Disease Risk Factors in Underserved Young Adult Populations: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 13305. [Google Scholar] [CrossRef] [PubMed]
- Kumanyika, S.K.; Obarzanek, E.; Stettler, N.; Bell, R.; Field, A.E.; Fortmann, S.P.; Franklin, B.A.; Gillman, M.W.; Lewis, C.E.; Poston, W.C. Population-based prevention of obesity: The need for comprehensive promotion of healthful eating, physical activity, and energy balance: A scientific statement from American Heart Association Council on Epidemiology and Prevention, Interdisciplinary Committee for Prevention (formerly the expert panel on population and prevention science). Circulation 2008, 118, 428–464. [Google Scholar] [PubMed]
- Sandmaier, M. Healthy Heart Handbook for Women; National Heart, Lung, & Blood Institute: Bethesda, ML, USA, 2005.
- World Health Organization. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 15 October 2022).
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The Role of Oxidative Stress in Cardiac Disease: From Physiological Response to Injury Factor. Oxid. Med. Cell. Longev. 2020, 2020, 5732956. [Google Scholar] [CrossRef] [PubMed]
- Urbanik, D.; Martynowicz, H.; Mazur, G.; Poręba, R.; Gać, P. Environmental Factors as Modulators of the Relationship between Obstructive Sleep Apnea and Lesions in the Circulatory System. J. Clin. Med. 2020, 9, 836. [Google Scholar] [CrossRef]
- Tietjens, J.R.; Claman, D.; Kezirian, E.J.; De Marco, T.; Mirzayan, A.; Sadroonri, B.; Goldberg, A.N.; Long, C.; Gerstenfeld, E.P.; Yeghiazarians, Y. Obstructive Sleep Apnea in Cardiovascular Disease: A Review of the Literature and Proposed Multidisciplinary Clinical Management Strategy. J. Am. Heart Assoc. 2019, 8, e010440. [Google Scholar] [CrossRef]
- Stevens, D.; Lane, D.A.; Harrison, S.L.; Lip, G.Y.H.; Kolamunnage-Dona, R. Modelling of longitudinal data to predict cardiovascular disease risk: A methodological review. BMC Med. Res. Methodol. 2021, 21, 283. [Google Scholar] [CrossRef]
- D’Agostino, R.B., Sr.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Coupland, C.; Brindle, P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: Prospective cohort study. BMJ 2017, 357, j2099. [Google Scholar] [CrossRef]
- Ranaware, A.M.; Banik, K.; Deshpande, V.; Padmavathi, G.; Roy, N.K.; Sethi, G.; Fan, L.; Kumar, A.P.; Kunnumakkara, A.B. Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer. Int. J. Mol. Sci. 2018, 19, 2362. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.P.; Girisa, S.; Banik, K.; Ghosh, S.; Swathi, P.; Deka, M.; Padmavathi, G.; Kotoky, J.; Sethi, G.; Fan, L.; et al. Potential application of zerumbone in the prevention and therapy of chronic human diseases. J. Funct. Foods 2019, 53, 248–258. [Google Scholar] [CrossRef]
