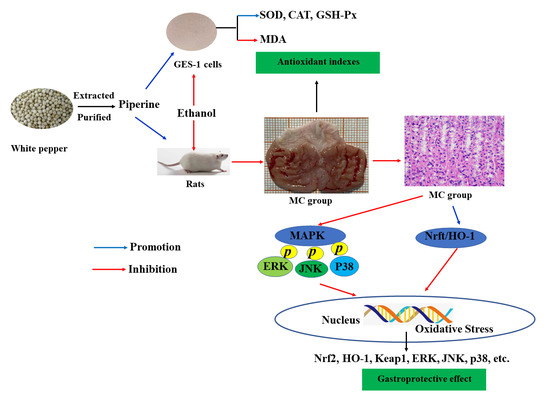
Protective Effects of Piperine on Ethanol-Induced Gastric Mucosa Injury by Oxidative Stress Inhibition
Abstract
1. Introduction
2. Material and Methods
2.1. Materials and Chemicals
2.2. Preparation of Piperine
2.3. Cell Experiment
2.3.1. Cell Culture Conditions
2.3.2. Piperine Cytotoxicity Analysis
2.3.3. Protective Effect of Piperine
2.3.4. SOD, CAT, GSH-Px and MDA Assays
2.4. Rat Experiment
2.4.1. Rat Treatment
2.4.2. Specimen Collection and Preparation
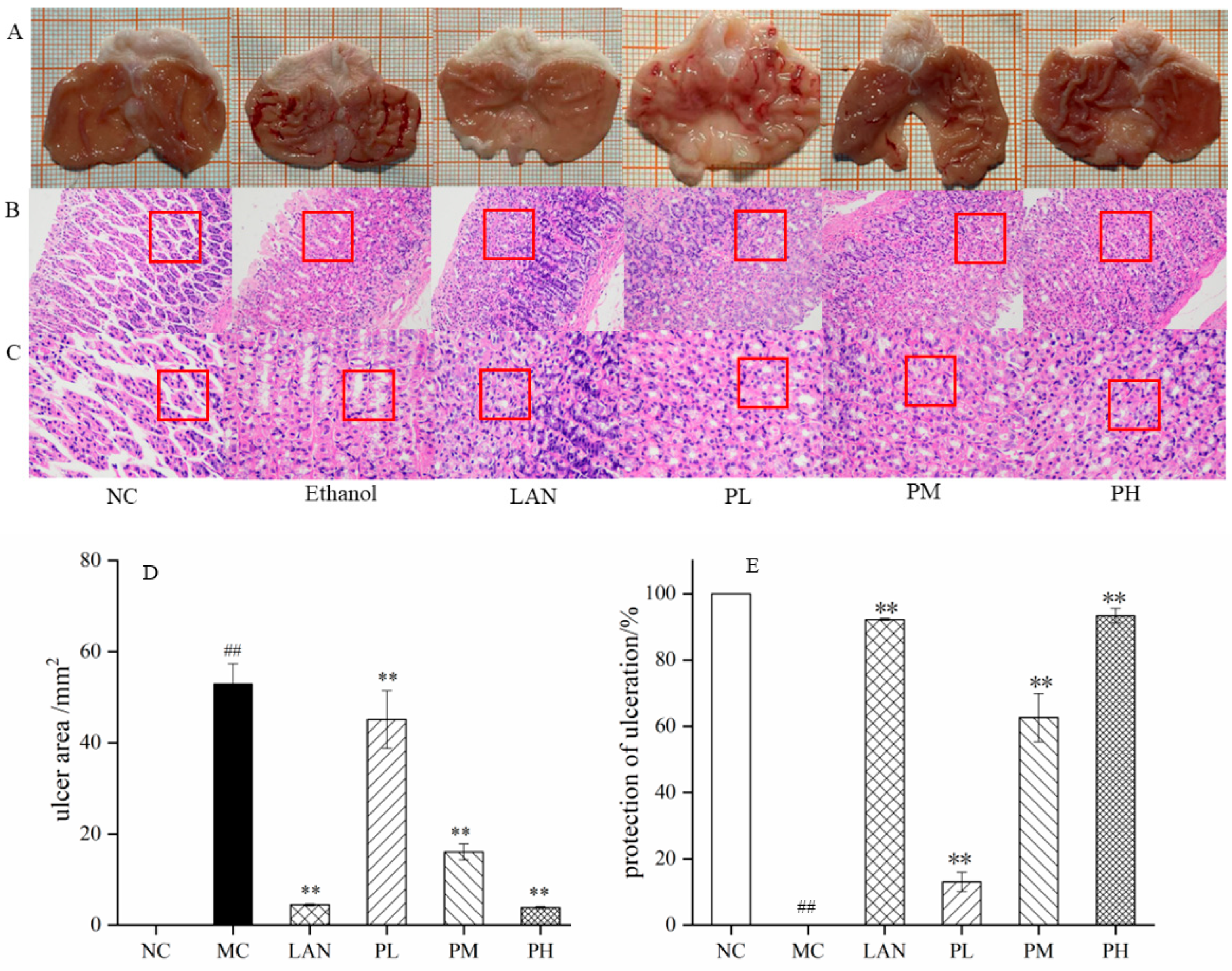
2.4.3. Histopathological Assessment
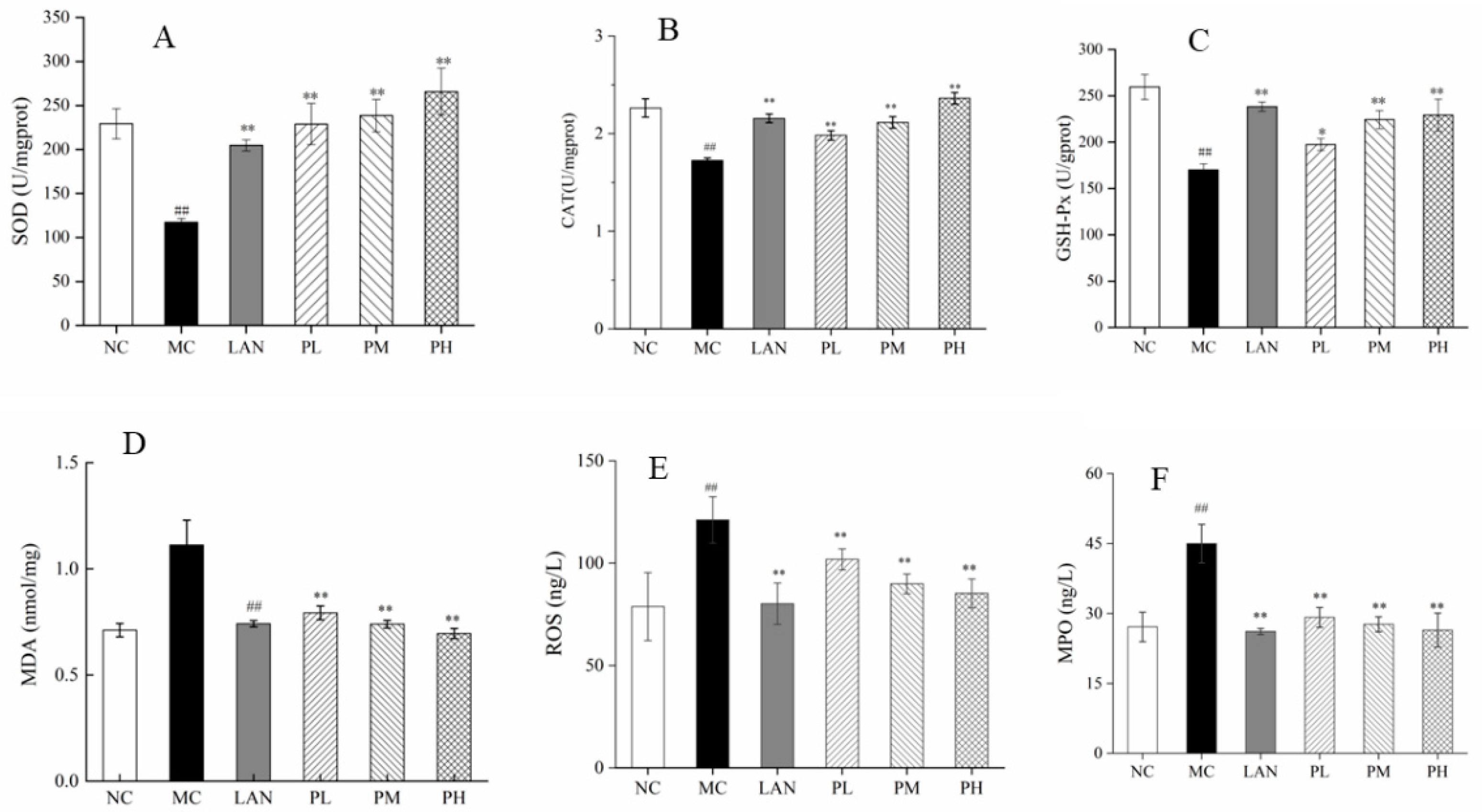
2.4.4. Evaluation of SOD, CAT, GSH-Px, MDA, ROS and MPO
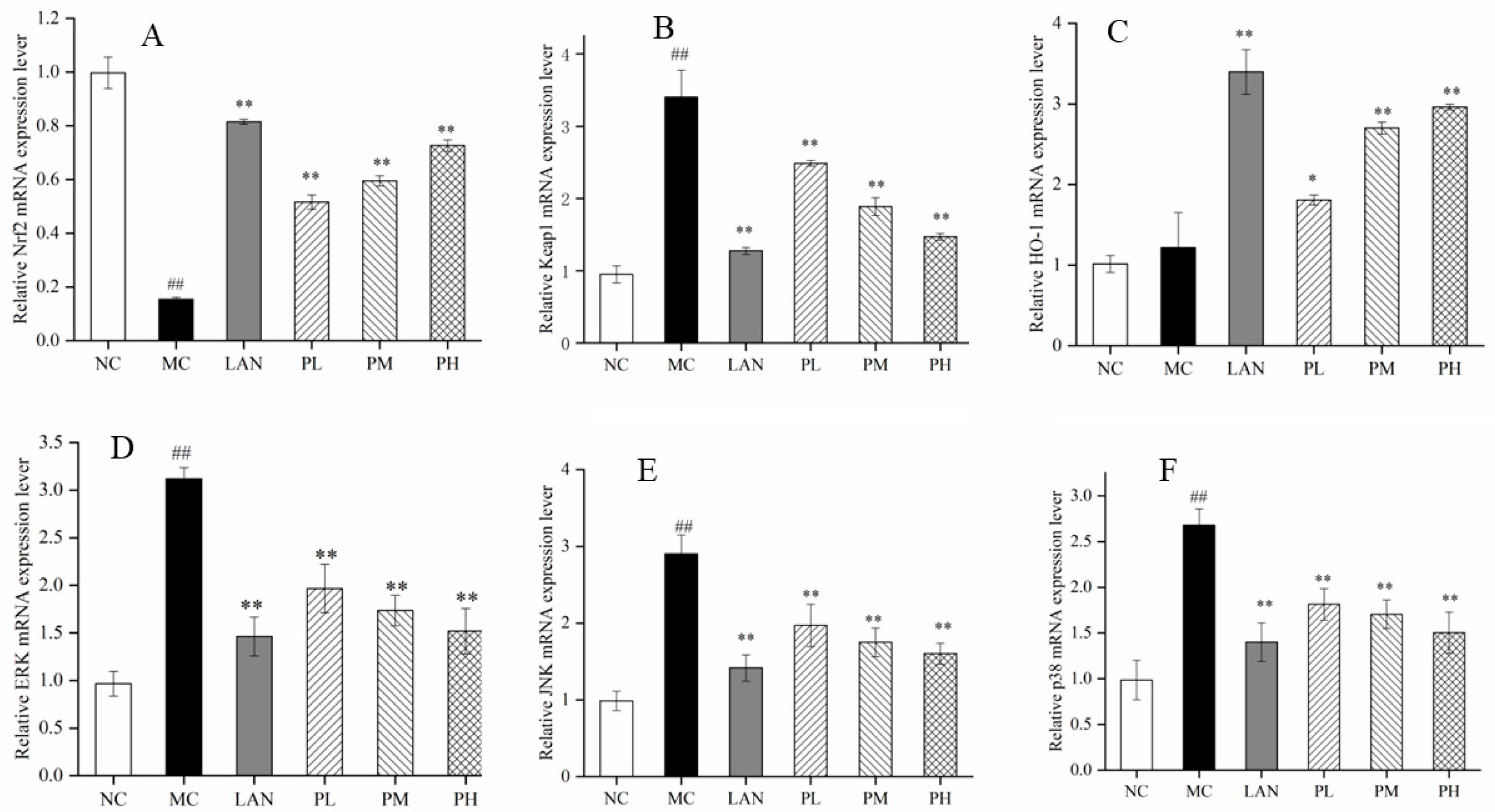
2.4.5. RT-qPCR Analysis
2.4.6. Western Blot Analysis
2.5. Statistical Analysis
3. Results
3.1. Proliferation of Piperine on GES-1 Cells
3.2. Effects of Piperine on the Antioxidant Parameters
3.3. Preventive Effect of Piperine on Ethanol-Stimulated Gastric Ulcers in Rats
3.4. Evaluation of SOD, CAT, GSH-Px, MDA, ROS and MPO
3.5. mRNA Expression Levels of Oxidation-Related Genes
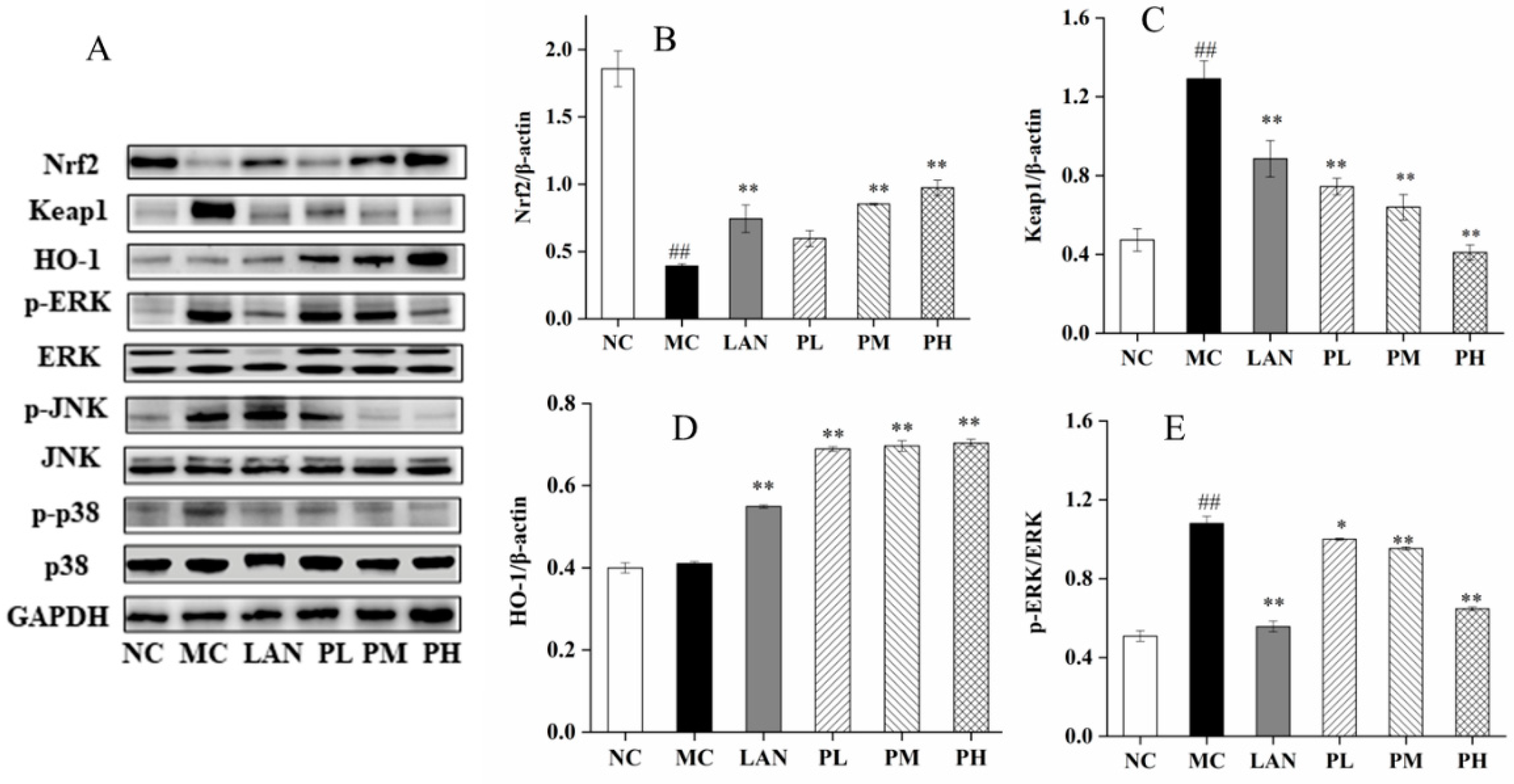
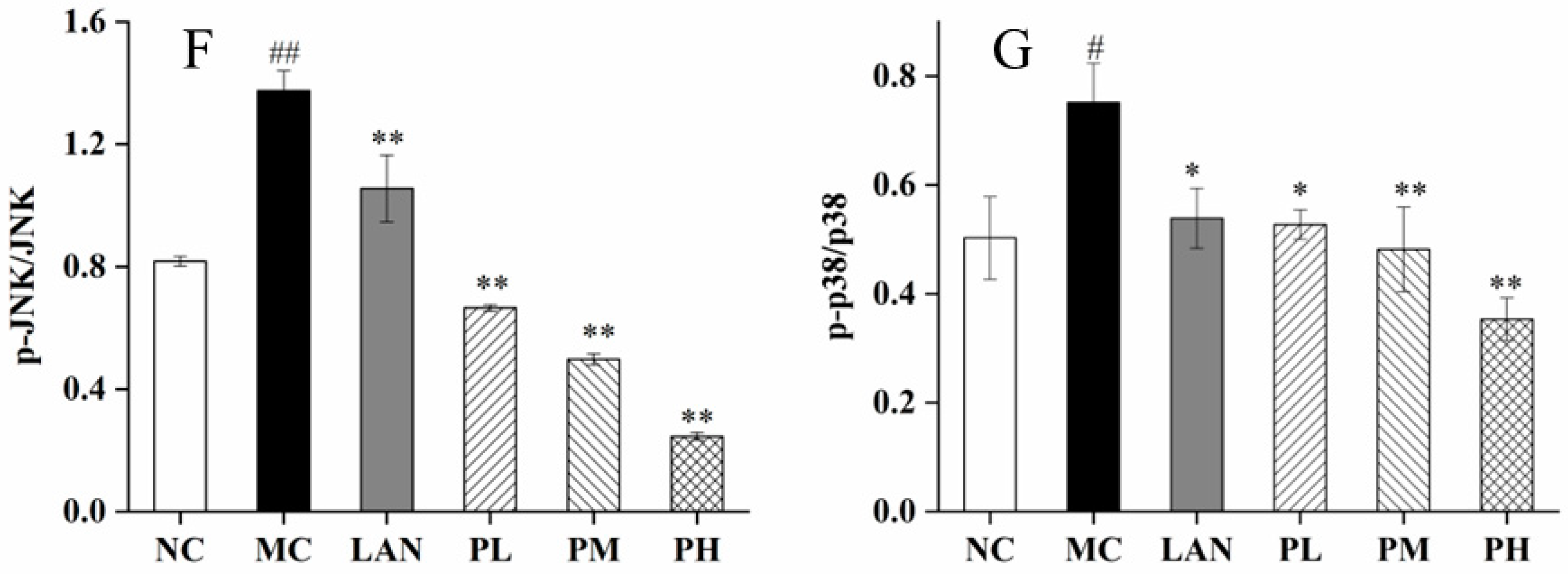
3.6. Analysis of Nrf2, HO-1, Keap1, ERK, JNK and p38 Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sriwiriyajan, S.; Tedasen, A.; Lailerd, N.; Boonyaphiphat, P.; Nitiruangjarat, A.; Deng, Y.; Graidist, P. Anti-cancer and cancer preventive effects of a piperine free Piper nigrum extract on N-nitrosomethylurea induced mammary tumorigenesis in rats. Cancer Prev. Res. 2016, 9, 74–82. [Google Scholar] [CrossRef]
- Yu, L.; Hu, X.; Xu, R.; Ba, Y.; Chen, X.; Wang, X.; Cao, B.; Wu, X. Amide alkaloids characterization and neuroprotective properties of Piper nigrum L.: A comparative study with fruits, pericarp, stalks and leaves. Food Chem. 2022, 368, 130832. [Google Scholar] [CrossRef] [PubMed]
- Parmar, V.S.; Jain, S.C.; Bisht, K.S.; Jain, R.; Taneja, P.; Jha, A.; Tyagi, O.; Prasad, A.; Wengel, J.; Olsen, C.E.; et al. Phytochemistry of the genus. Piper. Phytochem. 1997, 46, 597–673. [Google Scholar] [CrossRef]
