Glucagon-like Peptide 1 Receptor Activation Inhibits Microglial Pyroptosis via Promoting Mitophagy to Alleviate Depression-like Behaviors in Diabetic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Animals
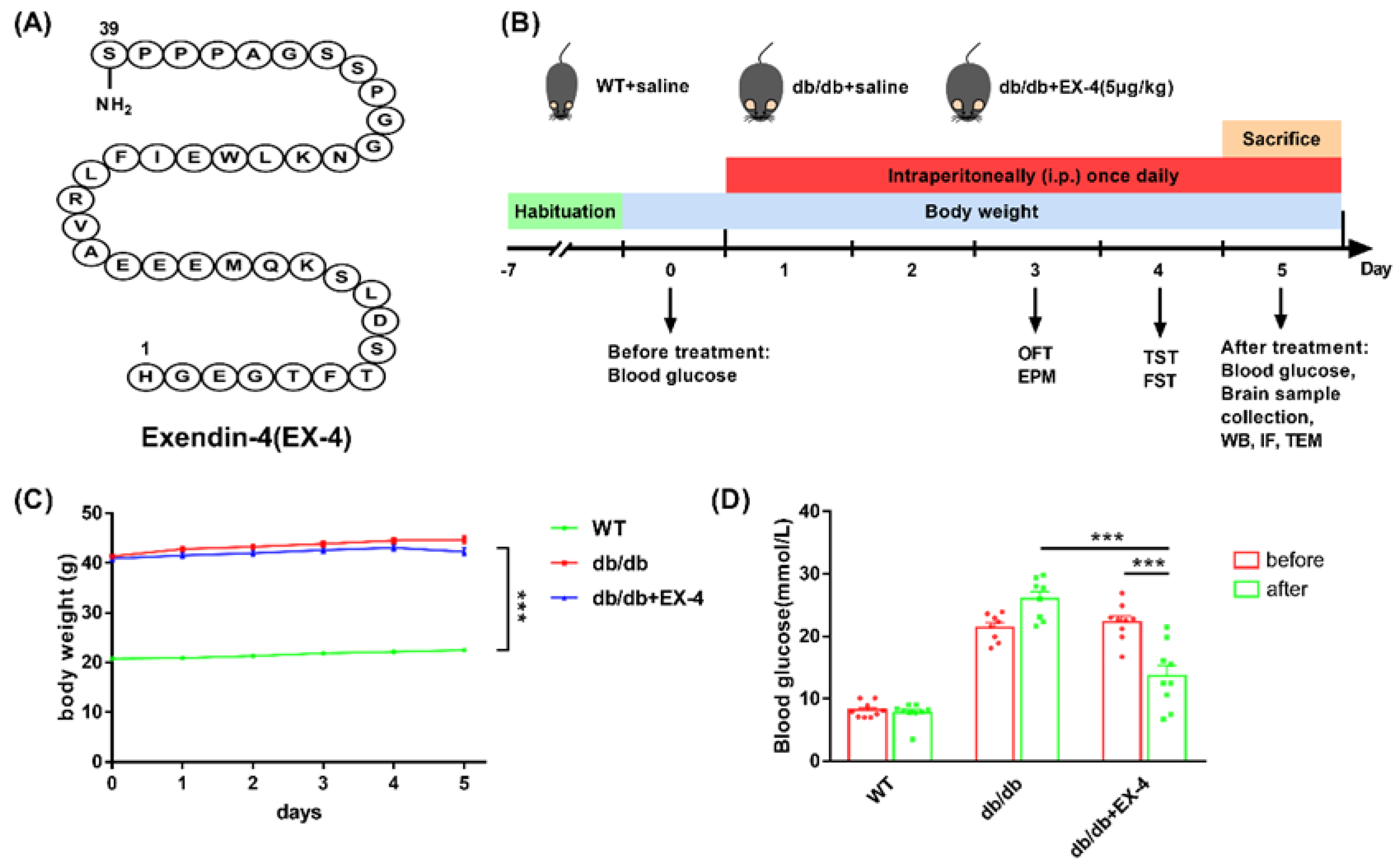
2.3. Animal Experimental Design
2.4. Behavioral Paradigms
2.4.1. Open Field Test (OFT)
2.4.2. Elevated plus Maze (EPM)
2.4.3. Tail Suspension Test (TST)
2.4.4. Forced Swimming Test (FST)
2.5. Cell Culture
2.6. Cell Viability Assay
2.7. Western Blot Analysis
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Mitochondrial Membrane Potential, Intracellular and Mitochondrial ROS Detection
2.10. Transmission Electron Microscopy (TEM)
2.11. Immunofluorescence
2.12. Statistical Analysis
3. Results
3.1. Activation of GLP-1R Attenuated Depression-Like Behaviors in db/db Mice
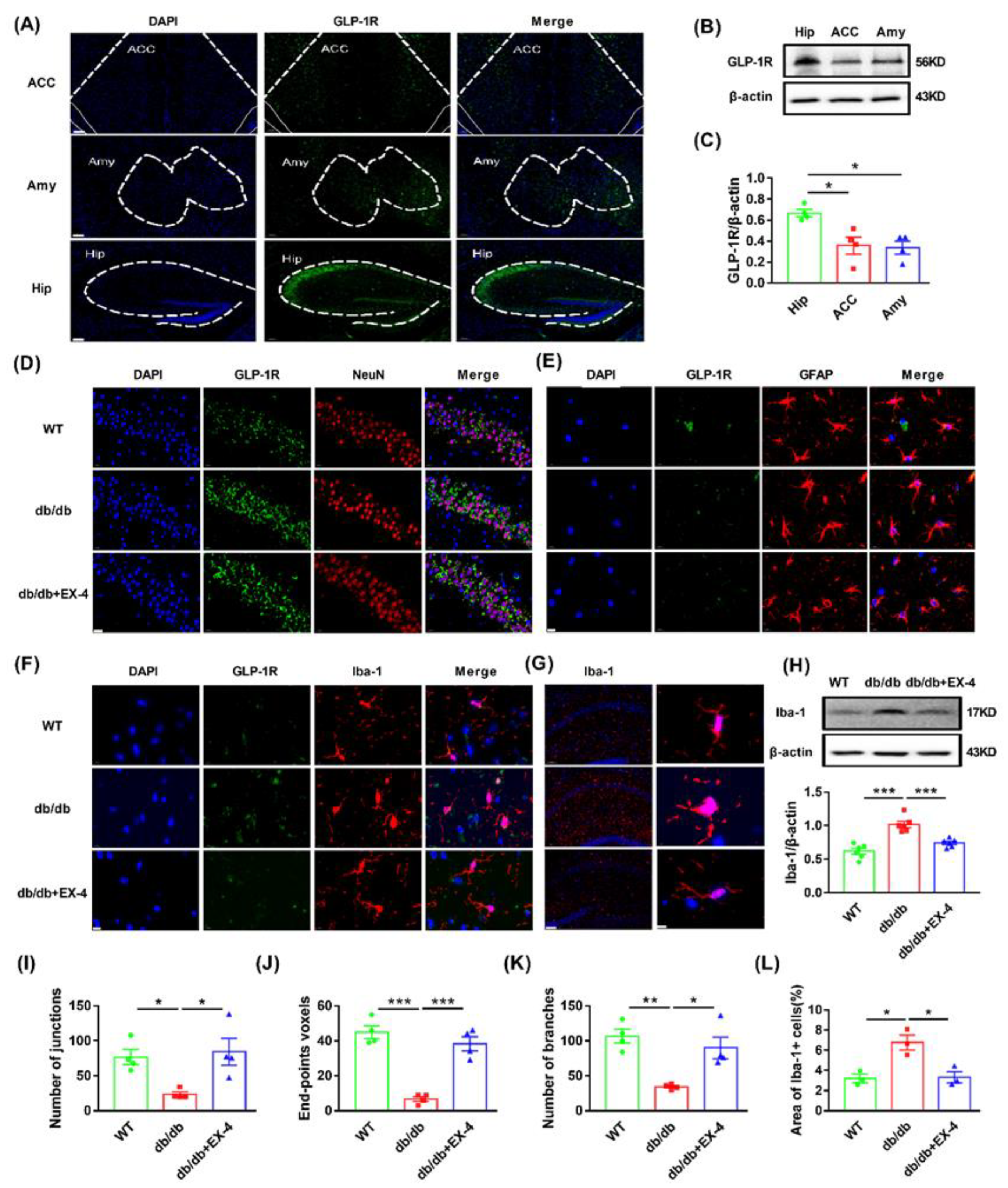
3.2. Microglial Activation in the Hippocampus Was Engaged in the Antidepressant Effects of GLP-1R
3.3. GLP-1R Activation Suppressed GSDMD-Mediated Pyroptosis in Hippocampal Microglia
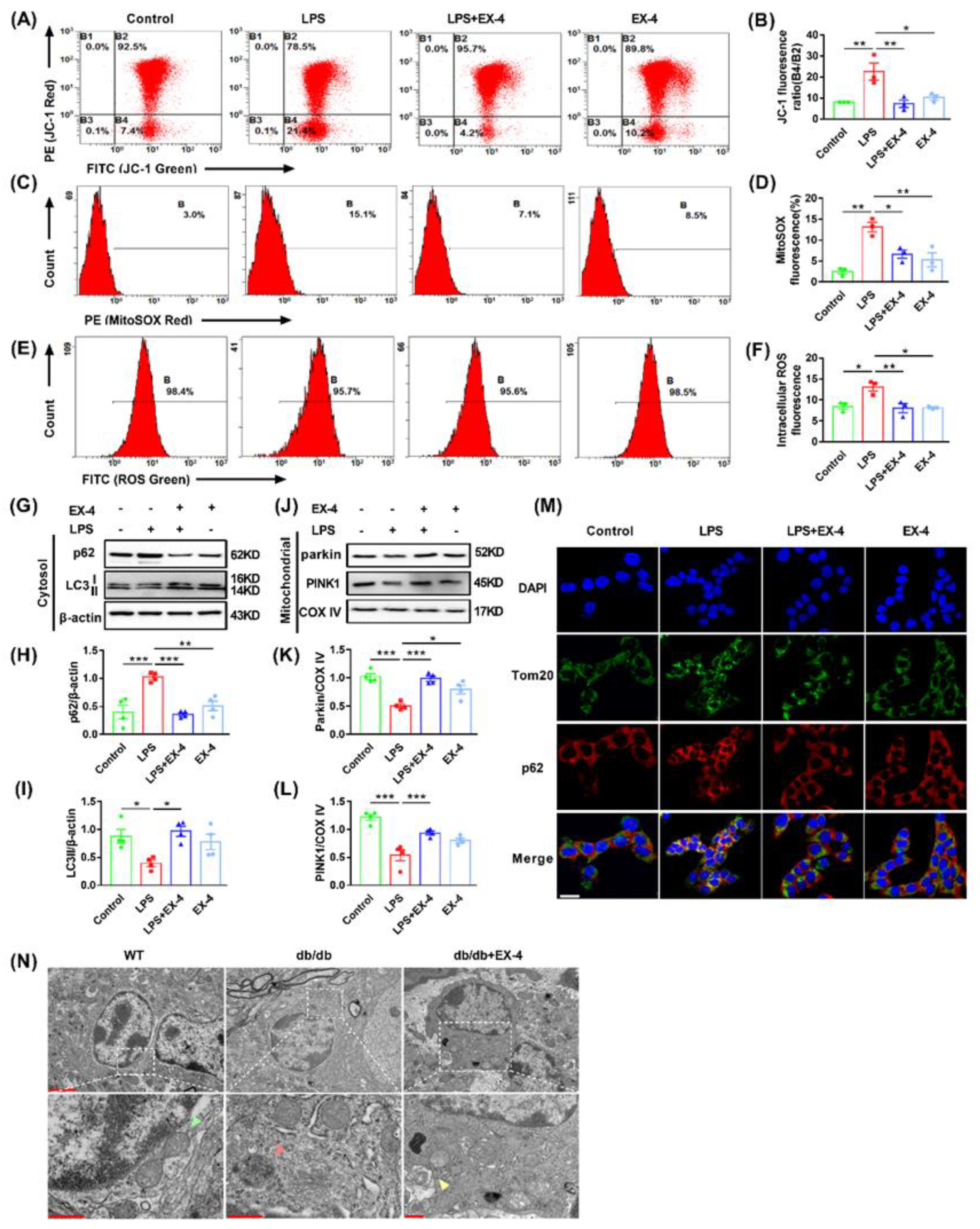
3.4. GLP-1R Activation Suppressed Pyroptosis Induced by LPS in N9 Microglia
3.5. GLP-1R Activation Ameliorated Mitochondrial and Intracellular ROS Generation by Promoting Mitophagy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Réus, G.Z.; Dos Santos, M.A.B.; Strassi, A.P.; Abelaira, H.M.; Ceretta, L.B.; Quevedo, J. Pathophysiological mechanisms involved in the relationship between diabetes and major depressive disorder. Life Sci. 2017, 183, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.J.; Freedland, K.E.; Clouse, R.E.; Lustman, P.J. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care 2001, 24, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.A.; Deschênes, S.S.; Khalil, M.N.; Danna, S.; Filion, K.B.; Schmitz, N. Measures of depression and risk of type 2 diabetes: A systematic review and meta-analysis. J. Affect. Disord. 2020, 265, 224–232. [Google Scholar] [CrossRef]