- Daimary, U.D.; Parama, D.; Rana, V.; Banik, K.; Kumar, A.; Harsha, C.; Kunnumakkara, A.B. Emerging roles of cardamonin, a multitargeted nutraceutical in the prevention and treatment of chronic diseases. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100008. [Google Scholar] [CrossRef] [PubMed]
- Henamayee, S.; Banik, K.; Sailo, B.L.; Shabnam, B.; Harsha, C.; Srilakshmi, S.; Vgm, N.; Baek, S.H.; Ahn, K.S.; Kunnumakkara, A.B. Therapeutic Emergence of Rhein as a Potential Anticancer Drug: A Review of Its Molecular Targets and Anticancer Properties. Molecules 2020, 25, 2278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shen, D.; Feng, Y.; Li, Y.; Liao, H. Pharmacological Actions, Molecular Mechanisms, Pharmacokinetic Progressions, and Clinical Applications of Hydroxysafflor Yellow A in Antidiabetic Research. J. Immunol. Res. 2021, 2021, 4560012. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.; Mondal, A.; Majumder, A.; Banik, A. Tea and its phytochemicals: Hidden health benefits & modulation of signaling cascade by phytochemicals. Food Chem. 2022, 371, 131098. [Google Scholar] [CrossRef]
- Wang, D.D.; Cassidy, A.; Ferruzzi, M.G.; Jacques, P.; Johnson, E.; Zhao, N.; Shams-White, M.; Karlsen, M.; Wallace, T.C.; Chung, M. Tea flavonoids and risk of cardiovascular and all-cause mortality: A systematic review and meta-analysis. Proc. Nutr. Soc. 2020, 79, 495–502. [Google Scholar] [CrossRef]
- Chieng, D.; Kistler, P.M. Coffee and tea on cardiovascular disease (CVD) prevention. Trends Cardiovasc. Med. 2021, 32, 399–405. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, Y.Y.; Wei, X.; Yu, F.F.; Zhou, Y.H.; He, J. Tea consumption and risk of cardiovascular outcomes and total mortality: A systematic review and meta-analysis of prospective observational studies. Eur. J. Epidemiol. 2015, 30, 103–113. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacol. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- da Silva Pinto, M. Tea: A new perspective on health benefits. Food Res. Int. 2013, 53, 558–567. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Nyambuya, T.M.; Orlando, P.; Silvestri, S.; Marcheggiani, F.; Cirilli, I.; Ziqubu, K.; Ndevahoma, F.; et al. Tea consumption and its effects on primary and secondary prevention of coronary artery disease: Qualitative synthesis of evidence from randomized controlled trials. Clin. Nutr. ESPEN 2021, 41, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S. Potential Bioactive Components and Health Promotional Benefits of Tea (Camellia sinensis). J. Am. Nutr. Assoc. 2022, 41, 65–93. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahdi, Z.K.A.; Ewadh, R.M.; Hindi, N.K.K. Health Benefits of Aqueous Extract of Black and Green Tea Leaves. In Bioactive Compounds in Nutraceutical and Functional Food for Good Human Health; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Keller, A.; Wallace, T.C. Tea intake and cardiovascular disease: An umbrella review. Ann. Med. 2021, 53, 929–944. [Google Scholar] [CrossRef] [PubMed]