- Zhu, F.; Mojel, R.; Li, G. Physicochemical properties of black pepper (Piper nigrum) starch. Carbohyd. Polym. 2018, 181, 986–993. [Google Scholar] [CrossRef]
- Yaffe, P.B.; Doucette, C.D.; Walsh, M.; Hoskin, D.W. Piperine impairs cell cycle progression and causes reactive oxygen species-dependent apoptosis in rectal cancer cells. Exp. Mol. Pathol. 2013, 94, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Bae, G.S.; Kim, M.S.; Jung, W.S.; Seo, S.W.; Yun, S.W.; Kim, S.G.; Park, R.K.; Kim, E.C.; Song, H.J.; Park, S.J. Inhibition of lipopolysaccharide-induced inflammatory responses by piperine. Eur. J. Pharmacol. 2010, 642, 154–162. [Google Scholar] [CrossRef]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit. Rev. Food Sci. 2019, 59, S210–S243. [Google Scholar] [CrossRef]
- Li, H.; Krstin, S.; Wang, S.; Wink, M. Capsaicin and Piperine Can Overcome Multidrug Resistance in Cancer Cells to Doxorubicin. Molecules 2018, 23, 557. [Google Scholar] [CrossRef]
- Vijayakumar, R.S.; Surya, D.; Rajagopal, S.; Nalini, N. Hypolipidemic effect of black pepper (Piper nigrum Linn.) in rats fed high fat diet. J. Clin. Biochem. Nutr. 2002, 32, 31–42. [Google Scholar] [CrossRef]
- Hritcu, L.; Noumedem, J.A.; Cioanca, O.; Hancianu, M.; Postu, P.; Mihasan, M. Anxiolytic and antidepressant profile of the methanolic extract of Piper nigrum fruits in beta-amyloid (1–42) rat model of Alzheimer’s disease. Behav. Brain Funct. 2015, 11, 13. [Google Scholar] [CrossRef]
- Escobedohinojosa, W.I.; Gomezchang, E.; Garcíamartínez, K.; Guerrero, R.A.; Cardosotaketa, A.; Romero, I. Gastroprotective mechanism and ulcer resolution effect of Cyrtocarpaprocera methanolic extract on ethanol-induced gastric injury. Evid.-Based Complement. Altern. Med. 2018, 4, 2862706. [Google Scholar] [CrossRef]
- Maity, R.; Mondal, P.; Giri, M.K.; Ghosh, C.; Mallick, C. Gastroprotective effect of hydromethanolic extract of Ayapana triplinervis leaves on indomethacin-induced gastric ulcer in male Wistar rats. J. Food Biochem. 2021, 45, e13859. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.M.; Song, C.H.; Park, S.J.; Park, D.C.; Cho, H.R.; Jung, G.W.; Bashir, K.M.; Ku, S.K.; Choi, J.S. Protective effects of a triple-fermented barley extract (FBe) against HCl/EtOH-inducedgastric mucosa damage in mice. Food Sci. Nutr. 2018, 6, 2036–2046. