Garcinone E Mitigates Oxidative Inflammatory Response and Protects against Experimental Autoimmune Hepatitis via Modulation of Nrf2/HO-1, NF-κB and TNF-α/JNK Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
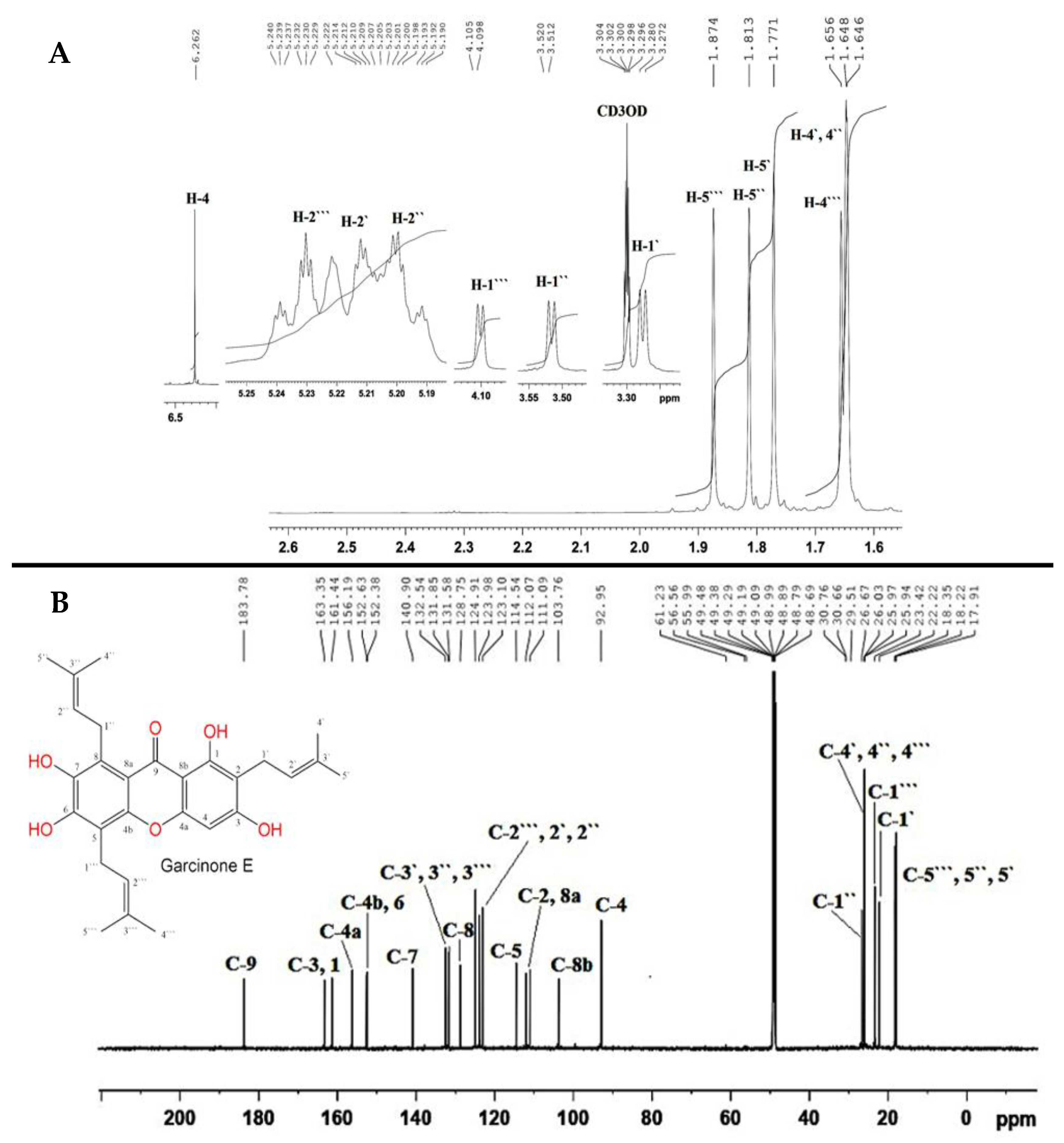
2.3. Extraction and Isolation
2.4. Animals
2.5. Chemicals and Reagents
2.6. Experimental Design
2.7. Biochemical Measurements
2.7.1. Hepatotoxicity Markers
2.7.2. MPO
2.7.3. Antioxidant Balance and Oxidative Stress
2.7.4. Nitric Oxide (NO)
2.8. ELISA Assay
2.8.1. Nrf2 and HO-1
2.8.2. Inflammatory and Apoptosis Mediators
2.9. Histopathology
2.10. IHC (Immunohistochemistry)
2.11. RT-PCR
2.12. Statistical Analysis
3. Results
3.1. GE-Alleviated Con-A-Induced Hepatic Injury
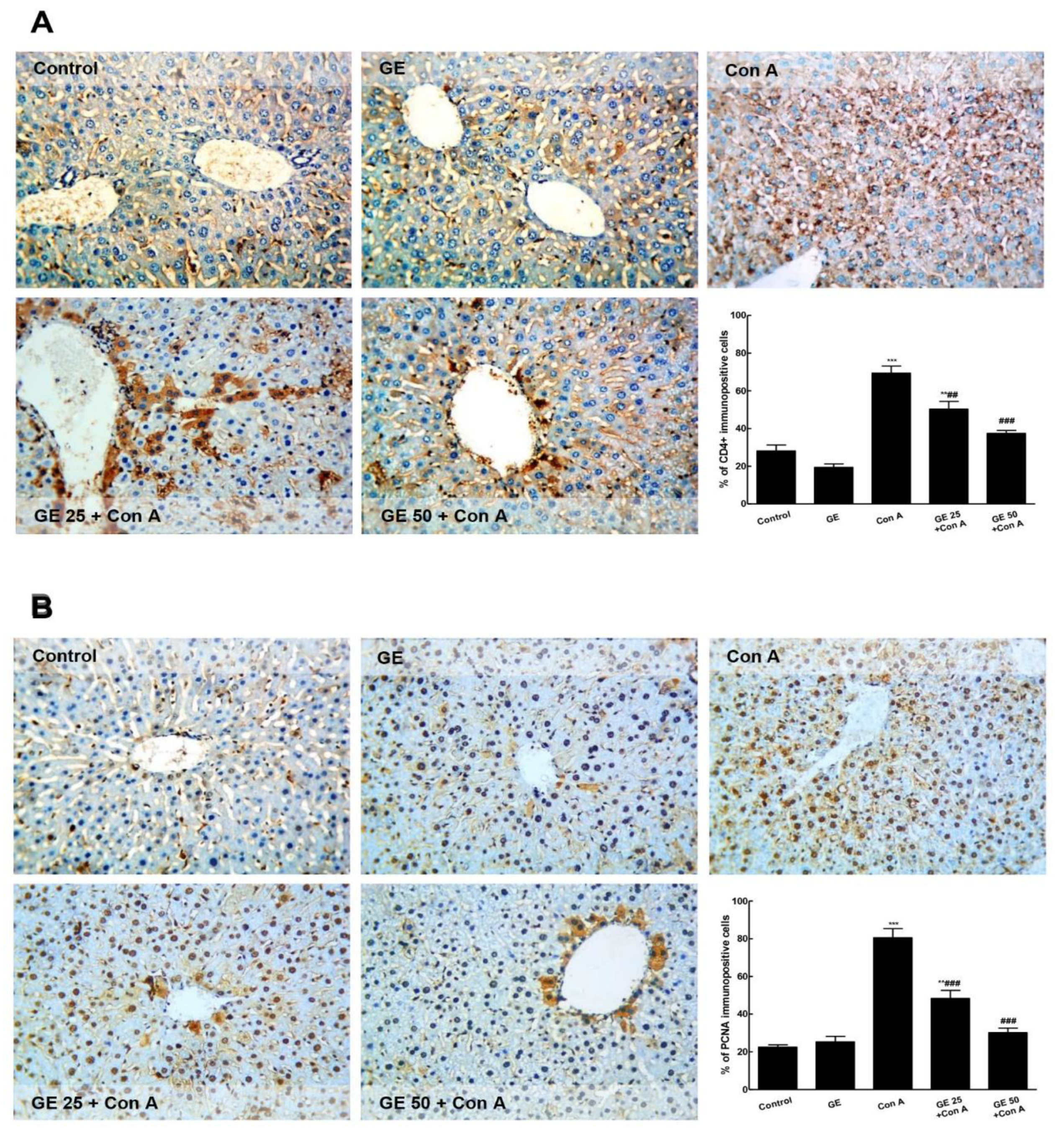
3.2. GE Decreased Immuno-Expression of CD4+ and PCNA in Con-A-Challenged Mice
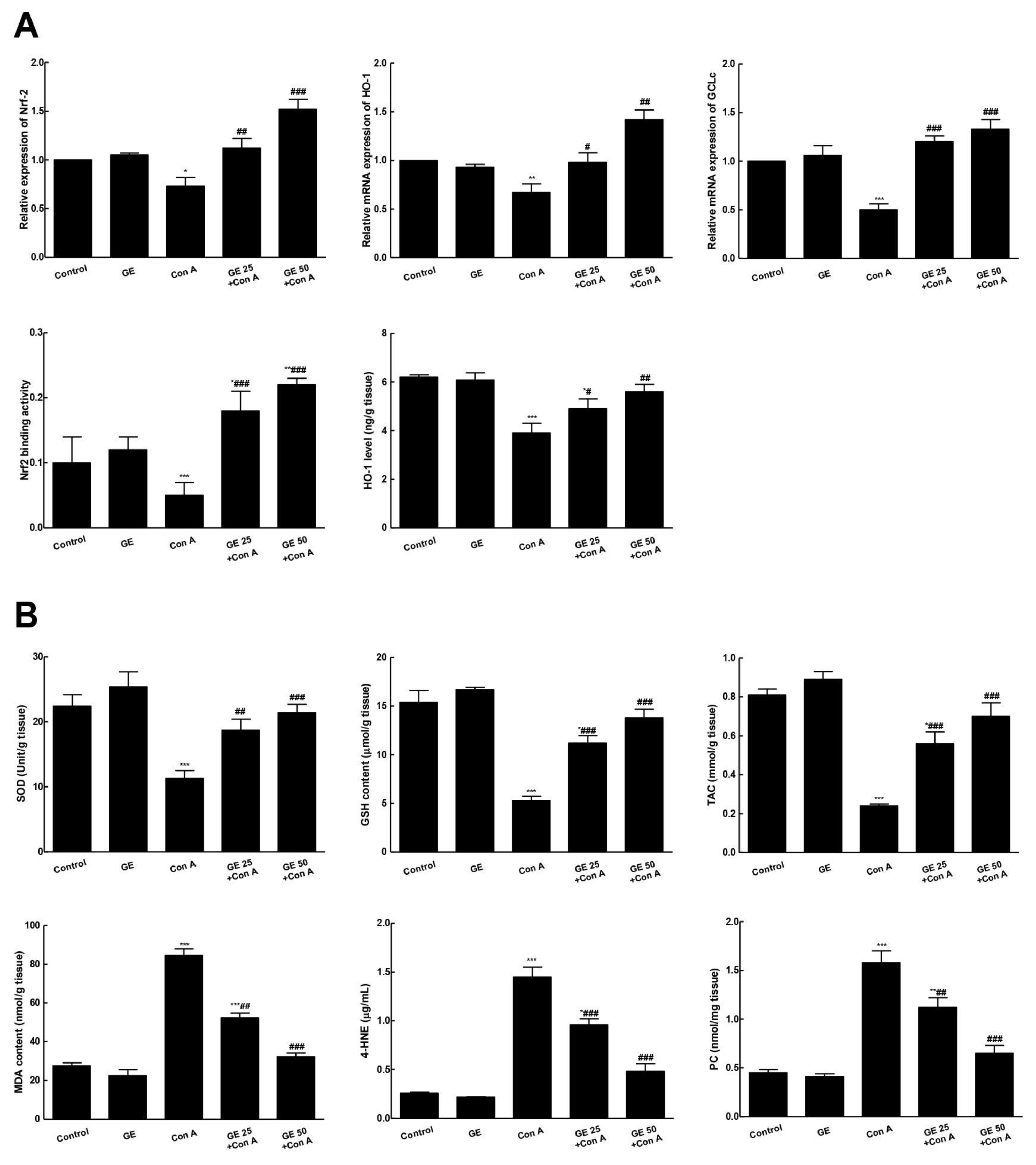
3.3. GE Enhanced Nrf2 Signaling and Antioxidants to Counteract Con-A-Induced Oxidative Response in Hepatic Tissue
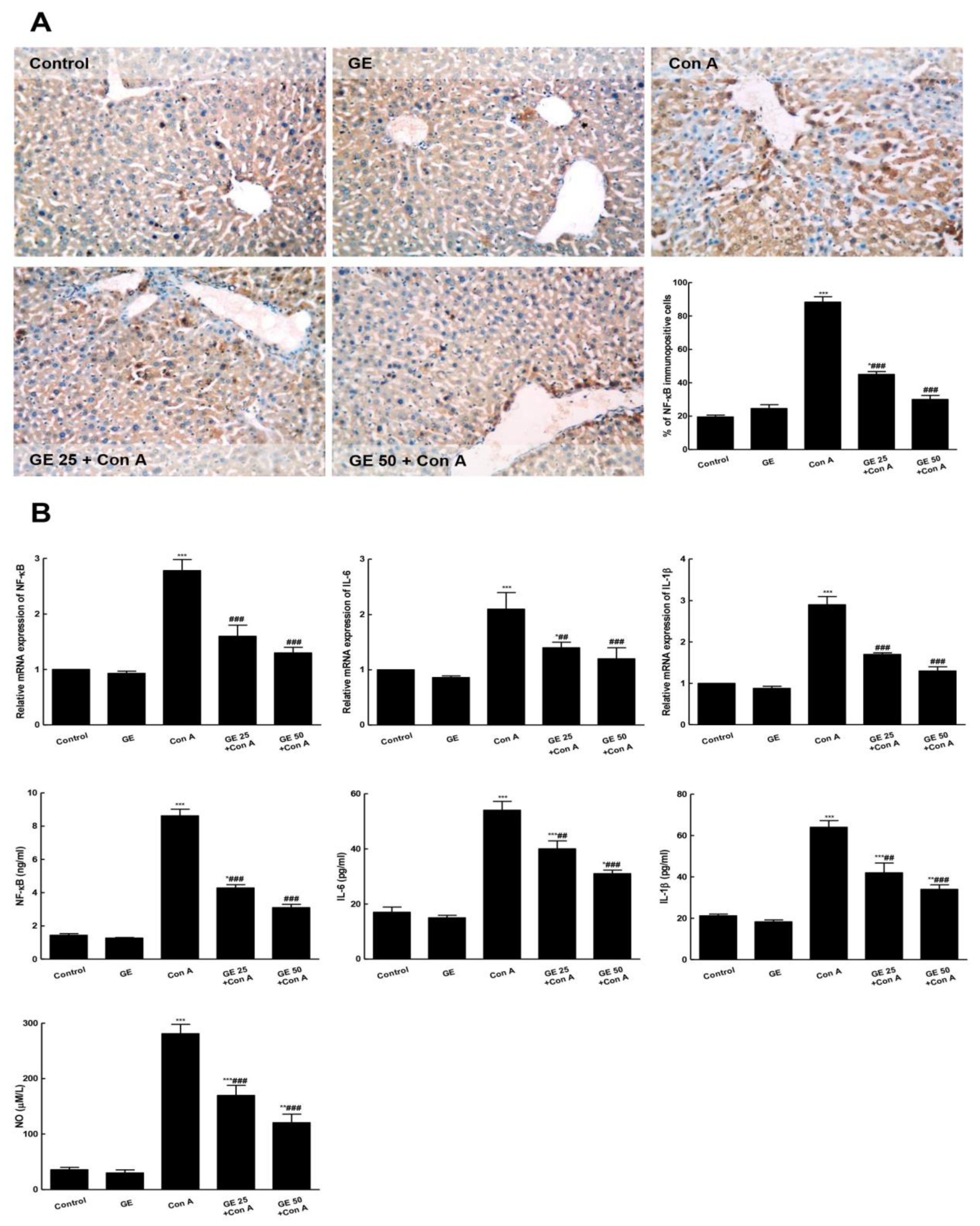
3.4. GE Suppressed Con-A-Induced NF-κB Activation and Downstream Inflammation Cascades
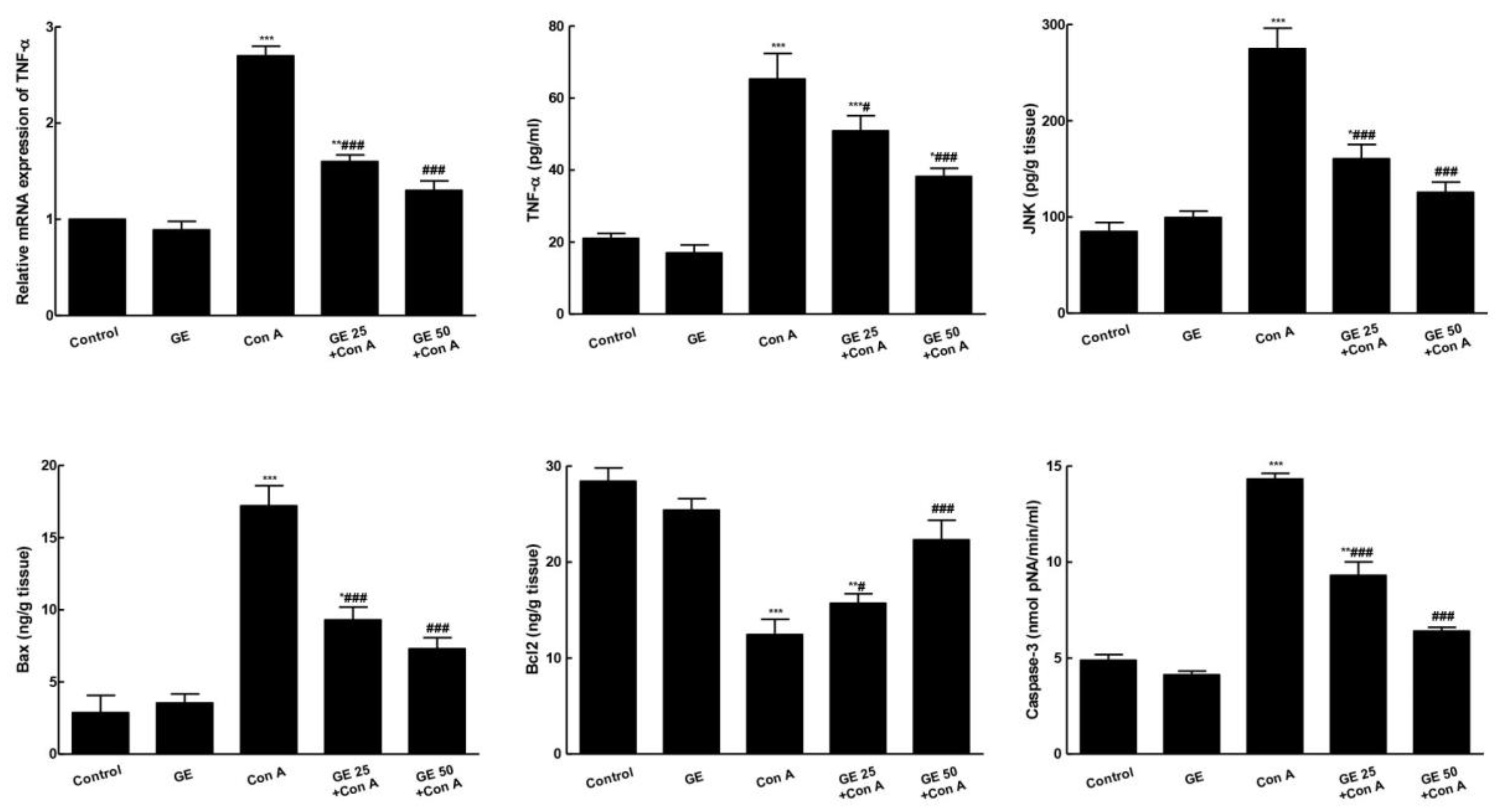