- van der Feltz-Cornelis, C.; Allen, S.F.; Holt, R.I.G.; Roberts, R.; Nouwen, A.; Sartorius, N. Treatment for comorbid depressive disorder or subthreshold depression in diabetes mellitus: Systematic review and meta-analysis. Brain Behav. 2021, 11, e01981. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, N.; Chaturvedi, S.K. Depressive symptoms and disorders in type 2 diabetes mellitus. Curr. Opin. Psychiatry 2019, 32, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Naicker, K.; Manuel, D.; Øverland, S.; Skogen, J.C.; Johnson, J.A.; Sivertsen, B.; Colman, I. Population attributable fractions for Type 2 diabetes: An examination of multiple risk factors including symptoms of depression and anxiety. Diabetol. Metab. Syndr. 2018, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Moulton, C.D.; Pickup, J.C.; Ismail, K. The link between depression and diabetes: The search for shared mechanisms. Lancet Diabetes Endocrinol. 2015, 3, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.M.; Perlman, G.; Kim, N.; Wu, C.Y.; Daher, V.; Zhou, A.; Mathers, E.H.; Anita, N.Z.; Lanctôt, K.L.; Herrmann, N.; et al. Depression in type 2 diabetes: A systematic review and meta-analysis of blood inflammatory markers. Psychoneuroendocrinology 2021, 134, 105448. [Google Scholar] [CrossRef]
- Zhang, J.; He, H.; Qiao, Y.; Zhou, T.; He, H.; Yi, S.; Zhang, L.; Mo, L.; Li, Y.; Jiang, W.; et al. Priming of microglia with IFN-γ impairs adult hippocampal neurogenesis and leads to depression-like behaviors and cognitive defects. Glia 2020, 68, 2674–2692. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, X.; Sun, B.; Jiang, W.; Liao, C.; Dai, X.; Chen, Y.; Chen, J.; Ding, R. Pretreatment with kaempferol attenuates microglia-mediate neuroinflammation by inhibiting MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury. Free Radic. Biol. Med. 2021, 168, 142–154. [Google Scholar] [CrossRef]
