The Acute Effect of Multi-Ingredient Antioxidant Supplementation following Ionizing Radiation
Abstract
1. Introduction
2. Materials and Methods
3. Results
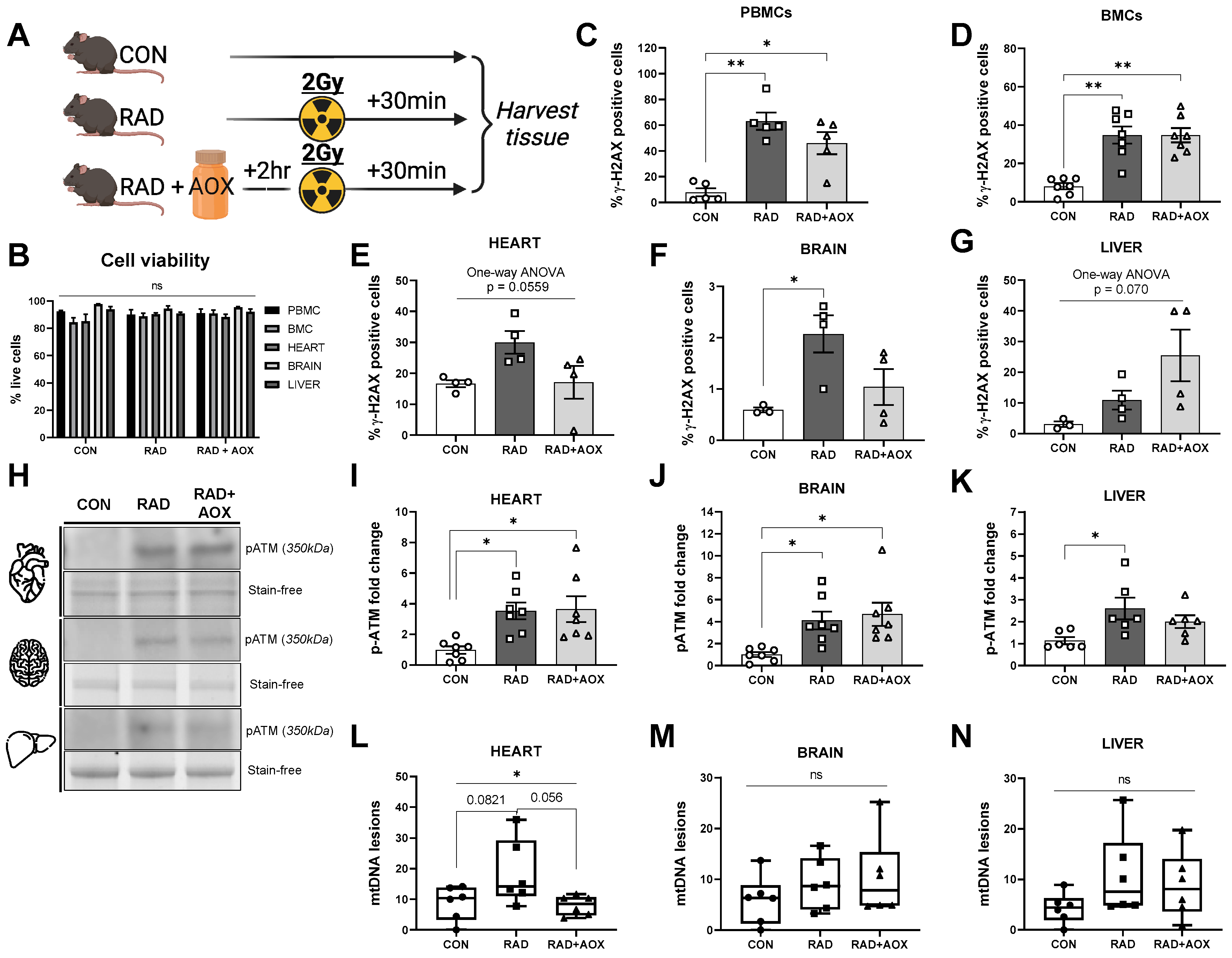
3.1. Ionizing Radiation Causes Acute DNA Damage across Multiple Tissues 30 Min following Exposure
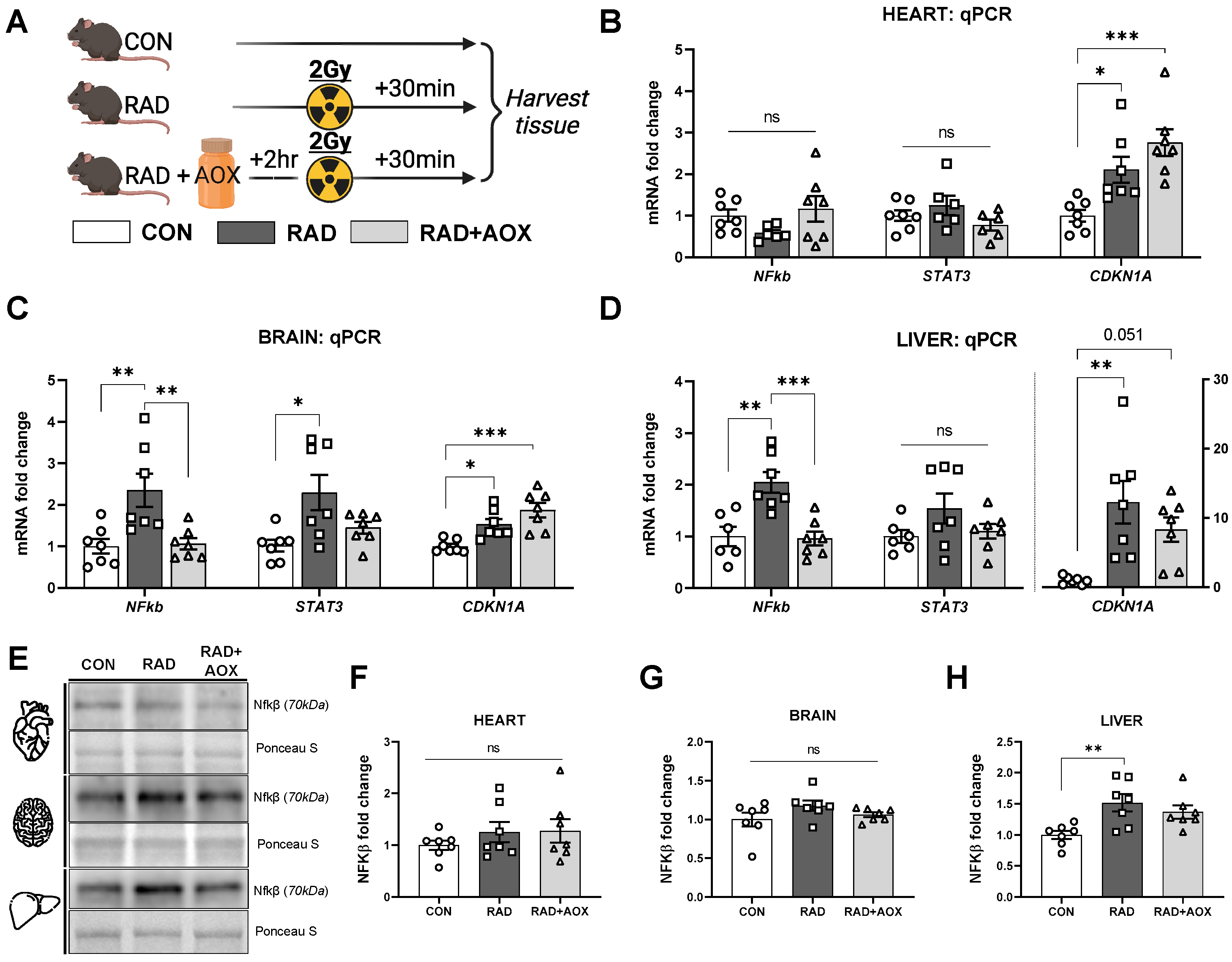
3.2. Multi-Ingredient Antioxidant Supplement Dampens the Inflammatory Stress Response Induced 24 h following Ionizing Radiation
3.3. Multi-Ingredient Antioxidant Supplement Attenuates Increase in Mitochondrial and Nuclear Encoded Mitochondrial Transcripts 24 h following Radiation Induced Damage
4. Discussion
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of Ionizing Radiation on Biological Molecules—Mechanisms of Damage and Emerging Methods of Detection. Antioxid Redox Signal 2014, 21, 260–292. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, H.; Kodama, S.; Matsuda, N.; Suzuki, K.; Watanabe, M. Involvement of Reactive Oxygen Species (ROS) in the induction of genetic instability by radiation. J. Radiat. Res. 2004, 45, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jenrow, K.A.; Brown, S.L. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat. Oncol. J. 2014, 32, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Burgio, E.; Piscitelli, P.; Migliore, L. Ionizing Radiation and Human Health: Reviewing Models of Exposure and Mechanisms of Cellular Damage. An Epigenetic Perspective. Int. J. Environ. Res. Public Health 2018, 15, 1971. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Choi, Y.; Ko, S.; Cha, E.S.; Kim, J.; Kim, Y.M.; Kong, K.A.; Seo, S.; Bang, Y.J.; Ha, Y.W. Projected lifetime cancer risks from occupational radiation exposure among diagnostic medical radiation workers in South Korea. BMC Cancer 2018, 18, 1206. [Google Scholar] [CrossRef]
- Vaiserman, A.; Koliada, A.; Zabuga, O.; Socol, Y. Health Impacts of Low-Dose Ionizing Radiation: Current Scientific Debates and Regulatory Issues. Dose-Response 2018, 16, 1559325818796331. [Google Scholar] [CrossRef]
- De González, A.B.; Darby, S. Risk of cancer from diagnostic X-rays: Estimates for the UK and 14 other countries. Lancet 2004, 363, 345–351. [Google Scholar] [CrossRef]
- Kamiya, K.; Ozasa, K.; Akiba, S.; Niwa, O.; Kodama, K.; Takamura, N.; Zaharieva, E.K.; Kimura, Y.; Wakeford, R. Long-term effects of radiation exposure on health. Lancet 2015, 386, 469–478. [Google Scholar] [CrossRef]