3.5. GE Inhibited TNF-α/JNK Signaling and Ameliorated Con-A-Induced Apoptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christen, U.; Hintermann, E. Pathogens and autoimmune hepatitis. Clin. Exp. Immunol. 2019, 195, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, Q.; Zhou, C.; Zhao, Y.; Li, R.; Zhang, Y. Demethyleneberberine attenuates concanavalin A-induced autoimmune hepatitis in mice through inhibition of NF-κB and MAPK signaling. Int. Immunopharmacol. 2020, 80, 106137. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Cao, L.; Luo, Y.; Feng, X.; Sun, L.; Wen, M.; Peng, S. Paeoniflorin protects against concanavalin A-induced hepatitis in mice. Int. Immunopharmacol. 2015, 24, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Sang, R.; Yu, Y.; Ge, B.; Xu, L.; Wang, Z.; Zhang, X. Taraxasterol from Taraxacum prevents concanavalin A-induced acute hepatic injury in mice via modulating TLRs/NF-κB and Bax/Bc1-2 signalling pathways. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3929–3937. [Google Scholar] [CrossRef] [PubMed]
- El-Agamy, D.S. Pirfenidone ameliorates concanavalin A-induced hepatitis in mice via modulation of reactive oxygen species/nuclear factor kappa B signalling pathways. J. Pharm. Pharmacol. 2016, 68, 1559–1566. [Google Scholar] [CrossRef]
- Xue, J.; Chen, F.; Wang, J.; Wu, S.; Zheng, M.; Zhu, H.; Liu, Y.; He, J.; Chen, Z. Emodin protects against concanavalin A-induced hepatitis in mice through inhibiting activation of the p38 MAPK-NF-κB signaling pathway. Cell Physiol Biochem. 2015, 35, 1557–1570. [Google Scholar] [CrossRef]