- Yang, J.; Kim, C.S.; Tu, T.H.; Kim, M.S.; Goto, T.; Kawada, T.; Choi, M.S.; Park, T.; Sung, M.K.; Yun, J.W.; et al. Quercetin Protects Obesity-Induced Hypothalamic Inflammation by Reducing Microglia-Mediated Inflammatory Responses via HO-1 Induction. Nutrients 2017, 9, 650. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.W.; Zhang, J.; Li, X.; Wang, Y.; Fu, Y.H.; Gao, X.Y. A new research hot spot: The role of NLRP3 inflammasome activation, a key step in pyroptosis, in diabetes and diabetic complications. Life Sci. 2020, 240, 117138. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Sun, M.; Li, R.; Zhang, X.; Tao, Y.; Yuan, Y.; Luo, X.; Xie, Z. Didymin Suppresses Microglia Pyroptosis and Neuroinflammation Through the Asc/Caspase-1/GSDMD Pathway Following Experimental Intracerebral Hemorrhage. Front. Immunol. 2022, 13, 810582. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Ke, S.; Tu, L.; Wang, Y.; Ye, S.; Kou, S.; Ren, L. Regulation of Autophagy Orchestrates Pyroptotic Cell Death in Molybdenum Disulfide Quantum Dot-Induced Microglial Toxicity. ACS Biomater. Sci. Eng. 2020, 6, 1764–1775. [Google Scholar] [CrossRef]
- Han, X.; Xu, T.; Fang, Q.; Zhang, H.; Yue, L.; Hu, G.; Sun, L. Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox Biol. 2021, 44, 102010. [Google Scholar] [CrossRef]
- Scaini, G.; Mason, B.L.; Diaz, A.P.; Jha, M.K.; Soares, J.C.; Trivedi, M.H.; Quevedo, J. Dysregulation of mitochondrial dynamics, mitophagy and apoptosis in major depressive disorder: Does inflammation play a role? Mol. Psychiatry 2022, 27, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Dehdashtian, E.; Mehrzadi, S.; Yousefi, B.; Hosseinzadeh, A.; Reiter, R.J.; Safa, M.; Ghaznavi, H.; Naseripour, M. Diabetic retinopathy pathogenesis and the ameliorating effects of melatonin; involvement of autophagy, inflammation and oxidative stress. Life Sci. 2018, 193, 20–33. [Google Scholar] [CrossRef]
- Brown, E.; Heerspink, H.J.L.; Cuthbertson, D.J.; Wilding, J.P.H. SGLT2 inhibitors and GLP-1 receptor agonists: Established and emerging indications. Lancet 2021, 398, 262–276. [Google Scholar] [CrossRef]