- Zablotska, L.B. 30 years After the Chernobyl Nuclear Accident: Time for Reflection and Re-evaluation of Current Disaster Preparedness Plans. J. Urban Health 2016, 93, 407–413. [Google Scholar] [CrossRef]
- Redon, C.E.; Dickey, J.S.; Bonner, W.M.; Sedelnikova, O.A. γ-H2AX as a biomarker of DNA damage induced by ionizing radiation in human peripheral blood lymphocytes and artificial skin. Adv. Space Res. 2009, 43, 1171–1178. [Google Scholar] [CrossRef]
- Olivieri, F.; Albertini, M.C.; Orciani, M.; Ceka, A.; Cricca, M.; Procopio, A.D.; Bonafè, M. DNA damage response (DDR) and senescence: Shuttled inflamma-miRNAs on the stage of inflamm-aging. Oncotarget 2015, 6, 35509–35521. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, F.; Coleman, M.A.; Jones, I.M.; Wyrobek, A.J. Candidate protein biodosimeters of human exposure to ionizing radiation. Int. J. Radiat. Biol. 2006, 82, 605–639. [Google Scholar] [CrossRef] [PubMed]
- Miquel, J. An update on the mitochondrial-DNA mutation hypothesis of cell aging. Mutat. Res./DNAging 1992, 275, 209–216. [Google Scholar] [CrossRef]
- Yakes, F.M.; Van Houten, B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. USA 1997, 94, 514–519. [Google Scholar] [CrossRef]
- Jia, S.; Ge, S.; Fan, X.; Leong, K.W.; Ruan, J. Promoting reactive oxygen species generation: A key strategy in nanosensitizer-mediated radiotherapy. Nanomedicine 2021, 16, 759–778. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Kuefner, M.A.; Brand, M.; Ehrlich, J.; Braga, L.; Uder, M.; Semelka, R.C. Effect of antioxidants on X-ray-induced γ-H2AX foci in human blood lymphocytes: Preliminary observations. Radiology 2012, 264, 59–67. [Google Scholar] [CrossRef]
- Velauthapillai, N.; Barfett, J.; Jaffer, H.; Mikulis, D.; Murphy, K. Antioxidants Taken Orally prior to Diagnostic Radiation Exposure Can Prevent DNA Injury. J. Vasc. Interv. Radiol. 2017, 28, 406–411. [Google Scholar] [CrossRef]
- Brand, M.; Sommer, M.; Ellmann, S.; Wuest, W.; May, M.S.; Eller, A.; Vogt, S.; Lell, M.M.; Kuefner, M.A.; Uder, M. Influence of Different Antioxidants on X-Ray Induced DNA Double-Strand Breaks (DSBs) Using γ-H2AX Immunofluorescence Microscopy in a Preliminary Study. PLoS ONE 2015, 10, e0127142. [Google Scholar] [CrossRef]