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Tang, H.; Guo, Y.; Bian, Z.; Yang, L.; Chen, Y.P. Hot Tea Consumption and Its Interactions With Alcohol and Tobacco Use on the Risk for Esophageal Cancer: A Population-Based Cohort Study. Ann. Intern. Med. 2018, 168, 489–497. [Google Scholar] [CrossRef] [PubMed]
- AlKreathy, H.M.; Alghamdi, M.K.; Esmat, A. Tetramethylpyrazine ameliorates indomethacin-induced gastric ulcer in rats: Impact on oxidative, inflammatory, and angiogenic machineries. Saudi Pharm. J. 2020, 28, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Luz, B.B.; Ferreira, D.M.; Dallazen, J.L.; Oliveira, A.F.; Telles, J.E.Q.; Beltrame, O.C.; Cipriani, T.H.; Werner, M.F. Effectiveness of the polyphenols-rich Sedum dendroideum infusion on gastric ulcer healing in rats: Roles of protective endogenous factors and antioxidant and anti-inflammatory mechanisms. J. Ethnopharmacol. 2021, 278, 114260. [Google Scholar] [CrossRef]
- Aziz, R.; Siddiqua, S.; Shahzad, A.M.; Shabbir, A.; Naseem, N. Oxyresveratrol ameliorates ethanol-induced gastric ulcer via downregulation of IL-6, TNF-α, NF-κB, and COX-2 levels, and upregulation of TFF-2 levels. Biomed. Pharmacother. 2019, 110, 554–560. [Google Scholar] [CrossRef]
- Hou, C.H.; Liu, L.Y.; Ren, J.Y.; Huang, M.; Yuan, E.D. Structural characterization of two Hericium erinaceus polysaccharides and their protective effects on the alcohol-induced gastric mucosal injury. Food Chem. 2022, 375, 131876. [Google Scholar] [CrossRef]
- Shohda, A.E.; Sherine, M.R.; Nancy, N.S. Gastroprotective effect of crocin in ethanol-induced gastric injury in rats. Chem.-Biol. Interact. 2015, 229, 26–35. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, H.Q.; Cui, H.L.; Li, Y.Y.; Li, C.Z. Gastroprotective and anti-ulcer effects of oxymatrine against several gastric ulcer models in rats: Possible roles of antioxidant, antiinflammatory, and prosurvival mechanisms. Phytother. Res. 2018, 32, 2047–2058. [Google Scholar] [CrossRef]
