Association between Dietary Inflammatory Index and Sarcopenia: A Meta-Analysis
Abstract
1. Introduction
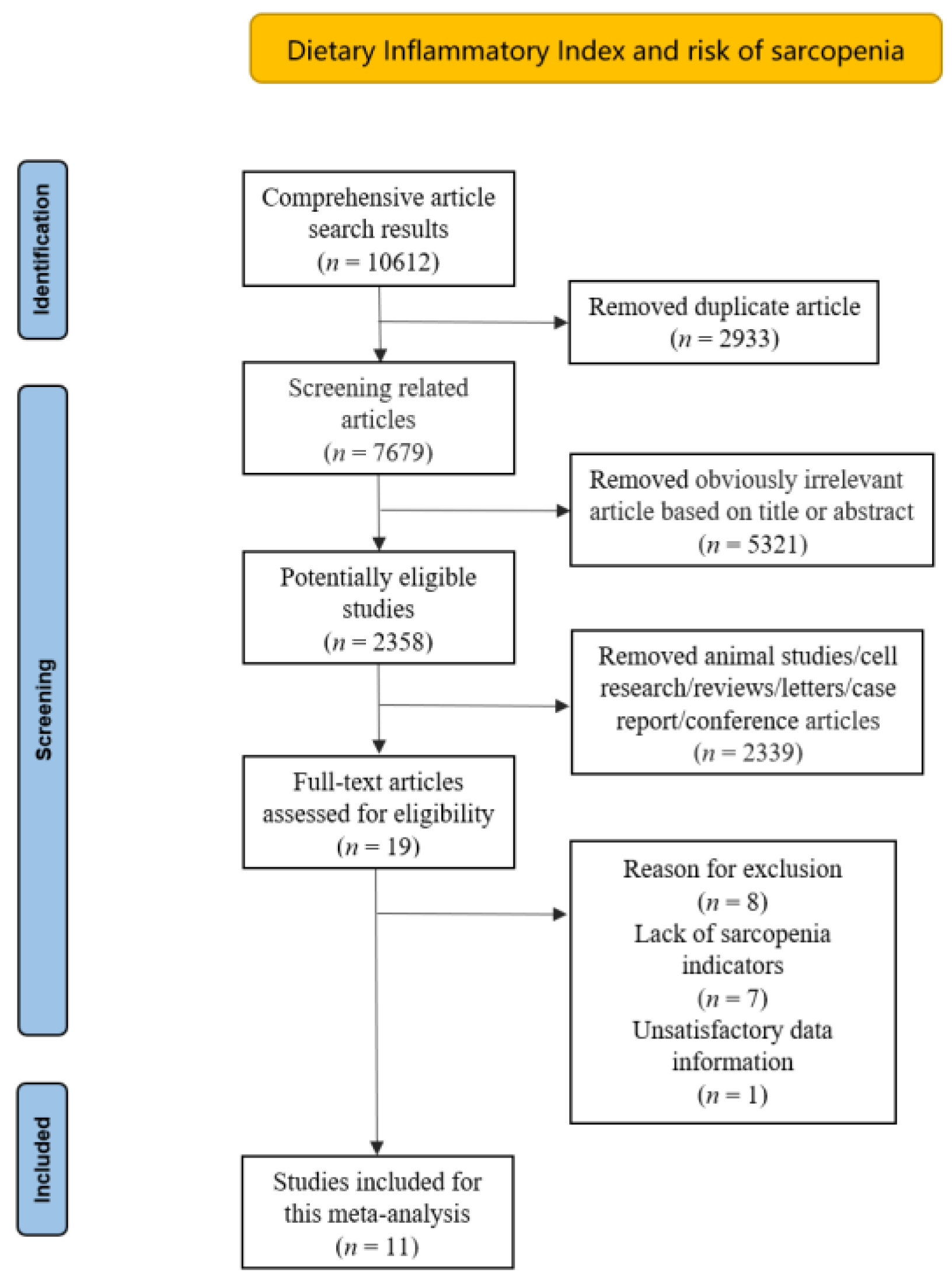
2. Materials and Methods
2.1. Sources and Methods of Data Retrieval
2.2. Inclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
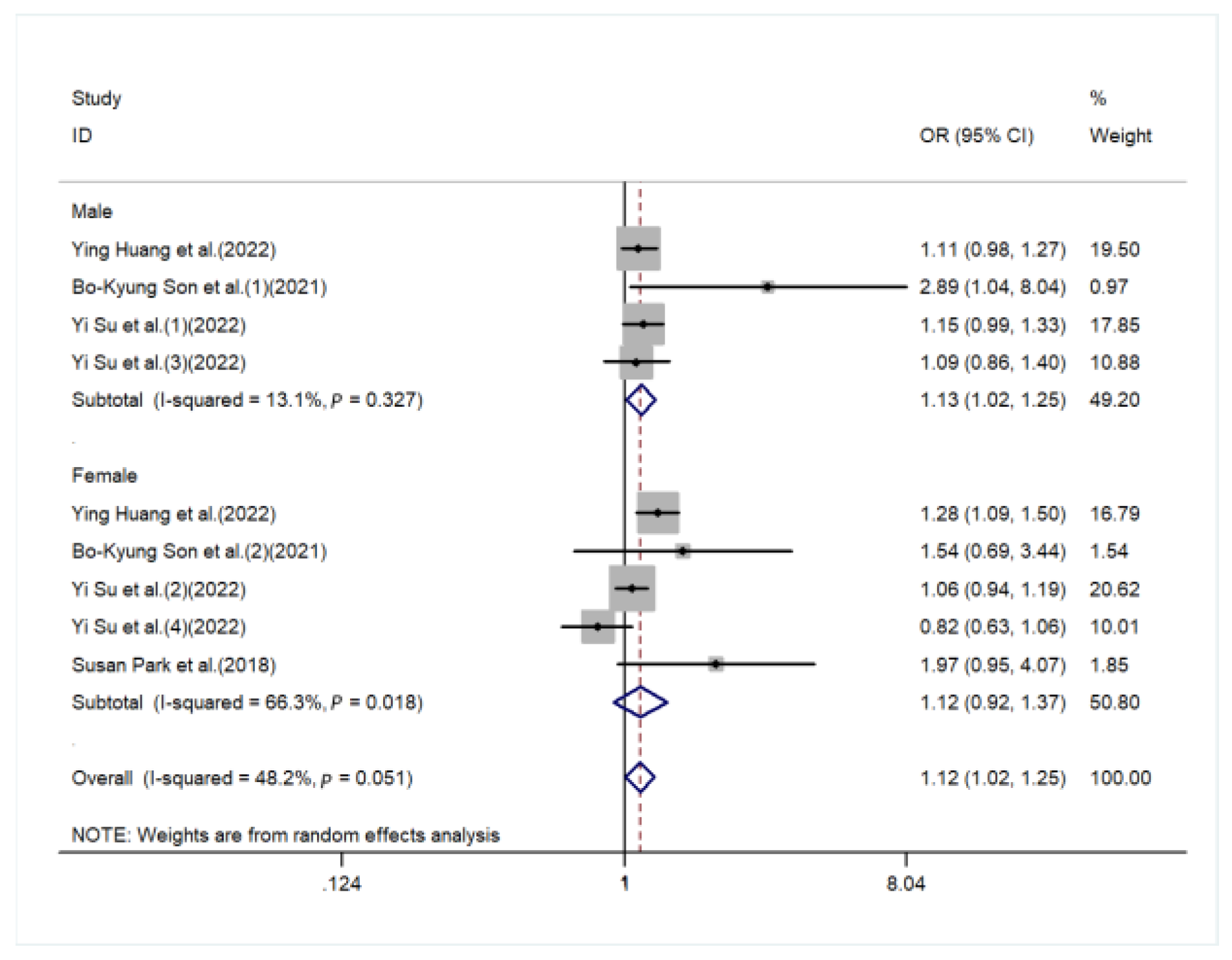
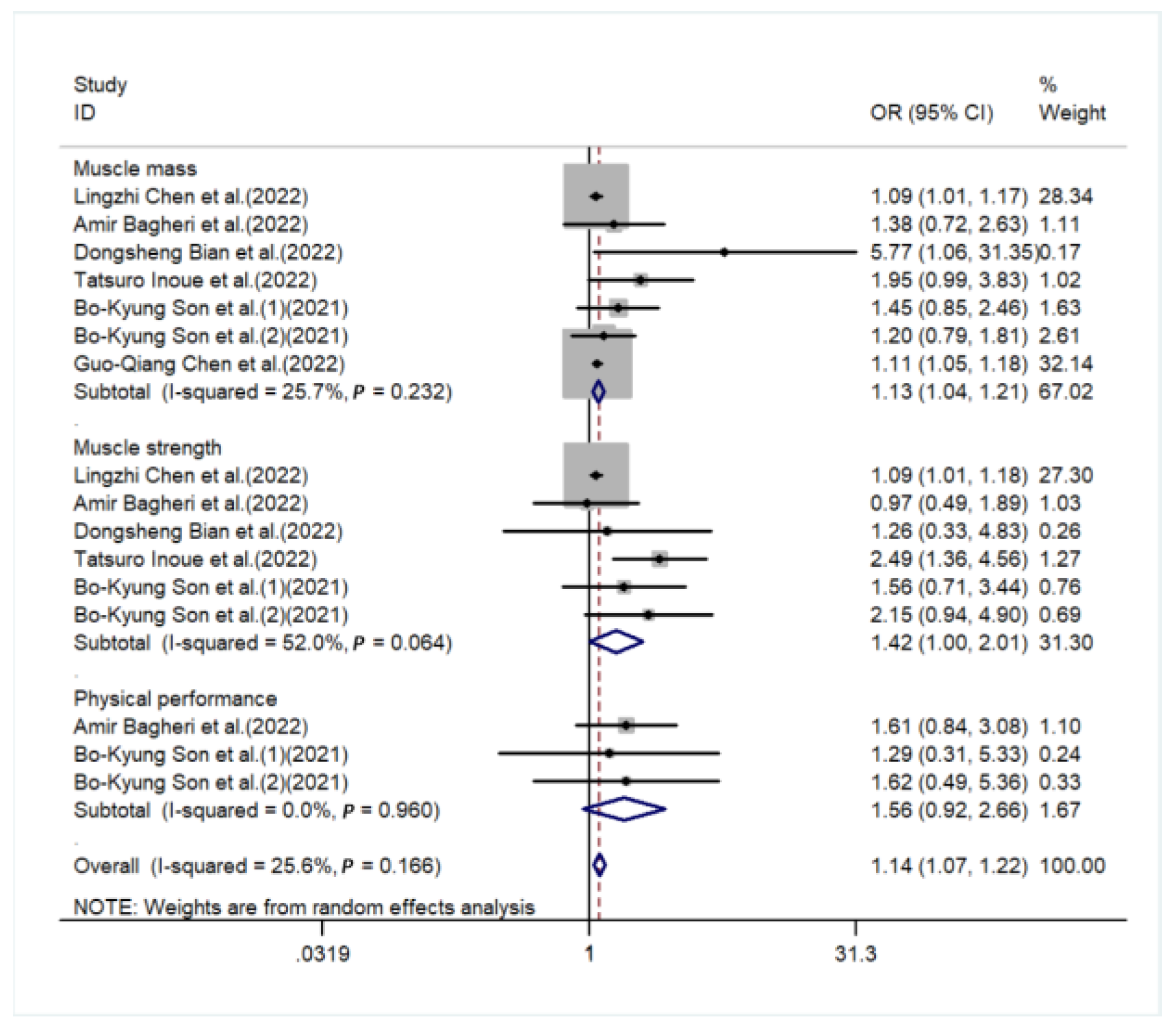
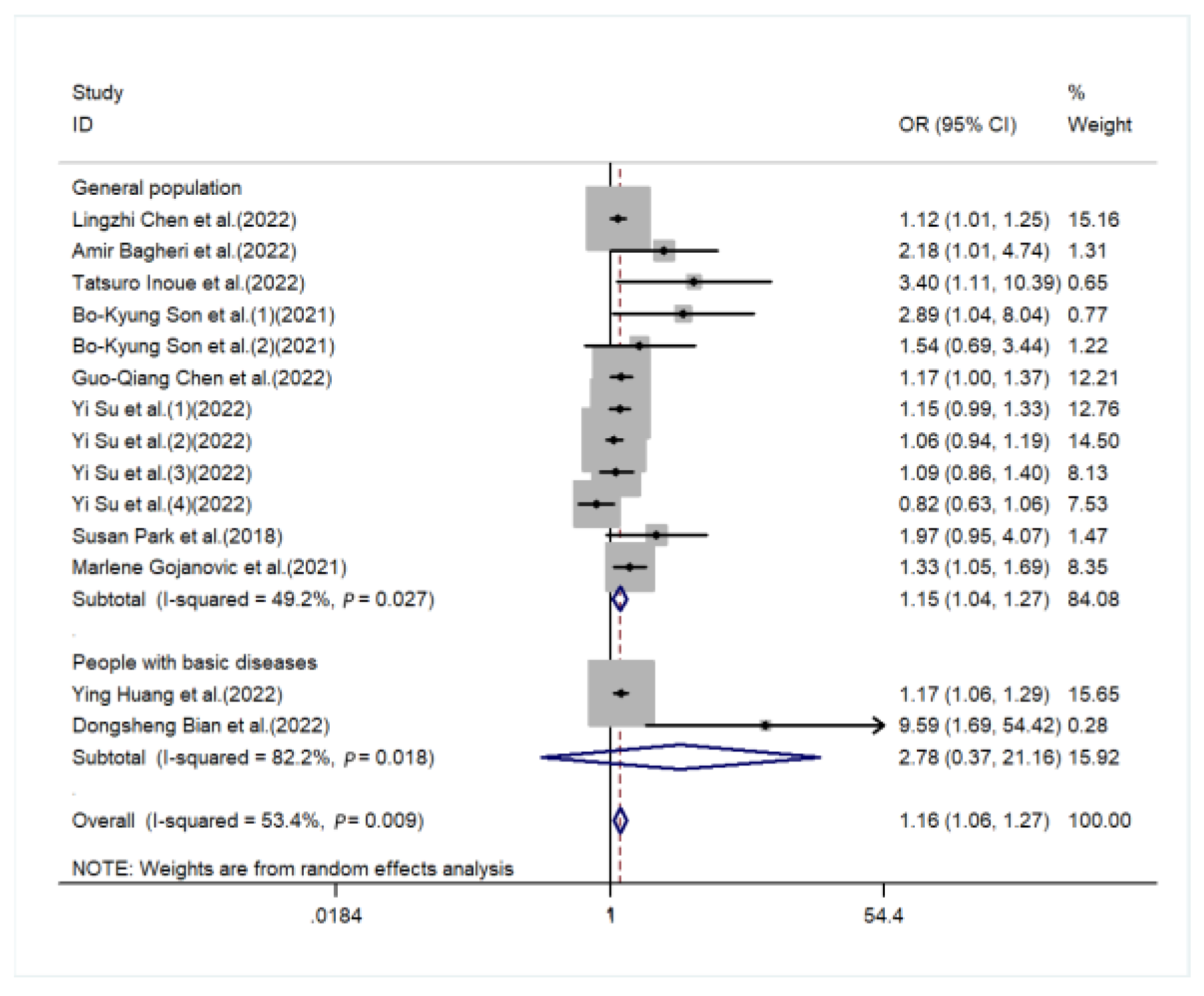
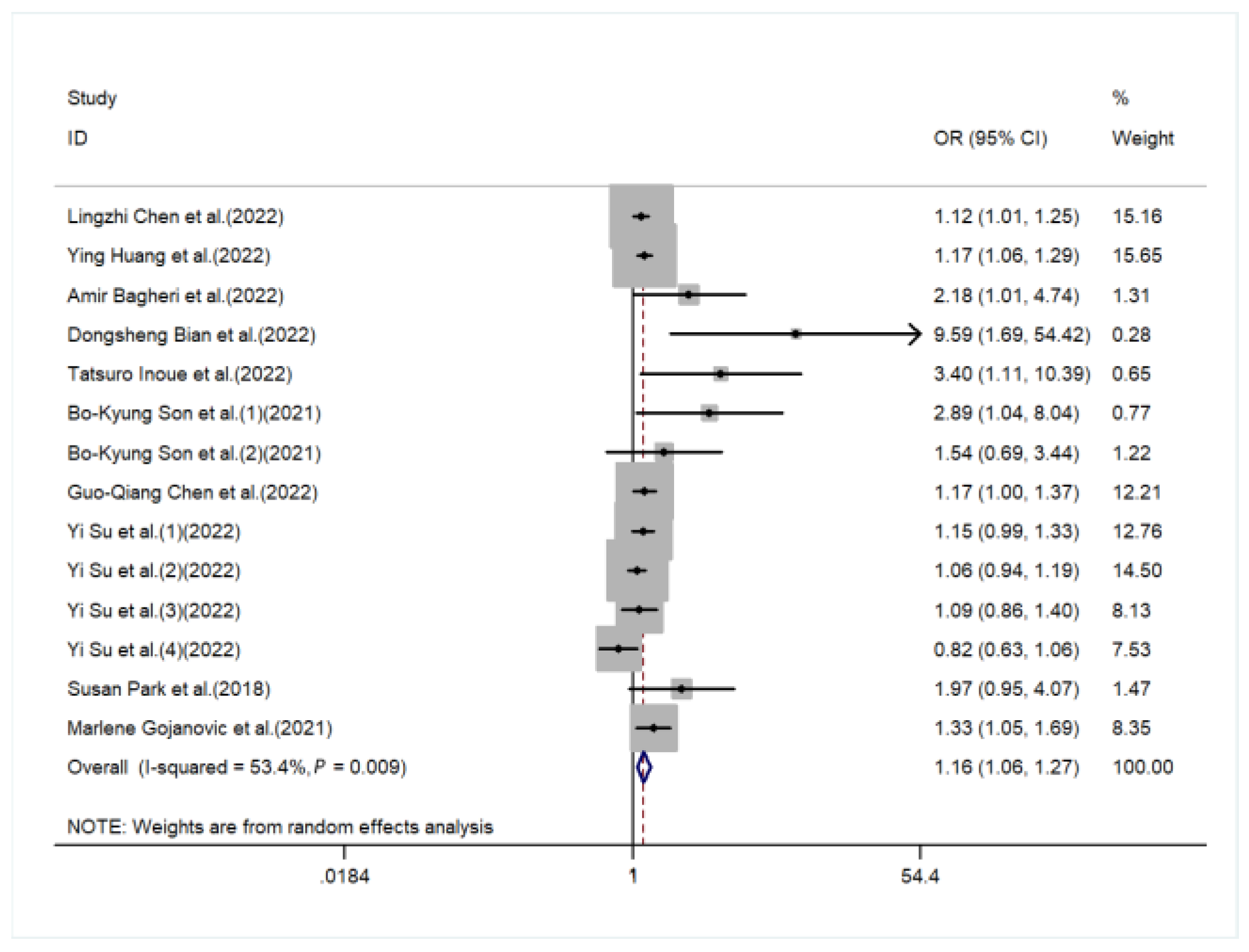
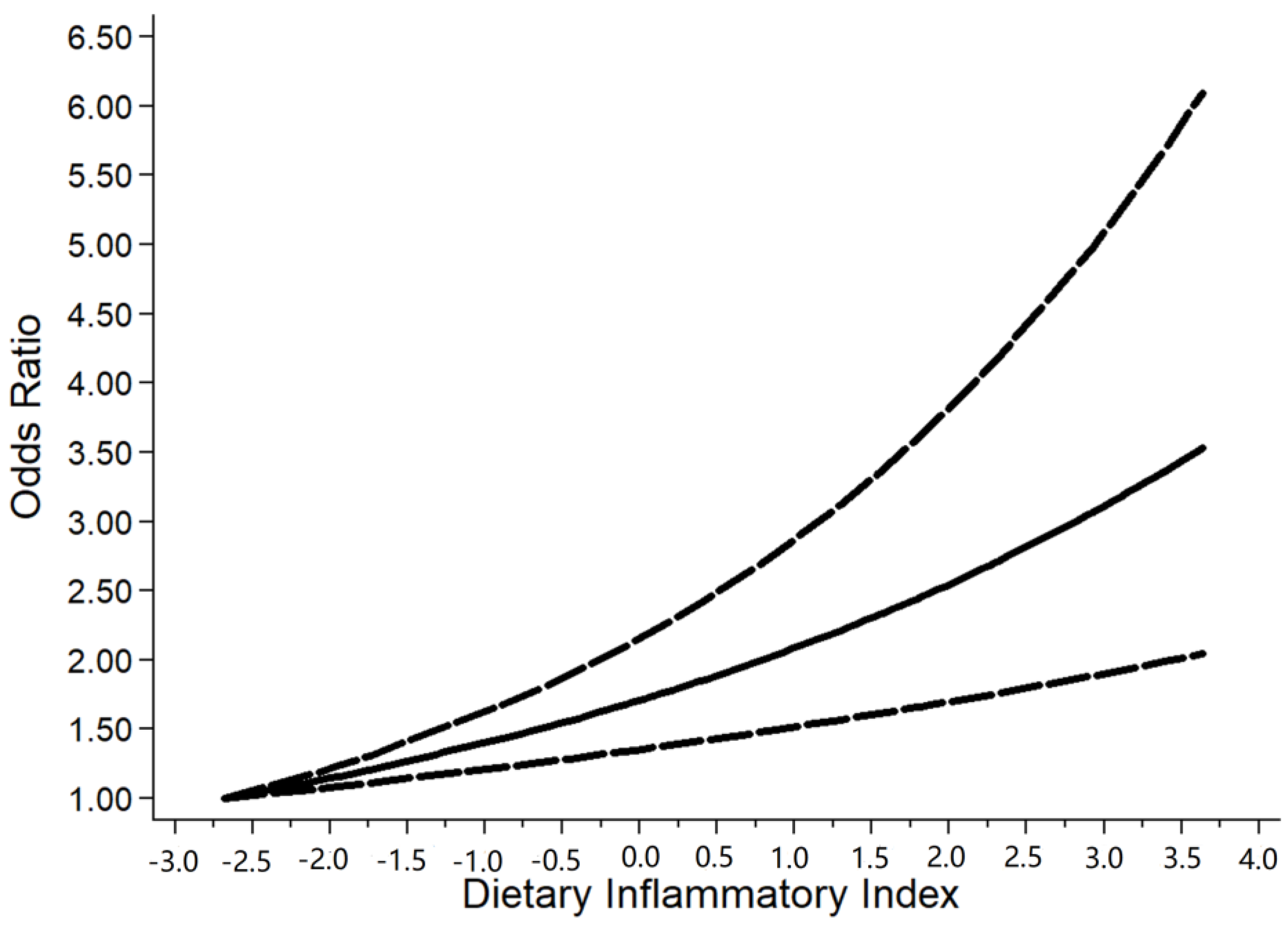
3. Result
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Harhay, M.O.; Harhay, M.N. Sarcopenia and mortality among a population-based sample of community-dwelling older adults. J. Cachexia Sarcopenia Muscle 2016, 7, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74. [Google Scholar] [CrossRef]
- Custodero, C.; Mankowski, R.T.; Lee, S.A.; Chen, Z.; Wu, S.; Manini, T.M.; Hincapie Echeverri, J.; Sabbà, C.; Beavers, D.P.; Cauley, J.A.; et al. Evidence-based nutritional and pharmacological interventions targeting chronic low-grade inflammation in middle-age and older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2018, 46, 42–59. [Google Scholar] [CrossRef]
- Wirth, M.D.; Shivappa, N.; Davis, L.; Hurley, T.G.; Ortaglia, A.; Drayton, R.; Blair, S.N.; Hébert, J.R. Construct Validation of the Dietary Inflammatory Index among African Americans. J. Nutr. Health Aging 2017, 21, 487–491. [Google Scholar] [CrossRef]
- Shivappa, N.; Wirth, M.D.; Hurley, T.G.; Hébert, J.R. Association between the dietary inflammatory index (DII) and telomere length and C-reactive protein from the National Health and Nutrition Examination Survey-1999-2002. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Tabung, F.; Hébert, J.R. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr. 2014, 17, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Steck, S.E.; Zhang, J.; Ma, Y.; Liese, A.D.; Agalliu, I.; Hingle, M.; Hou, L.; Hurley, T.G.; Jiao, L.; et al. Construct validation of the dietary inflammatory index among postmenopausal women. Ann. Epidemiol. 2015, 25, 398–405. [Google Scholar] [CrossRef]
- Granic, A.; Sayer, A.A.; Robinson, S.M. Dietary Patterns, Skeletal Muscle Health, and Sarcopenia in Older Adults. Nutrients 2019, 11, 745. [Google Scholar] [CrossRef]
