Dietary Methionine Restriction Alleviates Choline-Induced Tri-Methylamine-N-Oxide (TMAO) Elevation by Manipulating Gut Microbiota in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
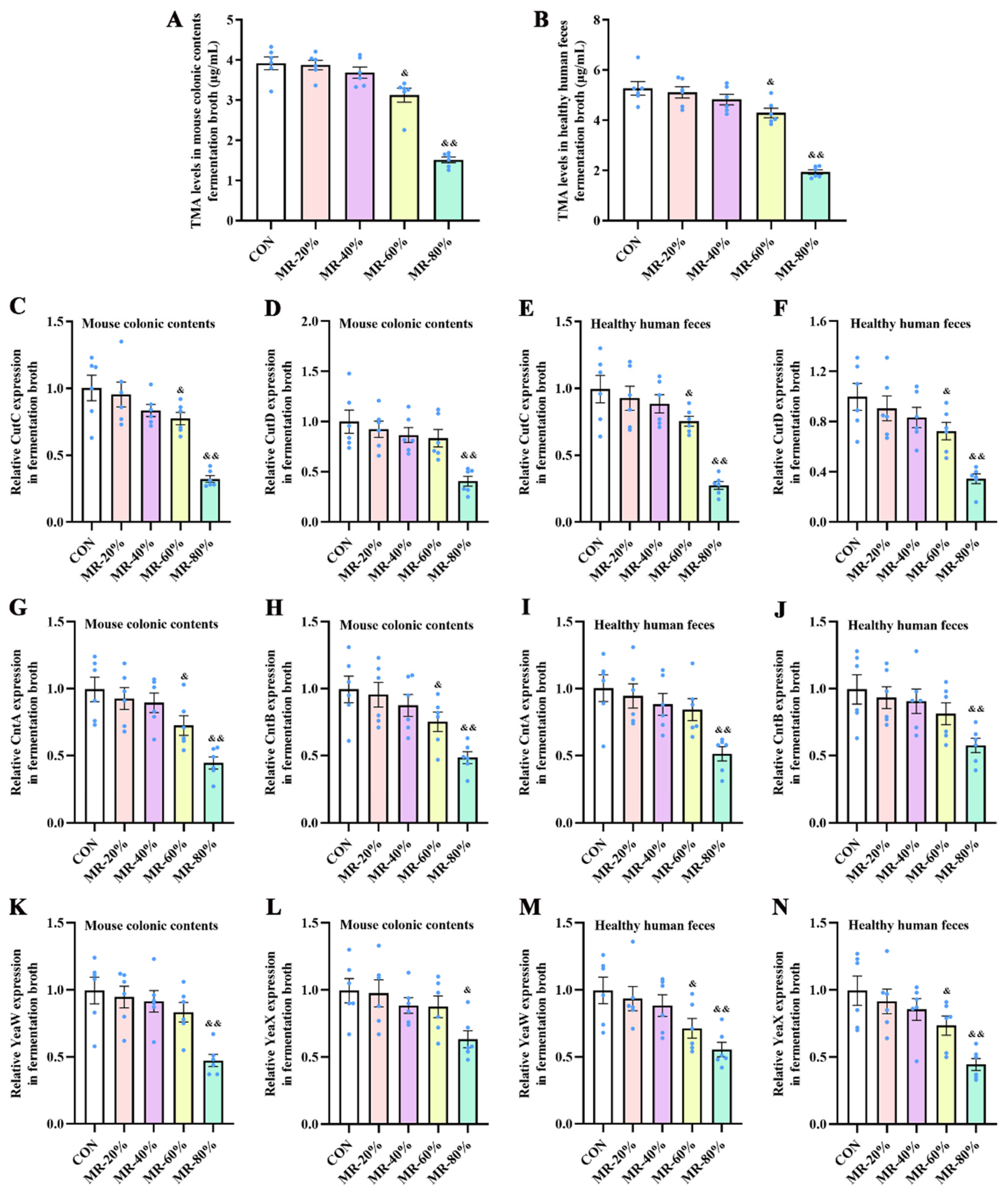
2.1.1. Experiment 1: Effects of Different Levels of MR on TMA Production by Bacteria from Colonic Contents of Healthy Mice and Feces from Healthy Humans
2.1.2. Experiment 2: Effects of MR on Circulating TMA/TMAO Levels and Gut Microbiota Composition in Mice
2.1.3. Experiment 3: Effects of MR/Sodium Butyrate Supplement on TMA Production from Escherichia fergusonii and Anaerococcus hydrogenalis
2.2. Detection of TMA/TMAO Levels
2.3. Detection of AS-Related Indicators
2.4. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.5. Assays for Activity of FMO3/CutC
2.6. Structural Analysis of Fecal Microbiota
2.7. Histological Analysis of Colon Tissues
2.8. Detection of SCFAs
2.9. Statistical Analysis
3. Results
3.1. MR Inhibited the TMA Production by the Intestinal Bacteria from Healthy Mice and Humans
3.2. MR Decreased Plasma TMA and TMAO Levels, Body Weight, and AS Index in H-CHO-Diet Fed-Mice
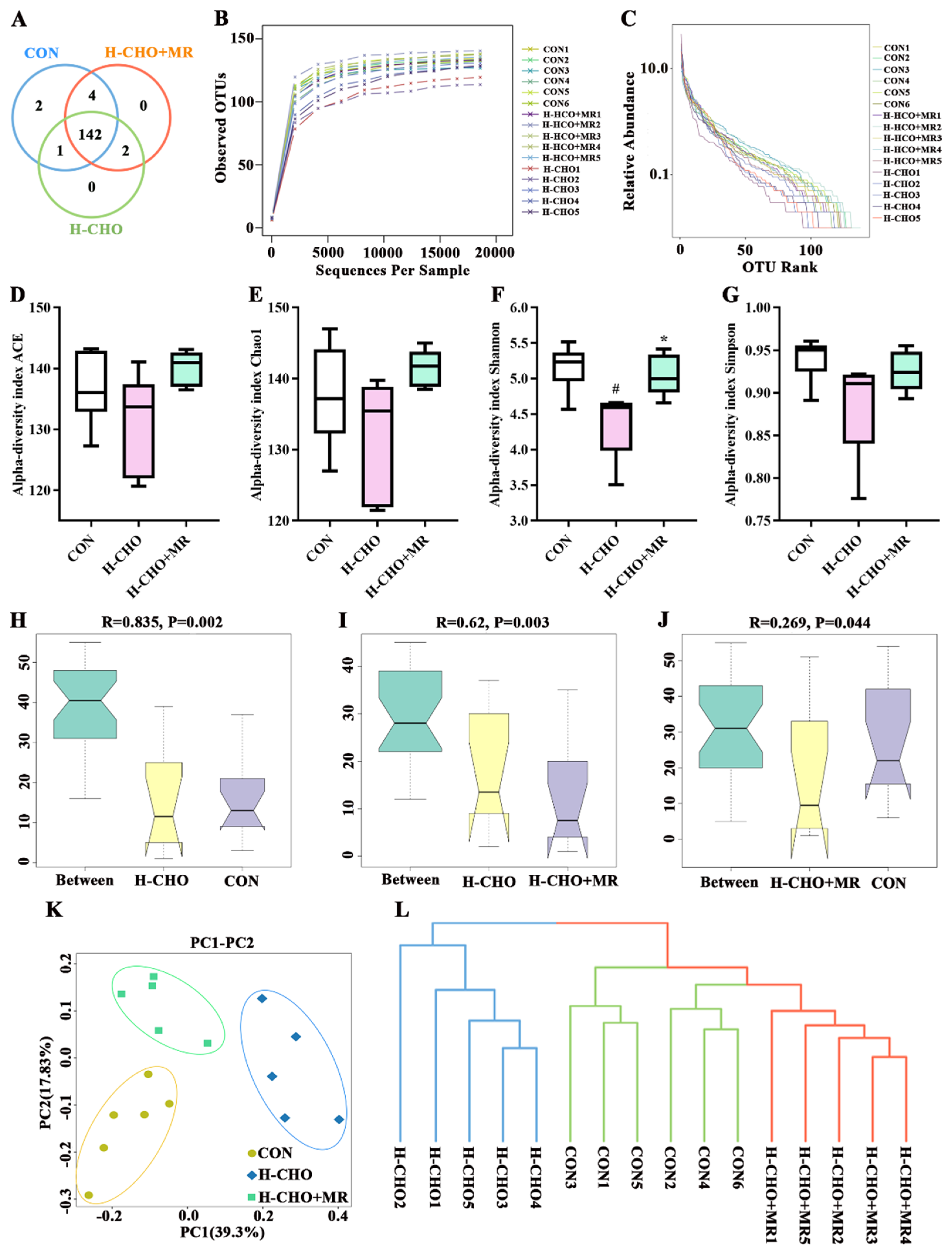
3.3. MR Improved Gut Microbiota Composition in H-CHO-Diet-Fed Mice
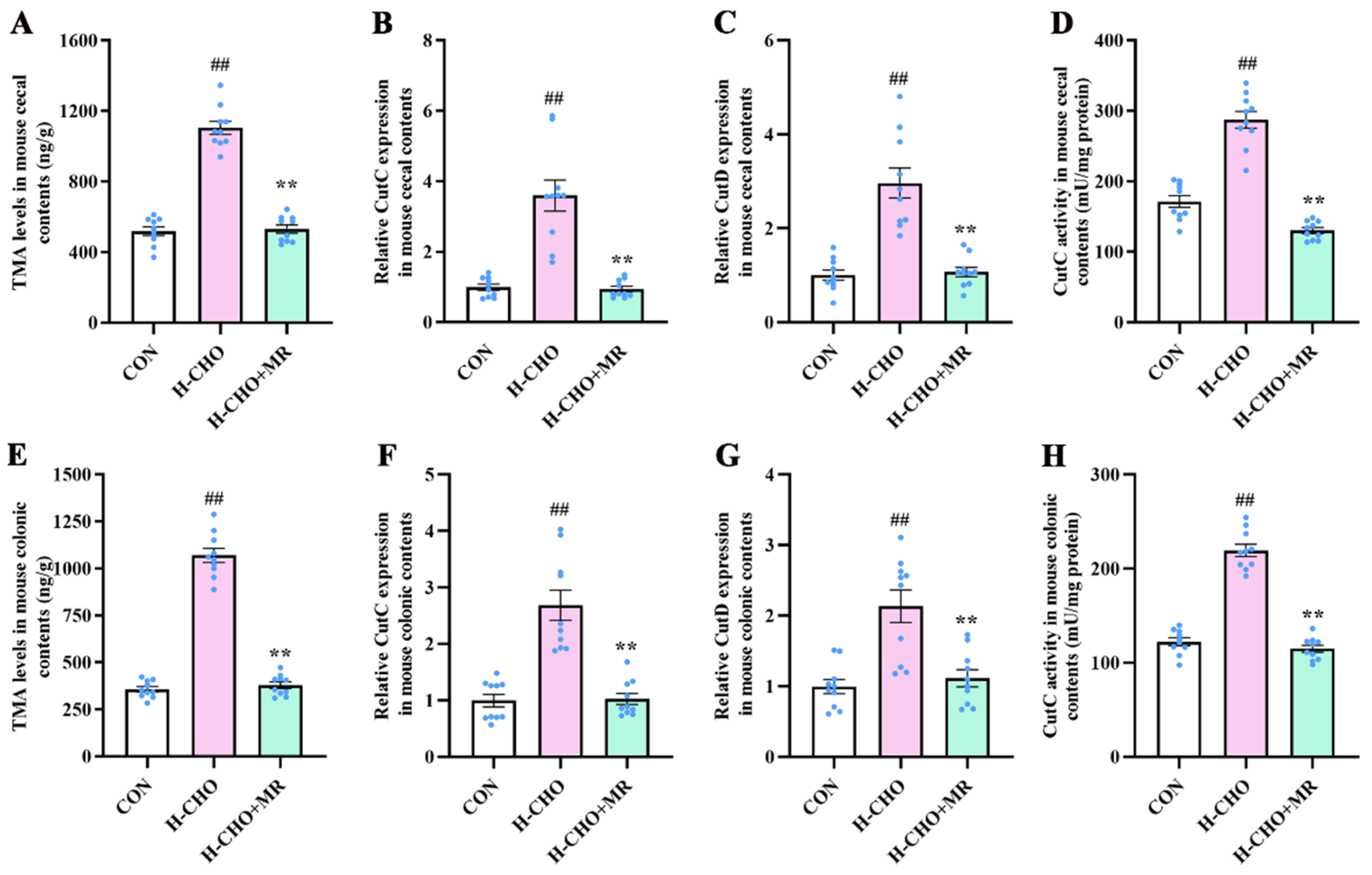
3.4. MR Decreased Intestinal TMA Levels in H-CHO-Diet-Fed Mice
3.5. MR Reduced TMA-Production by Bacteria Escherichia fergusonii and Anaerococcus hydrogenalis
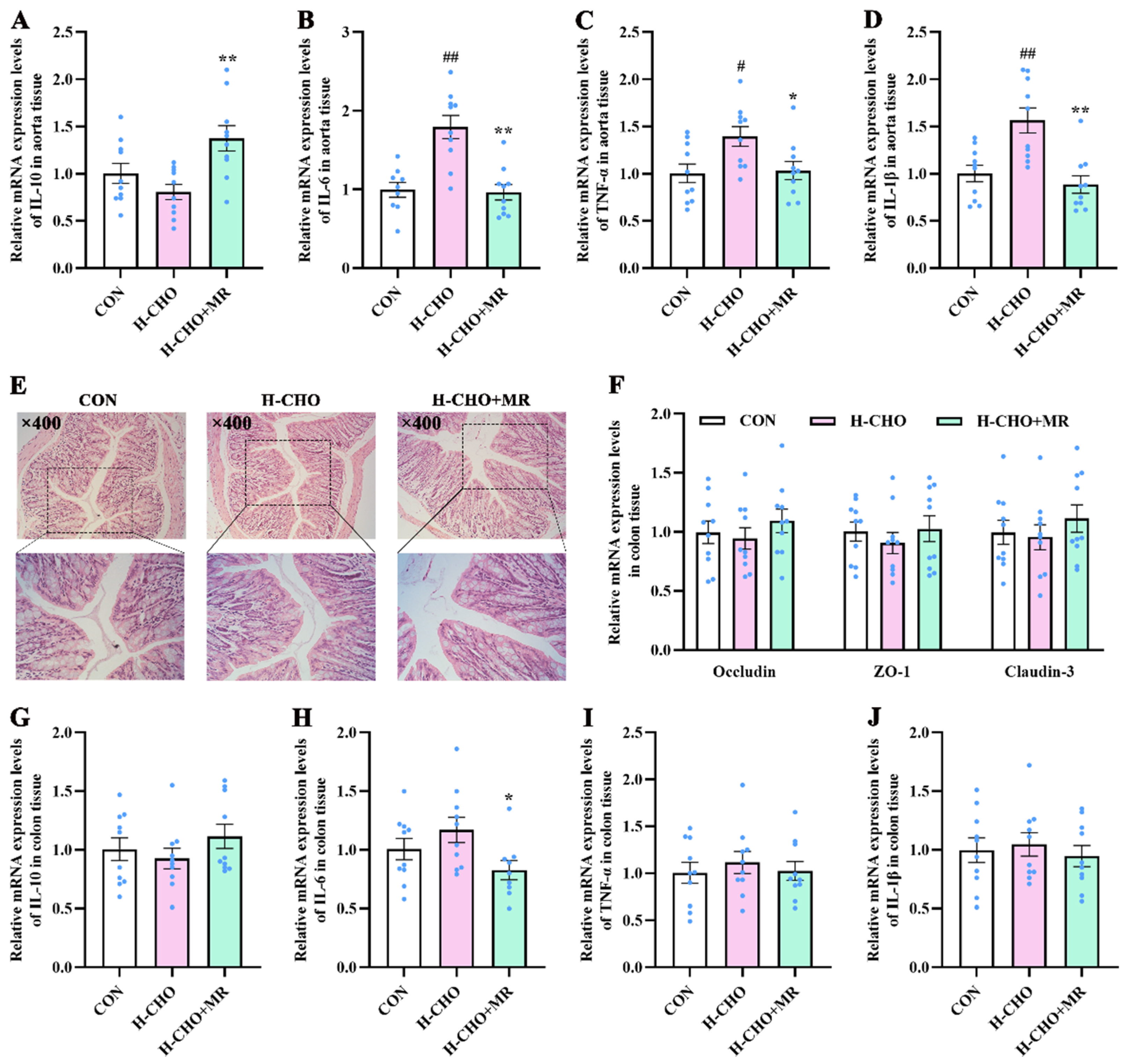
3.6. MR Reduced Inflammatory Response in Aorta Tissue of H-CHO-Diet-Fed Mice
3.7. MR Increased SCFA Levels in H-CHO-Diet-Fed Mice
3.8. Sodium Butyrate Supplementation Decreased TMA Production by Bacteria Escherichia fergusonii and Anaerococcus hydrogenalis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeisel, S.; Warrier, M. Trimethylamine N-Oxide, the Microbiome, and Heart and Kidney Disease. Annu. Rev. Nutr. 2017, 37, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Jameson, E.; Doxey, A.; Airs, R.; Purdy, K.; Murrell, J.; Chen, Y. Metagenomic data-mining reveals contrasting microbial populations responsible for trimethylamine formation in human gut and marine ecosystems. Microb. Genom. 2016, 2, e000080. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.; de Aguiar Vallim, T.; Wang, Z.; Shih, D.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef]
- Zeisel, S.; Mar, M.; Howe, J.; Holden, J. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef]
- Jia, Q.; Xie, Y.; Lu, C.; Zhang, A.; Lu, Y.; Lv, S.; Zhang, J. Endocrine organs of cardiovascular diseases: Gut microbiota. J. Cell. Mol. Med. 2019, 23, 2314–2323. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.; Koeth, R.; Levison, B.; Dugar, B.; Feldstein, A.; Britt, E.; Fu, X.; Chung, Y.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Organ, C.; Otsuka, H.; Bhushan, S.; Wang, Z.; Bradley, J.; Trivedi, R.; Polhemus, D.; Tang, W.; Wu, Y.; Hazen, S.; et al. Choline Diet and Its Gut Microbe-Derived Metabolite, Trimethylamine N-Oxide, Exacerbate Pressure Overload-Induced Heart Failure. Circ. Heart Fail. 2016, 9, e002314. [Google Scholar] [CrossRef]