- Rahman, Z.; Dwivedi, D.K.; Jena, G.B. Ethanol-induced gastric ulcer in rats and intervention of tert-butylhydroquinone: Involvement of Nrf2/HO-1 signalling pathway. Hum. Exp. Toxicol. 2020, 39, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Xie, H.; Yu, S.; Wang, S.; Yang, H. Piperine Derived from Piper nigrum L. Inhibits LPS-Induced Inflammatory through the MAPK and NF-κB Signalling Pathways in RAW264.7 Cells. Foods 2022, 11, 2990. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.Z.; Lin, I.H.; Yang, Y.C.; Chao, J.C. Gastroprotective effect of Lycium barbarum polysaccharides and C-phycocyanin in rats with ethanol-induced gastric ulcer. Int. J. Biol. Macromol. 2020, 165, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Z.; Liu, Y.H.; Liang, J.L.; Huang, Q.H.; Dou, Y.X.; Nie, J.; Zhou, J.Y.; Wu, X.; Chen, J.N.; Su, Z.R.; et al. Protective role of β-patchoulene from Pogostemon cablin against indomethacin-induced gastric ulcer in rats: Involvement of anti-inflammation and angiogenesis. Phytomedicine 2018, 39, 111–118. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Sui, D.Y.; Fu, W.W.; Sun, L.; Li, Y.G.; Yu, P.; Yu, X.; Zhou, Y.; Xu, H. Protective effects of polysaccharides from Panax ginseng on acute gastric ulcers induced by ethanol in rats. Food Funct. 2021, 12, 2741–2749. [Google Scholar] [CrossRef]
- Ma, N.; Sun, Y.; Yi, J.J.; Zhou, L.Y.; Cai, S.B. Chinese sumac (Rhus chinensis Mill.) fruits alleviate indomethacin-induced gastric ulcer in mice by improving oxidative stress, inflammation and apoptosis. J. Ethnopharmacol. 2022, 284, 114752. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, M.; Qing, Y.; Luo, Y.; Xia, G.; Li, Y. Study on immunostimulatory activity and extraction process optimization of polysaccharides from Caulerpa lentillifera. Int. J. Biol. Macromol. 2020, 143, 677–684. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, S.; Mei, F.; Zhao, M.; Xia, G.; Shen, X. Tilapia nilotica Head Lipids Improved Bone Loss by Regulating Inflammation and Serum Metabolism Through Gut Microbiota in Ovariectomized Rats. Front. Nutr. 2022, 8, 792793. [Google Scholar] [CrossRef]