- Cervo, M.M.; Shivappa, N.; Hebert, J.R.; Oddy, W.H.; Winzenberg, T.; Balogun, S.; Wu, F.; Ebeling, P.; Aitken, D.; Jones, G.; et al. Longitudinal associations between dietary inflammatory index and musculoskeletal health in community-dwelling older adults. Clin. Nutr. 2020, 39, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, M326–M332. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, D.; Costelli, P.; Sampaolesi, M.; Penna, F. Role of Inflammation in Muscle Homeostasis and Myogenesis. Mediat. Inflamm. 2015, 2015, 805172. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, M.J.; Bossuyt, J.E.; Boutron, P.M.; Hoffmann, I.; Mulrow, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.B.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 2372, n71. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Lo, C.K.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clin. Res. Ed.) 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Sultan, S.; Glasziou, P.; Akl, E.A.; Alonso-Coello, P.; Atkins, D.; Kunz, R.; Brozek, J.; Montori, V.; et al. GRADE guidelines: 9. Rating up the quality of evidence. J. Clin. Epidemiol. 2011, 64, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Chen, L.; Ming, J.; Chen, T.; Hébert, J.R.; Sun, P.; Zhang, L.; Wang, H.; Wu, Q.; Zhang, C.; Shivappa, N.; et al. Association between dietary inflammatory index score and muscle mass and strength in older adults: A study from National Health and Nutrition Examination Survey (NHANES) 1999-2002. Eur. J. Nutr. 2022, 61, 4077–4089. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, M.; Zhang, L.; Shi, J.; Yang, Y.; Liu, F.; Sun, L.; Xiao, L. Dietary Inflammatory Potential Is Associated with Sarcopenia Among Chronic Kidney Disease Population. Front. Nutr. 2022, 9, 856726. [Google Scholar] [CrossRef]
- Bagheri, A.; Soltani, S.; Hashemi, R.; Heshmat, R.; Motlagh, A.D.; Esmaillzadeh, A. Inflammatory potential of the diet and risk of sarcopenia and its components. Nutr. J. 2020, 19, 129. [Google Scholar] [CrossRef] [PubMed]