- Tang, W.; Wang, Z.; Levison, B.; Koeth, R.; Britt, E.; Fu, X.; Wu, Y.; Hazen, S. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Heianza, Y.; Ma, W.; Manson, J.; Rexrode, K.; Qi, L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J. Am. Heart Assoc. 2017, 6, e004947. [Google Scholar] [CrossRef]
- al-Waiz, M.; Mikov, M.; Mitchell, S.; Smith, R. The exogenous origin of trimethylamine in the mouse. Metab. Clin. Exp. 1992, 41, 135–136. [Google Scholar] [CrossRef]
- Koeth, R.; Levison, B.; Culley, M.; Buffa, J.; Wang, Z.; Gregory, J.; Org, E.; Wu, Y.; Li, L.; Smith, J.; et al. γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014, 20, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.; Zhang, Q.; Mi, M. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio 2016, 7, e02210-15. [Google Scholar] [CrossRef]
- Schmedes, M.; Brejnrod, A.; Aadland, E.; Kiilerich, P.; Kristiansen, K.; Jacques, H.; Lavigne, C.; Graff, I.; Eng, Ø.; Holthe, A.; et al. The Effect of Lean-Seafood and Non-Seafood Diets on Fecal Metabolites and Gut Microbiome: Results from a Randomized Crossover Intervention Study. Mol. Nutr. Food Res. 2019, 63, e1700976. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.-Y.; Huang, F.-Q.; Lao, X.; Lu, Y.; Gao, X.; Alolga, R.N.; Yin, K.; Zhou, X.; Wang, Y.; Liu, B. Integrated metagenomics identifies a crucial role for trimethylamine-producing Lachnoclostridium in promoting atherosclerosis. NPJ Biofilms Microbiomes 2022, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Thibodeaux, C.; van der Donk, W. Converging on a mechanism for choline degradation. Proc. Natl. Acad. Sci. USA 2012, 109, 21184–21185. [Google Scholar] [CrossRef]
- Iglesias-Carres, L.; Essenmacher, L.; Racine, K.; Neilson, A. Development of a High-Throughput Method to Study the Inhibitory Effect of Phytochemicals on Trimethylamine Formation. Nutrients 2021, 13, 1466. [Google Scholar] [CrossRef]
- Krüger, R.; Merz, B.; Rist, M.; Ferrario, P.; Bub, A.; Kulling, S.; Watzl, B. Associations of current diet with plasma and urine TMAO in the KarMeN study: Direct and indirect contributions. Mol. Nutr. Food Res. 2017, 61, 1700363. [Google Scholar] [CrossRef]
- Simó, C.; García-Cañas, V. Dietary bioactive ingredients to modulate the gut microbiota-derived metabolite TMAO. New opportunities for functional food development. Food Funct. 2020, 11, 6745–6776. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, L.; Jiang, X.; Xue, C.; Chi, N.; Zhang, T.; Wang, Y. Effects of dietary choline, betaine, and L-carnitine on the generation of trimethylamine-N-oxide in healthy mice. J. Food Sci. 2020, 85, 2207–2215. [Google Scholar] [CrossRef]
- Longchamp, A.; Mirabella, T.; Arduini, A.; MacArthur, M.; Das, A.; Treviño-Villarreal, J.; Hine, C.; Ben-Sahra, I.; Knudsen, N.; Brace, L.; et al. Amino Acid Restriction Triggers Angiogenesis via GCN2/ATF4 Regulation of VEGF and H2S Production. Cell 2018, 173, 117–129. [Google Scholar] [CrossRef]