- Mei, F.; Meng, K.; Gu, Z.; Yun, Y.; Zhang, W.; Zhang, C.; Zhong, Q.; Pan, F.; Shen, X.; Xia, G.; et al. Arecanut (Areca catechu L.) seed polyphenol-ameliorated osteoporosis by altering gut microbiome via LYZ and the immune system in estrogen-deficient rats. J. Agric. Food Chem. 2021, 69, 246–258. [Google Scholar] [CrossRef]
- Zhao, M.; Mei, F.; Lu, J.; Xiang, Q.; Xia, G.; Zhang, X. Gadus morhua Eggs Sialoglycoprotein Prevent Estrogen Deficiency- Induced High Bone Turnover by Controlling OPG/RANKL/TRAF6 Pathway and Serum Metabolism. Front. Nutr. 2022, 9, 871521. [Google Scholar] [CrossRef]
- Lafarga, T.; Hayes, M. Bioactive protein hydrolysates in the functional food ingredient industry: Overcoming current challenges. Food Rev. Int. 2016, 33, 217–246. [Google Scholar] [CrossRef]
- Liao, B.; Huang, H. Structural characterization of a novel polysaccharide from Hericium erinaceus and its protective effects against H2O2-induced injury in human gastric epithelium cells. J. Funct. Foods 2019, 56, 265–275. [Google Scholar] [CrossRef]
- Pan, J.; He, S.; Xu, H.; Zhan, X.; Yang, X.; Xiao, H.; Shi, H.; Ren, J. Oxidative stress disturbs energy metabolism of mitochondria in ethanol-induced gastric mucosa injury. World J. Gastroenterol. 2008, 14, 5857–5867. [Google Scholar] [CrossRef] [PubMed]
- Casimirri, E.; Stendardo, M.; Bonci, M.; Andreoli, R.; Bottazzi, B.; Leone, R.; Schito, M.; Vaccari, A.; Papi, A.; Contoli, M.; et al. Biomarkers of oxidative-stress and inflammation in exhaled breath condensate from hospital cleaners. Biomarkers 2016, 21, 115–122. [Google Scholar] [CrossRef]
- Oyagi, A.; Ogawa, K.; Kakino, M.; Hara, H. Protective effects of a gastrointestinal agent containing Korean red ginseng on gastric ulcer models in mice. BMC Complement. Altern. Med. 2010, 10, 45. [Google Scholar] [CrossRef]
- Liu, R.; Hao, Y.; Zhu, N.; Liu, X.; Kang, J.; Mao, R.; Chao, H.; Li, Y. The gastroprotective effect of small molecule oligopeptides isolated from Walnut (Juglans regia L.) against ethanol-induced gastric mucosal injury in rats. Nutrients 2020, 12, 1138. [Google Scholar] [CrossRef]