- Bendotti, G.; Montefusco, L.; Lunati, M.E.; Usuelli, V.; Pastore, I.; Lazzaroni, E.; Assi, E.; Seelam, A.J.; El Essawy, B.; Jang, J.; et al. The anti-inflammatory and immunological properties of GLP-1 Receptor Agonists. Pharmacol. Res. 2022, 182, 106320. [Google Scholar] [CrossRef]
- Diz-Chaves, Y.; Herrera-Pérez, S.; González-Matías, L.C.; Lamas, J.A.; Mallo, F. Glucagon-Like Peptide-1 (GLP-1) in the Integration of Neural and Endocrine Responses to Stress. Nutrients 2020, 12, 3304. [Google Scholar] [CrossRef]
- Peng, Y.; Lin, H.; Tian, S.; Liu, S.; Li, J.; Lv, X.; Chen, S.; Zhao, L.; Pu, F.; Chen, X.; et al. Glucagon-like peptide-1 receptor activation maintains extracellular matrix integrity by inhibiting the activity of mitogen-activated protein kinases and activator protein-1. Free Radic. Biol. Med. 2021, 177, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.N.; Stein, L.M.; Fortin, S.M.; Hayes, M.R. The role of glia in the physiology and pharmacology of glucagon-like peptide-1: Implications for obesity, diabetes, neurodegeneration and glaucoma. Br. J. Pharmacol. 2022, 179, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Ventorp, F.; Bay-Richter, C.; Nagendra, A.S.; Janelidze, S.; Matsson, V.S.; Lipton, J.; Nordström, U.; Westrin, Å.; Brundin, P.; Brundin, L. Exendin-4 Treatment Improves LPS-Induced Depressive-Like Behavior without Affecting Pro-Inflammatory Cytokines. J. Parkinsons Dis. 2017, 7, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Koshal, P.; Kumar, P. Effect of Liraglutide on Corneal Kindling Epilepsy Induced Depression and Cognitive Impairment in Mice. Neurochem. Res. 2016, 41, 1741–1750. [Google Scholar] [CrossRef]
- Barthas, F.; Sellmeijer, J.; Hugel, S.; Waltisperger, E.; Barrot, M.; Yalcin, I. The anterior cingulate cortex is a critical hub for pain-induced depression. Biol. Psychiatry 2015, 77, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Barrot, M.; DiLeone, R.J.; Eisch, A.J.; Gold, S.J.; Monteggia, L.M. Neurobiology of depression. Neuron 2002, 34, 13–25. [Google Scholar] [CrossRef]