- Brown, S.L.; Kolozsvary, A.; Liu, J.; Jenrow, K.A.; Ryu, S.; Kim, J.H. Antioxidant Diet Supplementation Starting 24 Hours after Exposure Reduces Radiation Lethality. Radiat. Res. 2010, 173, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Özyurt, H.; Çevik, O.; Özgen, Z.; Özden, A.S.; Çadırcı, S.; Elmas, M.A.; Ercan, F.; Gören, M.Z.; Şener, G. Quercetin protects radiation-induced DNA damage and apoptosis in kidney and bladder tissues of rats. Free Radic. Res. 2014, 48, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.; Piskin, Ö.; Bas, Y.; Aydin, B.G.; Can, M.; Elmas, Ö.; Büyükuysal, Ç. Neuroprotective effects of Quercetin on radiation-induced brain injury in rats. J. Radiat. Res. 2018, 59, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.-L.; Han, X.-D.; Li, Y.; Chu, X.-F.; Miao, W.-M.; Zhang, J.-L.; Fan, S.-J. Astaxanthin attenuates total body irradiation-induced hematopoietic system injury in mice via inhibition of oxidative stress and apoptosis. Stem Cell Res. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Firdous, A.P.; Sindhu, E.R.; Ramnath, V.; Kuttan, R. Amelioration of radiation-induced damages in mice by carotenoid meso-zeaxanthin. Int. J. Radiat. Biol. 2012, 89, 171–181. [Google Scholar] [CrossRef]
- Sato, T.; Kinoshita, M.; Yamamoto, T.; Ito, M.; Nishida, T.; Takeuchi, M.; Saitoh, D.; Seki, S.; Mukai, Y. Treatment of Irradiated Mice with High-Dose Ascorbic Acid Reduced Lethality. PLoS ONE 2015, 10, e0117020. [Google Scholar] [CrossRef]
- Al-Meer, R.S.; El-Habit, O.H.M.; Al-Hazaa, A.A. Adaptive response to ionizing radiation and the role of vitamin B12 in amelioration radiation protection standards. J. King Saud. Univ. Sci. 2011, 23, 197–204. [Google Scholar] [CrossRef]
- Karami, M.; Asri-Rezaei, S.; Dormanesh, B.; Nazarizadeh, A. Comparative study of radioprotective effects of selenium nanoparticles and sodium selenite in irradiation-induced nephropathy of mice model. Int. J. Radiat. Biol. 2017, 94, 17–27. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, Z.; Weng, H.; Chen, Y.; Zhang, J.; Mei, S.; Wei, J.; Zhu, X.; Nong, Y.; Ruan, J.; et al. Folic Acid Preconditioning Alleviated Radiation-Induced Ovarian Dysfunction in Female Mice. Front. Nutr. 2022, 9, 1358. [Google Scholar] [CrossRef]
- Mohamed, H.A.; Said, R.S. Coenzyme Q10 attenuates inflammation and fibrosis implicated in radiation enteropathy through suppression of NF-kB/TGF-β/MMP-9 pathways. Int. Immunopharmacol. 2021, 92, 107347. [Google Scholar] [CrossRef]