- Raish, M.; Shahid, M.; Jardan, Y. Gastroprotective Effect of Sinapic Acid on Ethanol-Induced Gastric Ulcers in Rats: Involvement of Nrf2/HO-1 and NF-κB Signaling and Antiapoptotic Role. Front. Pharmacol. 2021, 12, 622815. [Google Scholar] [CrossRef]
- Huang, C.C.; Chen, Y.M.; Wang, D.C.; Chiu, C.C.; Lin, W.T.; Huang, C.Y.; Hsu, M.C. Cytoprotective effect of American ginseng in a rat ethanol gastric ulcer model. Molecules 2013, 19, 316–326. [Google Scholar] [CrossRef]
- Boutemine, I.M.; Amri, M.; Amir, Z.C.; Fitting, C.; Mecherara-Idjeri, S.; Layaida, K.; Sennoun, N.; Berkane, S.; Cavaillon, J.; Boukoffa, C. Gastro-protective, therapeutic and anti-inflammatory activities of Pistacia lentiscus L. fatty oil against ethanol-induced gastric ulcers in rats. J. Ethnopharmacol. 2018, 224, 273–282. [Google Scholar] [CrossRef]
- Sistani, K.N.; Arzi, A.; Rezaie, A.; Pashmforoosh, M.; Kordi, F. Gastroprotective effect of zingerone on ethanol-induced gastric ulcers in rats. Medicina 2019, 55, 64. [Google Scholar] [CrossRef]
- Liang, J.; Dou, Y.; Wu, X.; Li, H.; Wu, J.; Huang, Q.; Luo, D.; Yi, T.; Liu, Y.; Su, Z.; et al. Prophylactic efficacy of patchoulene epoxide against ethanol-induced gastric ulcer in rats: Influence on oxidative stress, inflammation and apoptosis. Chem.-Biol. Interact. 2018, 283, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; Bandyopadhyay, U.; Chattopadhyay, I.; Varadaraj, A.; Ali, E.; Banerjee, R.K. A novel antioxidant and antiapoptotic role of omeprazole to block gastric ulcer through scavenging of hydroxyl radical. J. Biol. Chem. 2003, 278, 10993–11001. [Google Scholar] [CrossRef] [PubMed]
- Kwiecień, S.; Brzozowski, T.; Konturek, P.C.H.; Konturek, S.J. The role of reactive oxygen species in action of nitric oxide-donors on stress-induced gastric mucosal lesions. J. Physiol. Pharmacol. 2003, 53, 761–773. [Google Scholar] [CrossRef]
- Huh, K.; Kwon, T.H.; Shin, U.S.; Kim, W.B.; Ahn, B.O.; Oh, T.Y.; Kim, J.A. Inhibitory effects of DA-9601 on ethanol-induced gastrohemorrhagic lesions and gastric xanthine oxidase activity in rats. J. Ethnopharmacol. 2003, 88, 269–273. [Google Scholar] [CrossRef]