- Wang, X.S.; Jiang, Y.L.; Lu, L.; Feng, B.; Ma, X.; Zhang, K.; Guan, S.Y.; Yang, L.; Fan, Q.Y.; Zhu, X.C.; et al. Activation of GIPR Exerts Analgesic and Anxiolytic-Like Effects in the Anterior Cingulate Cortex of Mice. Front. Endocrinol. 2022, 13, 887238. [Google Scholar] [CrossRef]
- Young, K.; Morrison, H. Quantifying Microglia Morphology from Photomicrographs of Immunohistochemistry Prepared Tissue Using ImageJ. J. Vis. Exp. 2018, 136, e57648. [Google Scholar] [CrossRef]
- Zhang, Z.; Bassam, B.; Thomas, A.G.; Williams, M.; Liu, J.; Nance, E.; Rojas, C.; Slusher, B.S.; Kannan, S. Maternal inflammation leads to impaired glutamate homeostasis and up-regulation of glutamate carboxypeptidase II in activated microglia in the fetal/newborn rabbit brain. Neurobiol. Dis. 2016, 94, 116–128. [Google Scholar] [CrossRef]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J. Neuroinflamm. 2021, 18, 258. [Google Scholar] [CrossRef]
- Pearson-Stuttard, J.; Holloway, S.; Polya, R.; Sloan, R.; Zhang, L.; Gregg, E.W.; Harrison, K.; Elvidge, J.; Jonsson, P.; Porter, T. Variations in comorbidity burden in people with type 2 diabetes over disease duration: A population-based analysis of real world evidence. EClinicalMedicine 2022, 52, 101584. [Google Scholar] [CrossRef] [PubMed]
- Semenkovich, K.; Brown, M.E.; Svrakic, D.M.; Lustman, P.J. Depression in type 2 diabetes mellitus: Prevalence, impact, and treatment. Drugs 2015, 75, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Jun, H.S. Anti-Inflammatory Effects of GLP-1-Based Therapies beyond Glucose Control. Mediat. Inflamm. 2016, 2016, 3094642. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kim, O.Y.; Song, J. Alleviation of Depression by Glucagon-Like Peptide 1 Through the Regulation of Neuroinflammation, Neurotransmitters, Neurogenesis, and Synaptic Function. Front. Pharmacol. 2020, 11, 1270. [Google Scholar] [CrossRef] [PubMed]