- Noh, Y.H.; Kim, K.-Y.; Shim, M.S.; Choi, S.-H.; Choi, S.; Ellisman, M.H.; Weinreb, R.N.; Perkins, G.A.; Ju, W.-K. Inhibition of oxidative stress by coenzyme Q10 increases mitochondrial mass and improves bioenergetic function in optic nerve head astrocytes. Cell Death Dis. 2013, 4, e820. [Google Scholar] [CrossRef] [PubMed]
- Manda, K.; Ueno, M.; Moritake, T.; Anzai, K. Alpha-Lipoic acid attenuates x-irradiation-induced oxidative stress in mice. Cell Biol. Toxicol. 2007, 23, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Weiss, J.F. Radioprotection by vitamin E: Injectable vitamin E administered alone or with WR-3689 enhances survival of irradiated mice. Int. J. Radiat. Oncol. Biol. Phys. 1992, 23, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.C.; MacDonald, J.R.; Mahoney, D.J.; Parise, G.; Beal, M.F.; Tarnopolsky, M.A. Beneficial effects of creatine, CoQ10, and lipoic acid in mitochondrial disorders. Muscle Nerve 2007, 35, 235–242. [Google Scholar] [CrossRef]
- Roguin, A.; Goldstein, J.; Bar, O. Brain tumours among interventional cardiologists: A cause for alarm. Report of four new cases from two cities and a review of the literature. EuroIntervention 2012, 7, 1081–1086. [Google Scholar] [CrossRef]
- Hatch, M.; Cardis, E. Somatic health effects of Chernobyl: 30 years on. Eur. J. Epidemiol. 2017, 32, 1047–1054. [Google Scholar] [CrossRef]
- Hauptmann, M.; Byrnes, G.; Cardis, E.; Bernier, M.O.; Blettner, M.; Dabin, J.; Engels, H.; Istad, T.S.; Johansen, C.; Kaijser, M.; et al. Brain cancer after radiation exposure from CT examinations of children and young adults: Results from the EPI-CT cohort study. Lancet Oncol. 2022; In Press. [Google Scholar] [CrossRef]
- Lehle, S.; Hildebrand, D.G.; Merz, B.; Malak, P.N.; Becker, M.S.; Schmezer, P.; Essmann, F.; Schulze-Osthoff, K.; Rothfuss, O. LORD-Q: A long-run real-time PCR-based DNA-damage quantification method for nuclear and mitochondrial genome analysis. Nucleic Acids Res. 2014, 42, e41. [Google Scholar] [CrossRef]
- Dannenmann, B.; Lehle, S.; Lorscheid, S.; Huber, S.M.; Essmann, F.; Schulze-Osthoff, K. Simultaneous quantification of DNA damage and mitochondrial copy number by long-run DNA-damage quantification (LORD-Q). Oncotarget 2017, 8, 112417–112425. [Google Scholar] [CrossRef]
- Belzile-Dugas, E.; Eisenberg, M.J. Radiation-induced cardiovascular disease: Review of an underrecognized pathology. J. Am. Heart Assoc. 2021, 10, e021686. [Google Scholar] [CrossRef]