- Karakus, B.F.; Odabasoglu, A.; Cakir, Z.; Halici, Y.; Bayir, M.; Halici, A.; Aslan, H.; Suleyman, H. The effects of methanol extract of Lobaria pulmonaria, a lichen species, on indometacin-induced gastric mucosal damage, oxidative stress and neutrophil infiltration. Phytother. Res. 2009, 23, 635–639. [Google Scholar] [CrossRef]
- Bonamin, F.; Moraes, R.C.; Santos, R.C.; Kushima, H.; Faria, F.M.; Silva, M.A.; Junior, I.V.; Nogueira, L.; Bauab, T.M.; Brito, A.S.; et al. The effect of a minor constituent of essential oil from Citrus aurantium: The role of β-myrcene in preventing peptic ulcer disease. Chem. Biol. Interact. 2014, 212, 11–19. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, J.; Ding, Y.; Ma, Y.; Shang, P.; Liu, T.; Hui, G.; Wang, L.; Wang, M.; Zhu, Z.; et al. Alpha-boswellic acid protects against ethanol-induced gastric injury in rats: Involvement of nuclear factor erythroid-2-related factor 2/heme oxygenase-1 pathway. J. Pharm. Pharmacol. 2016, 68, 514–522. [Google Scholar] [CrossRef]
- Badr, A.M.; El-Orabi, N.F.; Ali, R.A. The implication of the crosstalk of Nrf2 with NOXs, and HMGB1 in ethanol-induced gastric ulcer: Potential protective effect is afforded by Raspberry Ketone. PLoS ONE 2019, 14, e0220548. [Google Scholar] [CrossRef]
- Yanaka, A. Role of NRF2 in protection of the gastrointestinal tract against oxidative stress. J. Clin. Biochem. Nutr. 2018, 63, 18–25. [Google Scholar] [CrossRef]
- Abdelwahab, S.I. Protective mechanism of gallic acid and its novel derivative against ethanol-induced gastric ulcerogenesis: Involvement of immunomodulation markers, Hsp70 and Bcl-2-associated X protein. Int. Immunopharmacol. 2013, 16, 296–305. [Google Scholar] [CrossRef]
- You, Z.; Liu, S.P.; Du, P.; Wu, Y.H.; Zhang, S.Z. Advancements in MAPK signaling pathways and MAPK-targeted therapies for ameloblastoma: A review. J. Oral Pathol. Med. 2019, 48, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Son, H.J.; Eo, H.J.; Park, G.H.; Jeong, J.B. Heracleum moellendorffii root extracts exert immunostimulatory activity through TLR2/4-dependent MAPK activation in mouse macrophages, RAW264.7 cells. Food Sci. Nutr. 2021, 9, 514–521. [Google Scholar] [CrossRef]