- Bian, D.; Liu, X.; Wang, C.; Jiang, Y.; Gu, Y.; Zhong, J.; Shi, Y. Association between Dietary Inflammatory Index and Sarcopenia in Crohn’s Disease Patients. Nutrients 2022, 14, 901. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Shimizu, A.; Ueshima, J.; Murotani, K.; Nagano, A.; Ishida, Y.; Kinoshita, K.; Iizuka, Y.; Satake, S.; Matsui, Y.; et al. Diet-induced inflammation is associated with sarcopenia and muscle strength in older adults who visit a frailty clinic. Aging Clin. Exp. Res. 2022, 34, 2525–2532. [Google Scholar] [CrossRef] [PubMed]
- Son, B.K.; Akishita, M.; Yamanaka, T.; Toyoshima, K.; Tanaka, T.; Suthutvoravut, U.; Iijima, K. Association between inflammatory potential of the diet and sarcopenia/its components in community-dwelling older Japanese men. Arch. Gerontol. Geriatr. 2021, 97, 104481. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Wang, G.P.; Lian, Y. Relationships Between Depressive Symptoms, Dietary Inflammatory Potential, and Sarcopenia: Mediation Analyses. Front. Nutr. 2022, 9, 844917. [Google Scholar] [CrossRef]
- Su, Y.; Yeung, S.S.Y.; Chen, Y.M.; Leung, J.C.S.; Kwok, T.C.Y. The Associations of Dietary Inflammatory Potential with Musculoskeletal Health in Chinese Community-Dwelling Older People: The Mr. OS and Ms. OS (Hong Kong) Cohort Study. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2022, 37, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Elshorbagy, A.; Turner, C.; Refsum, H.; Kwok, T. The Association of Circulating Amino Acids and Dietary Inflammatory Potential with Muscle Health in Chinese Community-Dwelling Older People. Nutrients 2022, 14, 2471. [Google Scholar] [CrossRef]
- Park, S.; Na, W.; Sohn, C. Relationship between osteosarcopenic obesity and dietary inflammatory index in postmenopausal Korean women: 2009 to 2011 Korea National Health and Nutrition Examination Surveys. J. Clin. Biochem. Nutr. 2018, 63, 211–216. [Google Scholar] [CrossRef]
- Gojanovic, M.; Holloway-Kew, K.L.; Hyde, N.K.; Mohebbi, M.; Shivappa, N.; Hebert, J.R.; O’Neil, A.; Pasco, J.A. The Dietary Inflammatory Index Is Associated with Low Muscle Mass and Low Muscle Function in Older Australians. Nutrients 2021, 13, 1166. [Google Scholar] [CrossRef]
- Mitch, W.E.; Goldberg, A.L. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N. Engl. J. Med. 1996, 335, 1897–1905. [Google Scholar] [CrossRef]