- Fang, H.; Stone, K.; Wanders, D.; Forney, L.; Gettys, T. The Origins, Evolution, and Future of Dietary Methionine Restriction. Annu. Rev. Nutr. 2022, 42, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Lees, E.; Krol, E.; Shearer, K.; Mody, N.; Gettys, T.; Delibegovic, M. Effects of hepatic protein tyrosine phosphatase 1B and methionine restriction on hepatic and whole-body glucose and lipid metabolism in mice. Metab. Clin. Exp. 2015, 64, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Y.; Sun, J.; Zhang, J.; Guo, H.; Shi, Y.; Cheng, X.; Tang, X.; Le, G. Dietary methionine restriction reduces hepatic steatosis and oxidative stress in high-fat-fed mice by promoting H2S production. Food Funct. 2019, 10, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.; Xu, Y.; Luo, T.; Ge, Y.; Jiang, Y.; Shi, Y.; Sun, J.; Le, G. Dietary methionine restriction improves the gut microbiota and reduces intestinal permeability and inflammation in high-fat-fed mice. Food Funct. 2019, 10, 5952–5968. [Google Scholar] [CrossRef] [PubMed]
- Barcena, C.; Quiros, P.M.; Durand, S.; Mayoral, P.; Rodriguez, F.; Caravia, X.M.; Marino, G.; Garabaya, C.; Fernandez-Garcia, M.T.; Kroemer, G.; et al. Methionine Restriction Extends Lifespan in Progeroid Mice and Alters Lipid and Bile Acid Metabolism. Cell Rep. 2018, 24, 2392–2403. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Wu, G.; Sun, J.; Wang, Y.; Guo, H.; Shi, Y.; Cheng, X.; Tang, X.; Le, G. Dietary methionine restriction regulated energy and protein homeostasis by improving thyroid function in high fat diet mice. Food Funct. 2018, 9, 3718–3731. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, M.; Xu, Y.; Qian, J.; Le, G.; Xie, Y. High dietary methionine intake may contribute to the risk of nonalcoholic fatty liver disease by inhibiting hepatic H2S production. Food Res. Int. 2022, 158, 111507. [Google Scholar] [CrossRef]
- Wang, L.; Ren, B.; Hui, Y.; Chu, C.; Zhao, Z.; Zhang, Y.; Zhao, B.; Shi, R.; Ren, J.; Dai, X.; et al. Methionine Restriction Regulates Cognitive Function in High-Fat Diet-Fed Mice: Roles of Diurnal Rhythms of SCFAs Producing- and Inflammation-Related Microbes. Mol. Nutr. Food Res. 2020, 64, e2000190. [Google Scholar] [CrossRef]
- Wu, G.; Shi, Y.; Han, L.; Feng, C.; Ge, Y.; Yu, Y.; Tang, X.; Cheng, X.; Sun, J.; Le, G.W. Dietary Methionine Restriction Ameliorated Fat Accumulation, Systemic Inflammation, and Increased Energy Metabolism by Altering Gut Microbiota in Middle-Aged Mice Administered Different Fat Diets. J. Agric. Food Chem. 2020, 68, 7745–7756. [Google Scholar] [CrossRef]
- Martínez-del Campo, A.; Bodea, S.; Hamer, H.; Marks, J.; Haiser, H.; Turnbaugh, P.; Balskus, E. Characterization and detection of a widely distributed gene cluster that predicts anaerobic choline utilization by human gut bacteria. mBio 2015, 6, e00042-15. [Google Scholar] [CrossRef]