- Turnquist, C.; Harris, B.T.; Harris, C.C. Radiation-induced brain injury: Current concepts and therapeutic strategies targeting neuroinflammation. Neurooncol. Adv. 2020, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mettler, F.A.; Gus’kova, A.K.; Gusev, I. Health effects in those with acute radiation sickness from the Chernobyl accident. Health Phys. 2007, 93, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Bouville, A.; Chumak, V.; Inskip, P.D.; Kryuchkov, V.; Luckyanov, N. The chornobyl accident: Estimation of radiation doses received by the Baltic and Ukrainian cleanup workers. Radiat. Res. 2006, 1 Pt 2, 158–167. [Google Scholar] [CrossRef]
- Lawenda, B.D.; Kelly, K.M.; Ladas, E.J.; Sagar, S.M.; Vickers, A.; Blumberg, J.B. Should Supplemental Antioxidant Administration Be Avoided During Chemotherapy and Radiation Therapy? JNCI J. Natl. Cancer Inst. 2008, 100, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Bairati, I.; Meyer, F.; Gélinas, M.; Fortin, A.; Nabid, A.; Brochet, F.; Mercier, J.-P.; Têtu, B.; Harel, F.; Masse, B.; et al. A randomized trial of antioxidant vitamins to prevent second primary cancers in head and neck cancer patients. J. Natl. Cancer Inst. 2005, 97, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, R.; Vogt, S.; Kadenbach, B. Stress-mediated generation of deleterious ROS in healthy individuals—Role of cytochrome c oxidase. J. Mol. Med. 2020, 98, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Kadenbach, B.; Hüttemann, M.; Arnold, S.; Lee, I.; Bender, E. Mitochondrial energy metabolism is regulated via nuclear-coded subunits of cytochrome c oxidase. Free Radic. Biol. Med. 2000, 29, 211–221. [Google Scholar] [CrossRef]
- Birch-Machin, M.A.; Russell, E.V.; Latimer, J.A. Mitochondrial DNA damage as a biomarker for ultraviolet radiation exposure and oxidative stress. Br. J. Dermatol. 2013, 169 (Suppl. S2), 9–14. [Google Scholar] [CrossRef]
- Abu Rmilah, A.; Zhou, W.; Nelson, E.; Lin, L.; Amiot, B.; Nyberg, S.L. Understanding the marvels behind liver regeneration. Wiley Interdiscip. Rev. Dev. Biol. 2019, 8, e340. [Google Scholar] [CrossRef]
- Sender, R.; Milo, R. The distribution of cellular turnover in the human body. Nat. Med. 2021, 27, 45–48. [Google Scholar] [CrossRef]