- Haran, P.H.; Rivas, D.A.; Fielding, R.A. Role and potential mechanisms of anabolic resistance in sarcopenia. J. Cachexia Sarcopenia Muscle 2012, 3, 157–162. [Google Scholar] [CrossRef] [PubMed]
- de Luca, C.; Olefsky, J.M. Inflammation and insulin resistance. FEBS Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Toth, M.J.; Ades, P.A.; Tischler, M.D.; Tracy, R.P.; LeWinter, M.M. Immune activation is associated with reduced skeletal muscle mass and physical function in chronic heart failure. Int. J. Cardiol. 2006, 109, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Roubenoff, R.; Roubenoff, R.A.; Cannon, J.G.; Kehayias, J.J.; Zhuang, H.; Dawson-Hughes, B.; Dinarello, C.A.; Rosenberg, I.H. Rheumatoid cachexia: Cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J. Clin. Investig. 1994, 93, 2379–2386. [Google Scholar] [CrossRef]
- Casimir, G.J.; Duchateau, J. Gender differences in inflammatory processes could explain poorer prognosis for males. J. Clin. Microbiol. 2011, 49, 478. [Google Scholar] [CrossRef] [PubMed]
- Ershler, W.B.; Keller, E.T. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu. Rev. Med. 2000, 51, 245–270. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Ribeiro, R.C.; Webb, P.; Gustafsson, J.A.; Kushner, P.J.; Baxter, J.D.; Leitman, D.C. Estradiol repression of tumor necrosis factor-alpha transcription requires estrogen receptor activation function-2 and is enhanced by coactivators. Proc. Natl. Acad. Sci. USA 1999, 96, 15161–15166. [Google Scholar] [CrossRef]
- Cavicchia, P.P.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Hébert, J.R. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J. Nutr. 2009, 139, 2365–2372. [Google Scholar] [CrossRef]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke Off. J. Int. Stroke Soc. 2018, 13, 612–632. [Google Scholar] [CrossRef]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017, 96, 10–15. [Google Scholar] [CrossRef]
- Tay, L.; Ding, Y.Y.; Leung, B.P.; Ismail, N.H.; Yeo, A.; Yew, S.; Tay, K.S.; Tan, C.H.; Chong, M.S. Sex-specific differences in risk factors for sarcopenia amongst community-dwelling older adults. Age 2015, 37, 121. [Google Scholar] [CrossRef]