- Anderberg, R.H.; Richard, J.E.; Hansson, C.; Nissbrandt, H.; Bergquist, F.; Skibicka, K.P. GLP-1 is both anxiogenic and antidepressant; divergent effects of acute and chronic GLP-1 on emotionality. Psychoneuroendocrinology 2016, 65, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Rashidy-Pour, A.; Atkin, S.L.; Jamialahmadi, T.; Sahebkar, A. GLP-1 mimetics and cognition. Life Sci. 2021, 264, 118645. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Xia, J.; Xue, X.; Wang, H.; Qi, Z.; Ji, L. Leptin receptor knockout-induced depression-like behaviors and attenuated antidepressant effects of exercise are associated with STAT3/SOCS3 signaling. Brain Behav. Immun. 2017, 61, 297–305. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Loftis, J.M.; Huckans, M.; Morasco, B.J. Neuroimmune mechanisms of cytokine-induced depression: Current theories and novel treatment strategies. Neurobiol. Dis. 2010, 37, 519–533. [Google Scholar] [CrossRef]
- Dinel, A.L.; André, C.; Aubert, A.; Ferreira, G.; Layé, S.; Castanon, N. Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PLoS ONE 2011, 6, e24325. [Google Scholar] [CrossRef]
- Tagliari, B.; Tagliari, A.P.; Schmitz, F.; da Cunha, A.A.; Dalmaz, C.; Wyse, A.T. Chronic variable stress alters inflammatory and cholinergic parameters in hippocampus of rats. Neurochem. Res. 2011, 36, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Hayley, S.; Hakim, A.M.; Albert, P.R. Depression, dementia and immune dysregulation. Brain 2021, 144, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Cameron, H.A.; Glover, L.R. Adult neurogenesis: Beyond learning and memory. Annu. Rev. Psychol. 2015, 66, 53–81. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Liu, T.; Jiang, P.; Dang, R. The interaction between autophagy and neuroinflammation in major depressive disorder: From pathophysiology to therapeutic implications. Pharmacol. Res. 2021, 168, 105586. [Google Scholar] [CrossRef]
- Alcocer-Gómez, E.; Casas-Barquero, N.; Williams, M.R.; Romero-Guillena, S.L.; Cañadas-Lozano, D.; Bullón, P.; Sánchez-Alcazar, J.A.; Navarro-Pando, J.M.; Cordero, M.D. Antidepressants induce autophagy dependent-NLRP3-inflammasome inhibition in Major depressive disorder. Pharmacol. Res. 2017, 121, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lu, M.; Du, R.H.; Qiao, C.; Jiang, C.Y.; Zhang, K.Z.; Ding, J.H.; Hu, G. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol. Neurodegener. 2016, 11, 28. [Google Scholar] [CrossRef]