- Najafi, M.; Motevaseli, E.; Shirazi, A.; Geraily, G.; Rezaeyan, A.; Norouzi, F.; Rezapoor, S.; Abdollahi, H. Mechanisms of inflammatory responses to radiation and normal tissues toxicity: Clinical implications. Int. J. Radiat. Biol. 2018, 94, 335–356. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Gupta, D.; Arora, R. NF-kB as a key player in regulation of cellular radiation responses and identification of radiation countermeasures. Discoveries 2015, 3, e35. [Google Scholar] [CrossRef] [PubMed]
- Kang, A.R.; Cho, J.H.; Lee, N.G.; Kwon, J.H.; Song, J.Y.; Hwang, S.G.; Jung, I.S.; Kim, J.S.; Um, H.D.; Oh, S.C.; et al. Radiation-induced IL-1β expression and secretion promote cancer cell migration/invasion via activation of the NF-κB-RIP1 pathway. Biochem. Biophys. Res. Commun. 2021, 534, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Molavi Pordanjani, S.; Jalal Hosseinimehr, S. The Role of NF-kB Inhibitors in Cell Response to Radiation. Curr. Med. Chem. 2016, 23, 3951–3963. [Google Scholar] [CrossRef]
- Wattel, A.; Kamel, S.; Prouillet, C.; Petit, J.P.; Lorget, F.; Offord, E.; Brazier, M. Flavonoid quercetin decreases osteoclastic differentiation induced by RANKL via a mechanism involving NFκB and AP-1. J. Cell Biochem. 2004, 92, 285–295. [Google Scholar] [CrossRef]
- Wambi, C.; Sanzari, J.; Wan, X.S.; Nuth, M.; Davis, J.; Ko, Y.-H.; Sayers, C.M.; Baran, M.; Ware, J.H.; Kennedy, A.R. Hematopoietic Cells and Improve Animal Survival after Total-Body Irradiation. Radiat. Res. 2008, 169, 384–396. [Google Scholar] [CrossRef]

| Ingredient | Dose (mg/kg) | Vendor | Cat. Number |
|---|---|---|---|
| Quercetin | 41 | Cayman Chemical Company | 10005169 |
| CoQ10 | 27.5 | MyBioSource LLC | MBS165643 |
| α-Lipoic Acid | 27.5 | Sigma-Aldrich | T1395 |
| Vitamin E | 27.5 | Sigma-Aldrich | T3126 |
| Vitamin C | 41 | Sigma-Aldrich | A5960 |
| Astaxanthin | 0.82 | Sigma-Aldrich | SML0982 |
| Zeaxanthin | 0.51 | Cayman Chemical Company | 10009992 |
| Folate | 0.082 | Sigma-Aldrich | F7876 |
| Selenium | 0.0205 | Sigma-Aldrich | 229865-5G |
| Vitamin B12 | 0.0103 | Sigma-Aldrich | V6629-250MG |
| Primary Antibodies | ||||
| Antibody | Species | Vendor | Cat. Number | Dilution |
| pATM | M | Thermo Scientific | MA1-2020 | 1:3000 in 5% BSA in 1× TBS |
| γH2AX | R | CST | 9718S | 1:5000 in 5% BSA in 1× TBS |
| NF-kβ | R | CST | 4764T | 1:1000 in 5% BSA in 1× TBS |
| SODI/II | R | abcam | ab16831 | 1:1000 in 5% BSA in 1× TBS |
| Total OXPHOS | M | abcam | ab110411 | 1:1000 in 5% BSA in 1× TBS |
| Secondary Antibodies | ||||
| Antibody | Vendor | Cat. Number | Dilution | |
| Peroxidase AffiniPure Donkey Anti-Mouse IgG (H + L) | Jackson ImmunoResearch | 715-035-151 | 1:20,000 in 5% BSA in 1× TBS | |