- Wang, Z.; Roberts, A.; Buffa, J.; Levison, B.; Zhu, W.; Org, E.; Gu, X.; Huang, Y.; Zamanian-Daryoush, M.; Culley, M.; et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 2015, 163, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, X.; Su, C.; Xi, M.; Zhang, X.; Jiang, Z.; Wang, L.; Hong, B. Butyrate protects against high-fat diet-induced atherosclerosis via up-regulating ABCA1 expression in apolipoprotein E-deficiency mice. Br. J. Pharmacol. 2020, 177, 1754–1772. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Y.; Sun, J.; Zhang, Y.; Luo, T.; Li, B.; Jiang, Y.; Shi, Y.; Le, G. Dietary methionine restriction ameliorates the impairment of learning and memory function induced by obesity in mice. Food Funct. 2019, 10, 1411–1425. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Yang, Y.; Xu, Y.; Gao, Q.; Wu, G.; Jiang, Y.; Sun, J.; Shi, Y.; Le, G. Dietary methionine restriction improves glucose metabolism in the skeletal muscle of obese mice. Food Funct. 2019, 10, 2676–2690. [Google Scholar] [CrossRef]
- Shuai, W.; Wen, J.; Li, X.; Wang, D.; Li, Y.; Xiang, J. High-Choline Diet Exacerbates Cardiac Dysfunction, Fibrosis, and Inflammation in a Mouse Model of Heart Failure With Preserved Ejection Fraction. J. Card. Fail. 2020, 26, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Gacias, M.; Gaspari, S.; Santos, P.; Tamburini, S.; Andrade, M.; Zhang, F.; Shen, N.; Tolstikov, V.; Kiebish, M.; Dupree, J.; et al. Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior. eLife 2016, 5, e13442. [Google Scholar] [CrossRef]
- Kuka, J.; Liepinsh, E.; Makrecka-Kuka, M.; Liepins, J.; Cirule, H.; Gustina, D.; Loza, E.; Zharkova-Malkova, O.; Grinberga, S.; Pugovics, O.; et al. Suppression of intestinal microbiota-dependent production of pro-atherogenic trimethylamine N-oxide by shifting L-carnitine microbial degradation. Life Sci. 2014, 117, 84–92. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.; Xing, R.; Cui, X.; Xiao, Y.; Xie, L.; You, P.; Wang, T.; Zeng, L.; Peng, W.; et al. Hyperlipidemia induces typical atherosclerosis development in Ldlr and Apoe deficient rats. Atherosclerosis 2018, 271, 26–35. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Xiao, H.; Shi, Y.; Le, G.; Sun, J. Isolation of lactobacillus reuteri from Peyer’s patches and their effects on sIgA production and gut microbiota diversity. Mol. Nutr. Food Res. 2016, 60, 2020–2030. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, M.; Xu, Y.; Qian, J.; Le, G.; Xie, Y. Dietary Methionine via Dose-Dependent Inhibition of Short-Chain Fatty Acid Production Capacity Contributed to a Potential Risk of Cognitive Dysfunction in Mice. J. Agric. Food Chem. 2022, 70, 15225–15243. [Google Scholar] [CrossRef]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Cotter, P.; Healy, S.; Marques, T.; O’Sullivan, O.; Fouhy, F.; Clarke, S.; O’Toole, P.; Quigley, E.; Stanton, C.; et al. Composition and energy harvesting capacity of the gut microbiota: Relationship to diet, obesity and time in mouse models. Gut 2010, 59, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wang, Z.; Kennedy, D.; Wu, Y.; Buffa, J.; Agatisa-Boyle, B.; Li, X.; Levison, B.; Hazen, S. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Tan, C.; Xu, Y.; Liu, Y. Gut microbiota-derived trimethylamine-N-oxide: A bridge between dietary fatty acid and cardiovascular disease? Food Res. Int. 2020, 138, 109812. [Google Scholar] [CrossRef]