- Ward, R.; Li, W.; Abdul, Y.; Jackson, L.; Dong, G.; Jamil, S.; Filosa, J.; Fagan, S.C.; Ergul, A. NLRP3 inflammasome inhibition with MCC950 improves diabetes-mediated cognitive impairment and vasoneuronal remodeling after ischemia. Pharmacol. Res. 2019, 142, 237–250. [Google Scholar] [CrossRef]
- Li, Y.; Song, W.; Tong, Y.; Zhang, X.; Zhao, J.; Gao, X.; Yong, J.; Wang, H. Isoliquiritin ameliorates depression by suppressing NLRP3-mediated pyroptosis via miRNA-27a/SYK/NF-κB axis. J. Neuroinflamm. 2021, 18, 1. [Google Scholar] [CrossRef]
- De, R.; Mazumder, S.; Sarkar, S.; Debsharma, S.; Siddiqui, A.A.; Saha, S.J.; Banerjee, C.; Nag, S.; Saha, D.; Bandyopadhyay, U. Acute mental stress induces mitochondrial bioenergetic crisis and hyper-fission along with aberrant mitophagy in the gut mucosa in rodent model of stress-related mucosal disease. Free Radic. Biol. Med. 2017, 113, 424–438. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Zhang, J.; Ma, L.; Gu, J.; Ho, G. Contribution of neural cell death to depressive phenotypes of streptozotocin-induced diabetic mice. Dis. Model. Mech. 2014, 7, 723–730. [Google Scholar] [CrossRef]
- Shu, X.; Sun, Y.; Sun, X.; Zhou, Y.; Bian, Y.; Shu, Z.; Ding, J.; Lu, M.; Hu, G. The effect of fluoxetine on astrocyte autophagy flux and injured mitochondria clearance in a mouse model of depression. Cell Death Dis. 2019, 10, 577. [Google Scholar] [CrossRef] [PubMed]
- Jayatunga, D.P.W.; Hone, E.; Khaira, H.; Lunelli, T.; Singh, H.; Guillemin, G.J.; Fernando, B.; Garg, M.L.; Verdile, G.; Martins, R.N. Therapeutic Potential of Mitophagy-Inducing Microflora Metabolite, Urolithin A for Alzheimer’s Disease. Nutrients 2021, 13, 3744. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, L.; Lan, J.; Feng, X.; Li, P.; Wu, L.; Peng, Y. Molecular mechanisms of mitophagy and its roles in neurodegenerative diseases. Pharmacol. Res. 2021, 163, 105240. [Google Scholar] [CrossRef]