- Wolters, T.L.C.; Netea, M.G.; Hermus, A.; Smit, J.W.A.; Netea-Maier, R.T. IGF1 potentiates the pro-inflammatory response in human peripheral blood mononuclear cells via MAPK. J. Mol. Endocrinol. 2017, 59, 129–139. [Google Scholar] [CrossRef]
- Bian, A.; Ma, Y.; Zhou, X.; Guo, Y.; Wang, W.; Zhang, Y.; Wang, X. Association between sarcopenia and levels of growth hormone and insulin-like growth factor-1 in the elderly. BMC Musculoskelet. Disord. 2020, 21, 214. [Google Scholar] [CrossRef] [PubMed]
- Cartier, A.; Côté, M.; Lemieux, I.; Pérusse, L.; Tremblay, A.; Bouchard, C.; Després, J.P. Sex differences in inflammatory markers: What is the contribution of visceral adiposity? Am. J. Clin. Nutr. 2009, 89, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Chon, J.; Lee, S.A.; Yoo, M.C.; Yun, Y.; Chung, S.J.; Kim, M.; Lee, E.T.; Kyu Choi, M.; Won, C.W.; et al. Central obesity is associated with lower prevalence of sarcopenia in older women, but not in men: A cross-sectional study. BMC Geriatr. 2022, 22, 406. [Google Scholar] [CrossRef] [PubMed]
- Senior, H.E.; Henwood, T.R.; Beller, E.M.; Mitchell, G.K.; Keogh, J.W. Prevalence and risk factors of sarcopenia among adults living in nursing homes. Maturitas 2015, 82, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Wong, M.; Leung, J.; Lee, J.; Auyeung, T.W.; Woo, J. Incidence, reversibility, risk factors and the protective effect of high body mass index against sarcopenia in community-dwelling older Chinese adults. Geriatr. Gerontol. Int. 2014, 14 Suppl 1, 15–28. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef]
- Chien, M.Y.; Huang, T.Y.; Wu, Y.T. Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community-dwelling elderly people in Taiwan. J. Am. Geriatr. Soc. 2008, 56, 1710–1715. [Google Scholar] [CrossRef]
- Bahat, G.; Turkmen, B.O.; Aliyev, S.; Catikkas, N.M.; Bakir, B.; Karan, M.A. Cut-off values of skeletal muscle index and psoas muscle index at L3 vertebra level by computerized tomography to assess low muscle mass. Clin. Nutr. 2021, 40, 4360–4365. [Google Scholar] [CrossRef]