- Wang, K.; Song, Z.; Wang, H.; Li, Q.; Cui, Z.; Zhang, Y. Angelica sinensis polysaccharide attenuates concanavalin A-induced liver injury in mice. Int. Immunopharmacol. 2016, 31, 140–148. [Google Scholar] [CrossRef]
- Miao, L.; Tao, H.; Peng, Y.; Wang, S.; Zhong, Z.; El-Seedi, H.; Dragan, S.; Zengin, G.; San Cheang, W.; Wang, Y.; et al. The anti-inflammatory potential of Portulaca oleracea, L. (purslane) extract by partial suppression on NF-κB and MAPK activation. Food Chem. 2019, 290, 239–245. [Google Scholar] [CrossRef]
- El-Agamy, D.S.; Shaaban, A.A.; Almaramhy, H.H.; Elkablawy, S.; Elkablawy, M.A. Pristimerin as a novel hepatoprotective agent against experimental autoimmune hepatitis. Front. Pharmacol. 2018, 9, 292. [Google Scholar] [CrossRef]
- Liu, T.; Xia, Y.; Li, J.; Li, S.; Feng, J.; Wu, L.; Zhang, R.; Xu, S.; Cheng, K.; Zhou, Y.; et al. Shikonin Attenuates Concanavalin A-Induced Acute Liver Injury in Mice via Inhibition of the JNK Pathway. Mediators Inflamm. 2016, 2016, 2748367. [Google Scholar] [CrossRef]
- El-Kashef, D.H.; Abdelrahman, R.S. Montelukast ameliorates Concanavalin A-induced autoimmune hepatitis in mice via inhibiting TNF-α/JNK signaling pathway. Toxicol. Appl. Pharmacol. 2020, 393, 114931. [Google Scholar] [CrossRef] [PubMed]