| Peroxidase AffiniPure Donkey Anti-Rabbit IgG (H + L) | Jackson ImmunoResearch | 711-035-152 | 1:20,000 in 5% BSA in 1× TBS | |
| Gene Symbol | Gene Name | Vendor | Assay ID |
|---|---|---|---|
| B2M | Beta-2-microglobulin | Thermo Fisher | Mm00437762_m1 |
| NF-kβ2 | Nuclear factor of kappa light polypeptide gene enhancer in B cells 2 | Thermo Fisher | Mm00479807_m1 |
| CDKN1A | Cyclin-dependent kinase inhibitor 1A (P21) | Thermo Fisher | Mm00432448_m1 |
| STAT3 | Signal transducer and activator of transcription 3 | Thermo Fisher | Mm01219775_m1 |
| COX1 | Cytochrome c oxidase subunit 1 | Thermo Fisher | Mm04225243_g1 |
| COX4I1 | Cytochrome c oxidase subunit 4I1 | Thermo Fisher | Mm01250094_m1 |
| Locus | Base Pairs | Primer Denotation | Primer Sequence |
|---|---|---|---|
| mtDNA (L) mouse | 3921 | MM.mtDNA.F | 5′-TCCTACTGGTCCGATTCCAC-3′ |
| MM.mtDNA.L.R | 5′-CGGTCTATGGAGGTTTGCAT-3′ | ||
| mtDNA (S) mouse | 74 | MM.mtDNA.F | 5′-TCCTACTGGTCCGATTCCAC-3′ |
| MM.mtDNA.S.R | 5′-GGCTCCGAGGCAAAGTATAG-3′ | ||
| Col1a1 (L) murine | 2637 | MM.col1a1.L1.F | 5′-CCGTTTGTCCCATTACTGCT-3′ |
| MM.col1a1.L1.R | 5′-AGCAAGGACGAGGACTTTGA-3′ | ||
| Col1a1 (S) murine | 60 | MM.col1a1.S.F | 5′-AAAGTGGGAATCTGGACACG-3′ |
| MM.col1a1.S.R | 5′-CAGAGGCCTTATTTCATTTTCG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xhuti, D.; Rebalka, I.A.; Minhas, M.; May, L.; Murphy, K.; Nederveen, J.P.; Tarnopolsky, M.A. The Acute Effect of Multi-Ingredient Antioxidant Supplementation following Ionizing Radiation. Nutrients 2023, 15, 207. https://doi.org/10.3390/nu15010207
Xhuti D, Rebalka IA, Minhas M, May L, Murphy K, Nederveen JP, Tarnopolsky MA. The Acute Effect of Multi-Ingredient Antioxidant Supplementation following Ionizing Radiation. Nutrients. 2023; 15(1):207. https://doi.org/10.3390/nu15010207
Chicago/Turabian StyleXhuti, Donald, Irena A. Rebalka, Mahek Minhas, Linda May, Kieran Murphy, Joshua P. Nederveen, and Mark A. Tarnopolsky. 2023. "The Acute Effect of Multi-Ingredient Antioxidant Supplementation following Ionizing Radiation" Nutrients 15, no. 1: 207. https://doi.org/10.3390/nu15010207
APA StyleXhuti, D., Rebalka, I. A., Minhas, M., May, L., Murphy, K., Nederveen, J. P., & Tarnopolsky, M. A. (2023). The Acute Effect of Multi-Ingredient Antioxidant Supplementation following Ionizing Radiation. Nutrients, 15(1), 207. https://doi.org/10.3390/nu15010207