- Beaudart, C.; Rolland, Y.; Cruz-Jentoft, A.J.; Bauer, J.M.; Sieber, C.; Cooper, C.; Al-Daghri, N.; Araujo de Carvalho, I.; Bautmans, I.; Bernabei, R.; et al. Assessment of Muscle Function and Physical Performance in Daily Clinical Practice: A position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Calcif. Tissue Int. 2019, 105, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Amakye, W.K.; Zhang, Z.; Wei, Y.; Shivappa, N.; Hebert, J.R.; Wang, J.; Su, Y.; Mao, L. The relationship between dietary inflammatory index (DII) and muscle mass and strength in Chinese children aged 6-9 years. Asia Pac. J. Clin. Nutr. 2018, 27, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
| Study | Year | Region | Design | Participant Characteristics | Sample Size | Age | Male (%) | DII Analysis | Diagnosis of Sarcopenia | Variables Adjusted | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ling zhi Chen et al. | 2022 | United States | cross sectional | non-institutionalized community-dwelling residents in the United States | 1863 | 62.1 | 49.00 | Categorized (T3:T1) Continuous | A combination of low muscle mass and low muscle strength was used as a representation of sarcopenia | age (years), sex, race, education, marital status, nativity, smoking, physical activity level and BMI, Chronic disease, Energy, and Protein | 9 |
| Ying Huang et al. | 2022 | United States | cross sectional | CKD participants with complete data for DII and sarcopenia in the United States | 2569 | 55.6 | 45.11 | Categorized (T3:T1) Continuous | FNIH | age, gender, race, income, physical activity, smoking, alcohol drinking, diabetes, hypertension, overweight, central obesity, dyslipidemia, cancer, arthritis, heart disease, eGFR, ACR, hypoalbuminemia, low energy intake, low protein intake, CRP, WBC, NLR, and NHANES strata | 8 |
| Amir Bagheri et al. | 2022 | Tehran, Iran | cross sectional | represents the older population in the district 6 of Tehran (age > 55) | 300 | 66.8 | 50.00 | Categorized (T3:T1) | EWGSOP | age, sex, energy intake, physical activity, smoking, alcohol consumption, medication use, and positive history of disease | 9 |