- Ramireddy, L.; Tsen, H.; Chiang, Y.; Hung, C.; Chen, F.; Yen, H. The gene expression and bioinformatic analysis of choline trimethylamine-lyase (CutC) and its activating enzyme (CutD) for gut microbes and comparison with their TMA production levels. Curr. Res. Microb. Sci. 2021, 2, 100043. [Google Scholar] [CrossRef]
- Petriello, M.; Hoffman, J.; Sunkara, M.; Wahlang, B.; Perkins, J.; Morris, A.; Hennig, B. Dioxin-like pollutants increase hepatic flavin containing monooxygenase (FMO3) expression to promote synthesis of the pro-atherogenic nutrient biomarker trimethylamine N-oxide from dietary precursors. J. Nutr. Biochem. 2016, 33, 145–153. [Google Scholar] [CrossRef]
- Koeth, R.; Wang, Z.; Levison, B.; Buffa, J.; Org, E.; Sheehy, B.; Britt, E.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Y.; Li, B.; Xie, Y.; Shi, Y.; Le, G. Dietary methionine restriction improves gut microbiota composition and prevents cognitive impairment in D-galactose-induced aging mice. Food Funct. 2022, 13, 12896–12914. [Google Scholar] [CrossRef]
- Romano, K.; Vivas, E.; Amador-Noguez, D.; Rey, F. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 2015, 6, e02481. [Google Scholar] [CrossRef]
- Zhang, X.; Gérard, P. Diet-gut microbiota interactions on cardiovascular disease. Comput. Struct. Biotechnol. J. 2022, 20, 1528–1540. [Google Scholar] [CrossRef]
- Falony, G.; Vieira-Silva, S.; Raes, J. Microbiology Meets Big Data: The Case of Gut Microbiota-Derived Trimethylamine. Annu. Rev. Microbiol. 2015, 69, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Farré, R.; Fiorani, M.; Abdu Rahiman, S.; Matteoli, G. Intestinal Permeability, Inflammation and the Role of Nutrients. Nutrients 2020, 12, 1185. [Google Scholar] [CrossRef]
- Jaworska, K.; Huc, T.; Samborowska, E.; Dobrowolski, L.; Bielinska, K.; Gawlak, M.; Ufnal, M. Hypertension in rats is associated with an increased permeability of the colon to TMA, a gut bacteria metabolite. PLoS ONE 2017, 12, e0189310. [Google Scholar] [CrossRef] [PubMed]
- Manor, O.; Zubair, N.; Conomos, M.; Xu, X.; Rohwer, J.; Krafft, C.; Lovejoy, J.; Magis, A. A Multi-omic Association Study of Trimethylamine N-Oxide. Cell Rep. 2018, 24, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yin, A.; Li, H.; Wang, R.; Wu, G.; Shen, J.; Zhang, M.; Wang, L.; Hou, Y.; Ouyang, H.; et al. Dietary Modulation of Gut Microbiota Contributes to Alleviation of Both Genetic and Simple Obesity in Children. EBioMedicine 2015, 2, 968–984. [Google Scholar] [CrossRef]
- Zhu, B.; Zhai, Y.; Ji, M.; Wei, Y.; Wu, J.; Xue, W.; Tao, W.; Wu, H. Alisma orientalis Beverage Treats Atherosclerosis by Regulating Gut Microbiota in ApoE Mice. Front. Pharmacol. 2020, 11, 570555. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xia, G.; He, Y.; Liao, S.; Yin, J.; Sheng, H.; Zhou, H. Distribution characteristics of trimethylamine N-oxide and its association with gut microbiota. J. South. Med. Univ. 2016, 36, 455–460. [Google Scholar]