- Gaeini, Z.; Bahadoran, Z.; Mirmiran, P.; Azizi, F. Tea, coffee, caffeine intake and the risk of cardio-metabolic outcomes: Findings from a population with low coffee and high tea consumption. Nutr. Metab. 2019, 16, 28. [Google Scholar] [CrossRef]
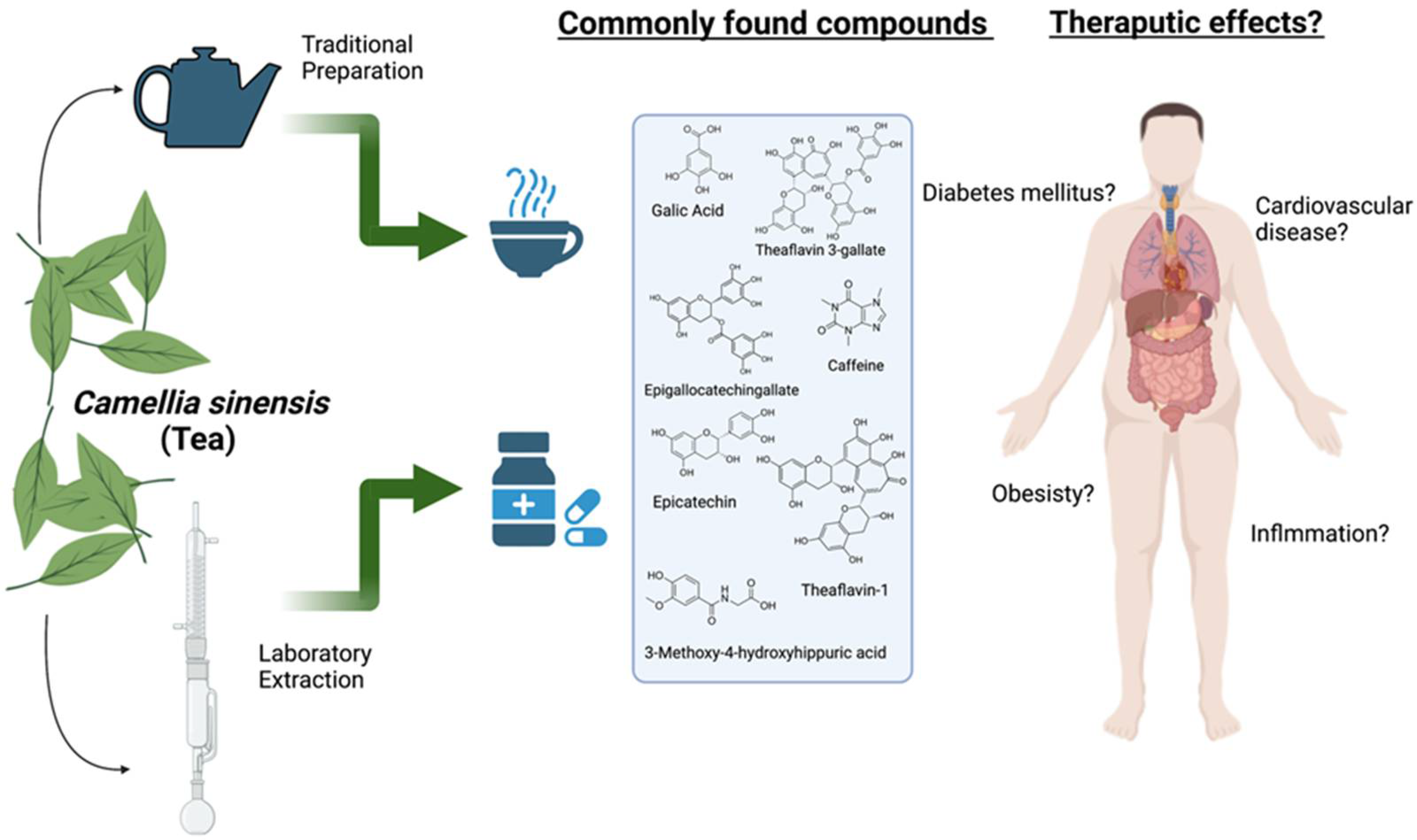
| Treatment | Dose of Tea | The Objective of the Study | Initial BMI of Subjects | Number of Participants | The Outcome of the Study | Ref |
|---|---|---|---|---|---|---|
| High dose green tea extract | 856.8 mg of EGCG for 12 weeks | lipid profile and obesity-related hormone | >27 kg/m2 | 115 women | Body weight ↓ BMI ↓ Total cholesterol ↓ LDL plasma levels ↓ Adiponectin↑ | [12] |
| Green tea along with a-glucosyl hesperidin | 146mg EGCG for 12 weeks | Anti-obesity effect | ≥25 kg/m2 | 60 healthy Japanese males and females aged between 30–75 years | Bodyweight ↓ Triglycerides ↓ Body fat percentage ↓ Visceral fat ↓ BMI ↓ LDL/HDL ratio ↓ | [101] |
| Minnesota decaffeinated green tea | 843 mg of EGCG for 12 months | Obesity-associated hormones, glucose homeostasis | ≥25 kg/m2 | 937 postmenopausal women | Non-significant relation of decaffeinated green tea with a reduction in body weight and BMI and no alteration in mean hormone concentration or energy intake. | [102] |
| Green tea as a dietary supplement in Thais | 250 mg capsule of green tea capsules from Herbal One Co., Ltd. for 12 weeks | Effect on weight reduction and associated metabolic risks | >25 kg/m2 | 60 overweight subjects aged 40–65 years | Weight loss ↓ Energy expenditure ↑ Fat oxidation ↑ Leptin ↓ | [103] |
| Green tea extract | 400 mg of green tea extract (491mg catechin containing 302 mg of EGCG) three times a day for 12 weeks. | To study the obesity-related hormonal peptides | >27 kg/m2 | 78 obese women aged between 16–60 years | Weight loss ↓ (non-significant compared to placebo) BMI ↓ (non-significant compared to placebo) Triglyceride ↓ LDL-cholesterol ↓ Adiponectin ↑ Ghrelin ↑ | [104] |
| Green tea supplement along with endurance training | 500 mg of green tea extract capsule (45% EGCG) once a day for 8 weeks. | Anti-inflammatory cytokines and adiponectin | >25 kg/m2 | 45 overweight men, aged 40–50 years | Bodyweight ↓ BMI ↓ Body fat percentage (BFP) ↓ Visceral fat area (VFA) ↓ IL-6 ↓ TNF-a (non-significant change) hs-CRP ↓ Adiponectin ↑ | [105] |
| Decaffeinated green tea extract | 530 mg of tea extract (containing 400 mg of catechin) twice daily for the following 6 weeks. | Ambulatory pressure and other metabolic markers | ≥28 and ≤38 kg/m2 | 137 males, aged 49–60 years. | Body weight ↓ LDL ↓ | [106] |
| Catechin- enriched green tea | 609.3 mg catechin and 68.7 mg caffeine for 12 weeks | Effect on visceral fat | <24 to ≥40 kg/m2 | 118 subjects aged 20–65 years | Visceral fat ↓ Average visceral fat area ↓ Bodyweight ↓ Body fat ↓ | [107] |