| Dongsheng Bian et al. | 2022 | China | cross sectional | CD patients who received anti-TNF-α therapy at Shanghai Rui jin Hospital | 140 | 32.5 | 72.14 | Categorized (T4:T1) | (AWGS) 2019 | sex, age, BMI, smoking status, alcohol consumption, nutritional status, disease activity, total energy intake, disease duration, Montreal Classification, Location | 7 |
| Tatsuro Inoue et al. | 2022 | Japan | cross sectional | ambulatory patients aged 65 years or older in Japan | 304 | 77.6 | 32.60 | Categorized (T4:T1) | (AWGS) 2019 | age, sex, comorbidity, BMI, physical activity | 7 |
| Bo-Kyung Son et al. | 2021 | Japan | cross sectional | community-dwelling older adults in Japan | 1254 | 74.6 | 51.80 | Categorized (T3:T1) | (AWGS) 2019 | age, education level, protein intake, physical activity, medical history, eating alone, Lubben Social Network Scale (LSNS) social ties | 9 |
| Guo-Qiang Chen et al. | 2022 | United States | cross sectional | non-institutionalized community-dwelling residents in the United States | 6082 | 37.2 | 51.99 | Categorized | FNIH | age, sex, race, educational level, marriage status, family poverty income ratio, smoking status, drinking status, physical activity level, BMI status, diabetes, and hypertension | 9 |
| Yi Su et al. (a) | 2022 | China | prospective cohort | Chinese people aged ≥65 years were recruited from local communities in Hong Kong (follow up for 14 years) | 2292 | 72.5 | 50.01 | Categorized (T3:T1) | (AWGS) 2019 | age, BMI, current smoking, previous fracture, hypertension, diabetes, chronic obstructive lung disease, cardiovascular disease, rheumatoid arthritis, nonsteroidal anti-inflammatory agent use, osteoporosis medication, vitamin D status, and physical activity level | 8 |
| Yi Su et al. (b) | 2022 | China | prospective cohort | Chinese people aged ≥65 years were recruited from local communities in Hong Kong (follow up for 4 years) | 2997 | 72.0 | 47.51 | Categorized (T3:T1) | (AWGS) 2019 | age, BMI, estimated glomerular filtration rate, current smoking, physical activity level, previous fracture, hypertension, diabetes, chronic obstructive lung disease, cardiovascular disease, rheumatoid arthritis, nonsteroidal anti-inflammatory agent use, and osteoporosis medication | 8 |