- Wang, X.L.; Feng, S.T.; Wang, Z.Z.; Chen, N.H.; Zhang, Y. Role of mitophagy in mitochondrial quality control: Mechanisms and potential implications for neurodegenerative diseases. Pharmacol. Res. 2021, 165, 105433. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, G.; Schwarz, T.L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013, 20, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.; Palikaras, K.; Lautrup, S.; Scheibye-Knudsen, M.; Tavernarakis, N.; Fang, E.F. Mitophagy and Neuroprotection. Trends Mol. Med. 2020, 26, 8–20. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Wang, X.; Qi, J.; Zhang, K.; Jiang, Y.; Feng, B.; Lv, T.; Yang, L.; Yang, Q.; Zhao, M.; et al. Glucagon-like Peptide 1 Receptor Activation Inhibits Microglial Pyroptosis via Promoting Mitophagy to Alleviate Depression-like Behaviors in Diabetic Mice. Nutrients 2023, 15, 38. https://doi.org/10.3390/nu15010038
Yang F, Wang X, Qi J, Zhang K, Jiang Y, Feng B, Lv T, Yang L, Yang Q, Zhao M, et al. Glucagon-like Peptide 1 Receptor Activation Inhibits Microglial Pyroptosis via Promoting Mitophagy to Alleviate Depression-like Behaviors in Diabetic Mice. Nutrients. 2023; 15(1):38. https://doi.org/10.3390/nu15010038
Chicago/Turabian StyleYang, Fan, Xinshang Wang, Jingyu Qi, Kun Zhang, Yongli Jiang, Ban Feng, Tao Lv, Le Yang, Qi Yang, Minggao Zhao, and et al. 2023. "Glucagon-like Peptide 1 Receptor Activation Inhibits Microglial Pyroptosis via Promoting Mitophagy to Alleviate Depression-like Behaviors in Diabetic Mice" Nutrients 15, no. 1: 38. https://doi.org/10.3390/nu15010038
APA StyleYang, F., Wang, X., Qi, J., Zhang, K., Jiang, Y., Feng, B., Lv, T., Yang, L., Yang, Q., Zhao, M., Liu, S., & Ma, X. (2023). Glucagon-like Peptide 1 Receptor Activation Inhibits Microglial Pyroptosis via Promoting Mitophagy to Alleviate Depression-like Behaviors in Diabetic Mice. Nutrients, 15(1), 38. https://doi.org/10.3390/nu15010038