- Wang, S.; Lv, D.; Jiang, S.; Jiang, J.; Liang, M.; Hou, F.; Chen, Y. Quantitative reduction in short-chain fatty acids, especially butyrate, contributes to the progression of chronic kidney disease. Clin. Sci. 2019, 133, 1857–1870. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Xu, J.; Xue, Z.; Zhang, M.; Pang, X.; Zhang, X.; Zhao, L. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci. Rep. 2015, 5, 14405. [Google Scholar] [CrossRef]
- Lv, Z.; Shan, X.; Tu, Q.; Wang, J.; Chen, J.; Yang, Y. Ginkgolide B treatment regulated intestinal flora to improve high-fat diet induced atherosclerosis in ApoE mice. Biomed. Pharmacother. 2021, 134, 111100. [Google Scholar] [CrossRef]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L.; et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 2020, 12, 1832857. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Li, S.; Orfila, C.; Shen, X.; Zhou, S.; Linhardt, R.; Ye, X.; Chen, S. Depolymerized RG-I-enriched pectin from citrus segment membranes modulates gut microbiota, increases SCFA production, and promotes the growth of Bifidobacterium spp., Lactobacillus spp. and Faecalibaculum spp. Food Funct. 2019, 10, 7828–7843. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia intestinalis: A Beneficial Gut Organism From the Discoveries in Genus and Species. Front. Cell. Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef]
- Zhang, Z.; Taylor, L.; Shommu, N.; Ghosh, S.; Reimer, R.; Panaccione, R.; Kaur, S.; Hyun, J.; Cai, C.; Deehan, E.; et al. A Diversified Dietary Pattern Is Associated With a Balanced Gut Microbial Composition of Faecalibacterium and Escherichia/Shigella in Patients With Crohn’s Disease in Remission. J. Crohn’s Colitis 2020, 14, 1547–1557. [Google Scholar] [CrossRef]
- Bergman, E. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yi, C.; Katiraei, S.; Kooijman, S.; Zhou, E.; Chung, C.; Gao, Y.; van den Heuvel, J.; Meijer, O.; Berbée, J.; et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 2018, 67, 1269–1279. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.; Martin, R.; Lefevre, M.; Cefalu, W.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Jiang, Q.; Yin, Y. Butyrate in Energy Metabolism: There Is Still More to Learn. Trends Endocrinol. Metab. TEM 2021, 32, 159–169. [Google Scholar] [CrossRef]
- Iversen, K.; Dicksved, J.; Zoki, C.; Fristedt, R.; Pelve, E.; Langton, M.; Landberg, R. The Effects of High Fiber Rye, Compared to Refined Wheat, on Gut Microbiota Composition, Plasma Short Chain Fatty Acids, and Implications for Weight Loss and Metabolic Risk Factors (the RyeWeight Study). Nutrients 2022, 14, 1669. [Google Scholar] [CrossRef]
- Spring, S.; Singh, A.; Zapata, R.; Chelikani, P.; Pezeshki, A. Methionine Restriction Partly Recapitulates the Sympathetically Mediated Enhanced Energy Expenditure Induced by Total Amino Acid Restriction in Rats. Nutrients 2019, 11, 707. [Google Scholar] [CrossRef]