- Elshal, M.; Hazem, S.H. Escin suppresses immune cell infiltration and selectively modulates Nrf2/HO-1, TNF-α/JNK, and IL-22/STAT3 signaling pathways in concanavalin A-induced autoimmune hepatitis in mice. Inflammopharmacology 2022, 30, 2317–2329. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Ibrahim, S.R.M.; El-Agamy, D.S.; Elsaed, W.M.; Sirwi, A.; Asfour, H.Z.; Koshak, A.E.; Elhady, S.S. Cucurbitacin E glucoside alleviates concanavalin A-induced hepatitis through enhancing SIRT1/Nrf2/HO-1 and inhibiting NF-ĸB/NLRP3 signaling pathways. J. Ethnopharmacol. 2022, 292, 115223. [Google Scholar] [CrossRef] [PubMed]
- AlSaadi, B.H.; AlHarbi, S.H.; Ibrahim, S.R.M.; El-Kholy, A.A.; El-Agamy, D.S.; Mohamed, G.A. Hepatoprotective activity of Costus speciosus against paracetamol-induced liver injury in mice. Afr. J. Tradit. Complement Altern. Med. 2018, 15, 35–41. [Google Scholar]
- Zhang, A.; Sun, H.; Wang, X. Recent advances in natural products from plants for treatment of liver diseases. Eur. J. Med. Chem. 2013, 63, 570–577. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Sirwi, A.; Eid, B.G.; Mohamed, S.G.A.; Mohamed, G.A. Summary of natural products ameliorate concanavalin A-induced liver injury: Structures, sources, pharmacological effects, and mechanisms of action. Plants 2021, 10, 228. [Google Scholar] [CrossRef]