| Green tea EGCG | 300 mg of EGCG for 12 weeks | Body composition related to obesity, markers for liver functional enzymes, and cardiometabolic risk factors | >30 to <40 kg/m2 | 38 obese premenopausal women | - Non-significant changes in all the factors in the treatment group compared to placebo in Bodyweight ↓ Energy ↓ Fat ↓ Total cholesterol ↓ LDL-cholesterol ↓ | [108] |
| Decaffeinated green tea | Green tea extract (400 mg EGCG and a-lipoic acid) for 8 weeks | fat oxidation, cardio health, and body composition | 25.0 to 29.9 kg/m2 | 27 obese subjects with regular physical activity | Fat oxidation ↑ Energy expenditure ↑ LDL-cholesterol ↓ Non-significant changes in cardio-metabolic indexes | [109] |
| Green tea | 856.8 mg EGCG, 236.1 mg ECG, 115.5 mg for 6 weeks EGC) | Effect on low-density lipoprotein-cholesterol (LDL-c) | ≥ 27 kg/m2 | 73 overweight women aged 18–65 years | LDL-cholesterol ↓ Leptin ↑ Non-significant changes in other markers related to obesity | [110] |
| Oolong tea | Oral uptake of 2 g of tea 4 times a day for 6 weeks | Effect on diet-induced obesity | N/A | 102 obese subjects, aged 35–65 years | Bodyweight ↓ (70% > 1kg; 22% of subjects >3 kgs) Subcutaneous fat ↓ Plasma triglyceride ↓ Total cholesterol ↓ | [75] |
| Green tea extract | EGCG at a dose of 150 mg twice a day for 8 weeks | EGCG on obesity, lipolysis, and browning of human white adipocytes | ≥25 kg/m2 | 30 Thai obese subjects aged above 18 years | Plasma triglyceride levels ↓ No significant changes in- BMI, Bodyweight, Lipolysis, Uncoupling protein1 (UCP1), PPAR-γ agonist genes (hence no effect on browning effect) | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brimson, J.M.; Prasanth, M.I.; Kumaree, K.K.; Thitilertdecha, P.; Malar, D.S.; Tencomnao, T.; Prasansuklab, A. Tea Plant (Camellia sinensis): A Current Update on Use in Diabetes, Obesity, and Cardiovascular Disease. Nutrients 2023, 15, 37. https://doi.org/10.3390/nu15010037
Brimson JM, Prasanth MI, Kumaree KK, Thitilertdecha P, Malar DS, Tencomnao T, Prasansuklab A. Tea Plant (Camellia sinensis): A Current Update on Use in Diabetes, Obesity, and Cardiovascular Disease. Nutrients. 2023; 15(1):37. https://doi.org/10.3390/nu15010037
Chicago/Turabian StyleBrimson, James Michael, Mani Iyer Prasanth, Kishoree Krishna Kumaree, Premrutai Thitilertdecha, Dicson Sheeja Malar, Tewin Tencomnao, and Anchalee Prasansuklab. 2023. "Tea Plant (Camellia sinensis): A Current Update on Use in Diabetes, Obesity, and Cardiovascular Disease" Nutrients 15, no. 1: 37. https://doi.org/10.3390/nu15010037
APA StyleBrimson, J. M., Prasanth, M. I., Kumaree, K. K., Thitilertdecha, P., Malar, D. S., Tencomnao, T., & Prasansuklab, A. (2023). Tea Plant (Camellia sinensis): A Current Update on Use in Diabetes, Obesity, and Cardiovascular Disease. Nutrients, 15(1), 37. https://doi.org/10.3390/nu15010037