- Wanders, D.; Burk, D.; Cortez, C.; Van, N.; Stone, K.; Baker, M.; Mendoza, T.; Mynatt, R.; Gettys, T. UCP1 is an essential mediator of the effects of methionine restriction on energy balance but not insulin sensitivity. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Seldin, M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.; Lusis, A.; Shih, D. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.; Hansson, G. Inflammation in atherosclerosis: From pathophysiology to practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- van den Munckhof, I.; Kurilshikov, A.; Ter Horst, R.; Riksen, N.; Joosten, L.; Zhernakova, A.; Fu, J.; Keating, S.; Netea, M.; de Graaf, J.; et al. Role of gut microbiota in chronic low-grade inflammation as potential driver for atherosclerotic cardiovascular disease: A systematic review of human studies. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2018, 19, 1719–1734. [Google Scholar] [CrossRef]
- Mahdavi, J.; Sondén, B.; Hurtig, M.; Olfat, F.; Forsberg, L.; Roche, N.; Angstrom, J.; Larsson, T.; Teneberg, S.; Karlsson, K.; et al. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 2002, 297, 573–578. [Google Scholar] [CrossRef]
- Moran, A. The role of endotoxin in infection: Helicobacter pylori and Campylobacter jejuni. Sub-Cell. Biochem. 2010, 53, 209–240. [Google Scholar] [CrossRef]
- Wu, D.; Cao, M.; Peng, J.; Li, N.; Yi, S.; Song, L.; Wang, X.; Zhang, M.; Zhao, J. The effect of trimethylamine N-oxide on Helicobacter pylori-induced changes of immunoinflammatory genes expression in gastric epithelial cells. Int. Immunopharmacol. 2017, 43, 172–178. [Google Scholar] [CrossRef]
- Sawayama, Y.; Ariyama, I.; Hamada, M.; Otaguro, S.; Machi, T.; Taira, Y.; Hayashi, J. Association between chronic Helicobacter pylori infection and acute ischemic stroke: Fukuoka Harasanshin Atherosclerosis Trial (FHAT). Atherosclerosis 2005, 178, 303–309. [Google Scholar] [CrossRef]
- Tang, R.; Jiang, Y.; Tan, A.; Ye, J.; Xian, X.; Xie, Y.; Wang, Q.; Yao, Z.; Mo, Z. 16S rRNA gene sequencing reveals altered composition of gut microbiota in individuals with kidney stones. Urolithiasis 2018, 46, 503–514. [Google Scholar] [CrossRef]
- Sánchez-Alcoholado, L.; Ordóñez, R.; Otero, A.; Plaza-Andrade, I.; Laborda-Illanes, A.; Medina, J.; Ramos-Molina, B.; Gómez-Millán, J.; Queipo-Ortuño, M. Gut Microbiota-Mediated Inflammation and Gut Permeability in Patients with Obesity and Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 6782. [Google Scholar] [CrossRef] [PubMed]
- Cruz, B.; Conceição, L.; Mendes, T.; Ferreira, C.; Gonçalves, R.; Peluzio, M. Use of the synbiotic VSL#3 and yacon-based concentrate attenuates intestinal damage and reduces the abundance of Candidatus Saccharimonas in a colitis-associated carcinogenesis model. Food Res. Int. 2020, 137, 109721. [Google Scholar] [CrossRef] [PubMed]
- Ables, G.; Johnson, J. Pleiotropic responses to methionine restriction. Exp. Gerontol. 2017, 94, 83–88. [Google Scholar] [CrossRef]