- Ee, G.C.; See, I.; Teh, S.S.; Daud, S. A new furanoxanthone from Garcinia mangostana. J. Asian Nat. Prod. Res. 2014, 16, 790–794. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Al-Abd, A.M.; El-halawany, A.M.; Abdallah, H.M.; Ibrahim, S.R.M. New xanthones and cytotoxic constituents from Garcinia mangostana fruit hulls against human hepatocellular, breast, and colorectal cancer cell lines. J. Ethnopharmacol. 2017, 198, 302–312. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Elfaky, M.A.; Al Haidari, R.A.; Zayed, M.F.; El-Kholy, A.A.; Ross, S.A. Garcixanthone A, a new cytotoxic xanthone from the pericarps of Garcinia mangostana. J. Asian Nat. Prod. Res. 2019, 21, 291–297. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Elfaky, M.A.; Zayed, M.F.; El-Kholy, A.A.; Abdelmageed, O.H.; Ross, S.A. Mangostanaxanthone VII, a new cytotoxic xanthone from Garcinia mangostana. Z. Naturforsch. 2018, 73, 185–189. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Abdallah, H.M.; El-Halawany, A.M.; Radwan, M.F.; Shehata, I.A.; Al-Harshany, E.M.; Zayed, M.F.; Mohamed, G.A. Garcixanthones B and C new xanthones from the pericarps of Garcinia mangostana and their cytotoxic activity. Phytochem. Lett. 2018, 25, 12–16. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Abdallah, H.M.; El-Halawany, A.M.; Nafady, A.M.; Mohamed, G.A. Mangostanaxanthone VIII, new xanthone from Garcinia mangostana and its cytotoxic activity. Nat. Prod. Res. 2019, 33, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; El-Agamy, D.S.; Abdallah, H.M.; Ahmed, N.; Elkablawy, M.A.; Mohamed, G.A. Protective activity of tovophyllin A a xanthone isolated from Garcinia mangostana pericarps against acetaminophen-induced hepatic damage: Role of Nrf2 activation. Food Fun. 2018, 9, 3291–3300. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Mohamed, G.A.; Khayat, M.T.; Ahmed, S.; Abo-Haded, H. Garcixanthone D, a new xanthone and other xanthones derivatives from Garcinia mangostana pericarps, their α-amylase inhibitory potential, and molecular docking study. Starch-Stärke 2019, 71, 1800354. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Khayat, M.T.; Ahmed, S.; Abo-Haded, H.; Alshali, K.Z. Mangostanaxanthone VIIII, a new xanthone from Garcinia mangostana pericarps, α-amylase inhibitory activity, and molecular docking studies. Braz. J. Pharmacog. 2019, 29, 206–212. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Khayat, M.T.; Ahmed, S.; Abo-Haded, H. α-Amylase inhibitors xanthones from Garcinia mangostana pericarps and its possible use for the treatment of diabetes with their molecular docking studies. J. Food Biochem. 2019, 43, e12844. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.M. New benzophenones and a dihydroflavanonol from Garcinia mangostana pericarps and their antioxidant and cytotoxic activities. Phytochem. Lett. 2020, 39, 43–48. [Google Scholar] [CrossRef]