- Liu, Y.T.; Hsieh, M.J.; Lin, J.T.; Gene, C.; Lin, C.C.; Lo, Y.S.; Chuang, Y.C.; Hsi, Y.T.; Chen, M.K.; Chou, M.C. Erianin induces cell apoptosis through ERK pathway in human nasopharyngeal carcinoma. Biomed. Pharmacother. 2019, 111, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Owuor, E.D.; Kong, A. Antioxidants and oxidants regulated signal transduction pathways. Biomed. Pharmacother. 2002, 64, 765–770. [Google Scholar] [CrossRef]


| Gene | Primer Sequence (5′-3′) | Orientation | Length/bp |
|---|---|---|---|
| GADPH | GACATGCCGCCTGGAGAAAC | Forward | 92 |
| AGCCCAGGATGCCCTTTAGT | Reverse | ||
| Nrf2 | GCCTTCCTCTGCTGCCATTAGTC | Forward | 110 |
| TGCCTTCAGTGTGCTTCTGGTTG | Reverse | ||
| HO-1 | CAGGTGTCCAGGGAAGGCTTTAAG | Forward | 96 |
| TGGGTTCTGCTTGTTTCGCTCTATC | Reverse | ||
| Keap1 | TGCTCAACCGCTTGCTGTATGC | Forward | 99 |
| TCATCCGCCACTCATTCCTCTCC | Reverse | ||
| ERK | CTGGCTGCTAGGAACATTCTGGTG | Forward | 84 |
| GTCATCCTGGAGGTAGCGAGAGAG | Reverse | ||
| JNK | CCACCACCAAAGATCCCTGACAAG | Forward | 117 |
| GACGCCATTCTTAGTTCGCTCCTC | Reverse | ||
| p38 | GATAAGAGGATCACAGCAGCCCAAG | Forward | 149 |
| TCGTAGGTCAGGCTCTTCCATTCG | Reverse |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Z.; Yu, S.; Wang, S.; Deng, H.; Guo, L.; Yang, H.; Xie, H. Protective Effects of Piperine on Ethanol-Induced Gastric Mucosa Injury by Oxidative Stress Inhibition. Nutrients 2022, 14, 4744. https://doi.org/10.3390/nu14224744
Duan Z, Yu S, Wang S, Deng H, Guo L, Yang H, Xie H. Protective Effects of Piperine on Ethanol-Induced Gastric Mucosa Injury by Oxidative Stress Inhibition. Nutrients. 2022; 14(22):4744. https://doi.org/10.3390/nu14224744
Chicago/Turabian StyleDuan, Zhouwei, Shasha Yu, Shiping Wang, Hao Deng, Lijun Guo, Hong Yang, and Hui Xie. 2022. "Protective Effects of Piperine on Ethanol-Induced Gastric Mucosa Injury by Oxidative Stress Inhibition" Nutrients 14, no. 22: 4744. https://doi.org/10.3390/nu14224744
APA StyleDuan, Z., Yu, S., Wang, S., Deng, H., Guo, L., Yang, H., & Xie, H. (2022). Protective Effects of Piperine on Ethanol-Induced Gastric Mucosa Injury by Oxidative Stress Inhibition. Nutrients, 14(22), 4744. https://doi.org/10.3390/nu14224744