- McCarty, M.; Barroso-Aranda, J.; Contreras, F. The low-methionine content of vegan diets may make methionine restriction feasible as a life extension strategy. Med. Hypotheses 2009, 72, 125–128. [Google Scholar] [CrossRef]
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food proteins from animals and plants: Differences in the nutritional and functional properties. Trends Food Sci. Technol. 2022, 119, 428–442. [Google Scholar] [CrossRef]
- Epner, D.; Morrow, S.; Wilcox, M.; Houghton, J. Nutrient intake and nutritional indexes in adults with metastatic cancer on a phase I clinical trial of dietary methionine restriction. Nutr. Cancer 2002, 42, 158–166. [Google Scholar] [CrossRef]
- Orentreich, N.; Matias, J.; DeFelice, A.; Zimmerman, J. Low methionine ingestion by rats extends life span. J. Nutr. 1993, 123, 269–274. [Google Scholar] [CrossRef]
- Richie, J.; Leutzinger, Y.; Parthasarathy, S.; Malloy, V.; Orentreich, N.; Zimmerman, J. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1994, 8, 1302–1307. [Google Scholar] [CrossRef]
- Gao, X.; Sanderson, S.M.; Dai, Z.; Reid, M.A.; Cooper, D.E.; Lu, M.; Richie, J.P., Jr.; Ciccarella, A.; Calcagnotto, A.; Mikhael, P.G.; et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature 2019, 572, 397–401. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Miyake, K.; Han, Q.; Li, S.; Tan, Y.; Igarashi, K.; Kiyuna, T.; Miyake, M.; Higuchi, T.; Oshiro, H.; et al. Oral recombinant methioninase (o-rMETase) is superior to injectable rMETase and overcomes acquired gemcitabine resistance in pancreatic cancer. Cancer Lett. 2018, 432, 251–259. [Google Scholar] [CrossRef]
- Tan, Y.; Zavala, J.; Xu, M.; Zavala, J.; Hoffman, R. Serum methionine depletion without side effects by methioninase in metastatic breast cancer patients. Anticancer Res. 1996, 16, 3937–3942. [Google Scholar] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, M.; Yang, Y.; Xu, Y.; Wang, X.; Li, B.; Le, G.; Xie, Y. Dietary Methionine Restriction Alleviates Choline-Induced Tri-Methylamine-N-Oxide (TMAO) Elevation by Manipulating Gut Microbiota in Mice. Nutrients 2023, 15, 206. https://doi.org/10.3390/nu15010206
Lu M, Yang Y, Xu Y, Wang X, Li B, Le G, Xie Y. Dietary Methionine Restriction Alleviates Choline-Induced Tri-Methylamine-N-Oxide (TMAO) Elevation by Manipulating Gut Microbiota in Mice. Nutrients. 2023; 15(1):206. https://doi.org/10.3390/nu15010206
Chicago/Turabian StyleLu, Manman, Yuhui Yang, Yuncong Xu, Xiaoyue Wang, Bo Li, Guowei Le, and Yanli Xie. 2023. "Dietary Methionine Restriction Alleviates Choline-Induced Tri-Methylamine-N-Oxide (TMAO) Elevation by Manipulating Gut Microbiota in Mice" Nutrients 15, no. 1: 206. https://doi.org/10.3390/nu15010206
APA StyleLu, M., Yang, Y., Xu, Y., Wang, X., Li, B., Le, G., & Xie, Y. (2023). Dietary Methionine Restriction Alleviates Choline-Induced Tri-Methylamine-N-Oxide (TMAO) Elevation by Manipulating Gut Microbiota in Mice. Nutrients, 15(1), 206. https://doi.org/10.3390/nu15010206