- Pedraza-Chaverri, J.; Cardenas-Rodriguez, N.; Orozco-Ibarra, M.; Perez-Rojas, J.M. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef]
- Chin, Y.W.; Kinghorn, A.D. Structural characterization, biological effects, and synthetic studies on xanthones from mangosteen (Garcinia mangostana), a popular botanical dietary supplement. Mini Rev. Org. Chem. 2008, 5, 355–364. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Vaseghi, G.; Pourfarzam, M.; Abdollahi, A. Are antioxidants helpful for disease prevention? Res. Pharm. Sci. 2010, 5, 1–8. [Google Scholar]
- Widowati, W.; Darsono, L.; Suherman, J.; Yelliantty, Y.; Maesaroh, M. High performance liquid chromatography (HPLC) analysis, antioxidant, antiaggregation of mangosteen peel extract (Garcinia mangostana, L.). Int. J. Biosci. Biochem. Bioinform. 2014, 4, 458. [Google Scholar] [CrossRef][Green Version]
- Gondokesumo, M.E.; Pardjianto, B.; Sumitro, S.B.; Widowati, W.; Handono, K. Xanthones analysis and antioxidant activity analysis (applying esr) of six different maturity levels of mangosteen rind extract (Garcinia mangostana Linn.). Pharmacog. J. 2019, 11, 369–373. [Google Scholar] [CrossRef]