| Susan Park et al. | 2018 | Korean | cross section | Korean postmenopausal women over 50 years old | 1344 | 62.3 | - | Categorized | Sarcopenia was diagnosed if the percentage-applied value acquired by dividing appendicular skeletal muscle mass (ASM) by weight | age, family income, regular exercise, education status, smoking, and female hormone supplements | 9 |
| Marlene Gojanovic et al. | 2021 | Australian | cross section | Individuals aged 60 years and over in Australian | 809 | 66.4 | 65.60 | Continuous | (AWGS) 2019 | race, sex, and body fat percentage, sex×age interaction term | 9 |
| Risk of Sarcopenia with Different Dietary Inflammatory Index Levels | |||
|---|---|---|---|
| Population: Subjects with sarcopenia vs. normal subjects | |||
| Settings: Seven studies were conducted in Asia; three studies were conducted in the United States; one study was conducted in Australia | |||
| Trials: Subjects with sarcopenia | |||
| Controls: Normal subjects | |||
| Outcomes | OR (95% CI) a | No of participants (studies) | Quality of the evidence Comments (GRADE) |
| sarcopenia | 1.16 (1.06,1.27) | 19,954 (eleven studies) | ⊕⊕⊕ MODERATE b |
| GRADE working group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect Abbreviations: OR, odds ratio; CI, confidence interval | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diao, H.; Yan, F.; He, Q.; Li, M.; Zheng, Q.; Zhu, Q.; Fang, F.; Cui, W. Association between Dietary Inflammatory Index and Sarcopenia: A Meta-Analysis. Nutrients 2023, 15, 219. https://doi.org/10.3390/nu15010219
Diao H, Yan F, He Q, Li M, Zheng Q, Zhu Q, Fang F, Cui W. Association between Dietary Inflammatory Index and Sarcopenia: A Meta-Analysis. Nutrients. 2023; 15(1):219. https://doi.org/10.3390/nu15010219
Chicago/Turabian StyleDiao, Houze, Feifei Yan, Qingzhen He, Mingyuan Li, Qingzhao Zheng, Qing Zhu, Fang Fang, and Weiwei Cui. 2023. "Association between Dietary Inflammatory Index and Sarcopenia: A Meta-Analysis" Nutrients 15, no. 1: 219. https://doi.org/10.3390/nu15010219
APA StyleDiao, H., Yan, F., He, Q., Li, M., Zheng, Q., Zhu, Q., Fang, F., & Cui, W. (2023). Association between Dietary Inflammatory Index and Sarcopenia: A Meta-Analysis. Nutrients, 15(1), 219. https://doi.org/10.3390/nu15010219