- Kusmayadi, A.; Adriani, L.; Abun, A.; Muchtaridi, M.; Tanuwiria, U.H. The effect of solvents and extraction time on total xanthone and antioxidant yields of mangosteen peel (Garcinia mangostana, L.) extract. Drug Invent. Today 2018, 10, 2572–2576. [Google Scholar]
- Fei, M.; Xie, Q.; Zou, Y.; He, R.; Zhang, Y.; Wang, J.; Bo, L.; Li, J.; Deng, X. Alpha-lipoic acid protects mice against concanavalin A-induced hepatitis by modulating cytokine secretion and reducing reactive oxygen species generation. Int. Immunopharmacol. 2016, 35, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.T.; Wolfe, D. Tissue processing and hematoxylin and eosin staining. In Histopathology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 31–43. [Google Scholar]
- Shehata, A.M.; Elbadawy, H.M.; Ibrahim, S.R.M.; Mohamed, G.A.; Elsaed, W.M.; Alhaddad, A.A.; Ahmed, N.; Abo-Haded, H.; El-Agamy, D.S. Alpha-mangostin as a new therapeutic candidate for concanavalin A-induced autoimmune hepatitis: Impact on the SIRT1/Nrf2 and NF-κB crosstalk. Plants 2022, 11, 2441. [Google Scholar] [CrossRef]
- Sakai, S.; Katsura, M.; Takayama, H.; Aimi, N.; Chokethaworn, N.; Suttajit, M. The structure of garcinone, E. Chem. Pharm. Bull. 1993, 41, 958–960. [Google Scholar] [CrossRef]
- Sheeja, K.; Lakshmi, S. Antimetastatic potential of garcinone E in human oral cancer cells. Asian Pac. J. Cancer Prev. 2019, 20, 65–72. [Google Scholar]
- Abdallah, H.M.; El-Bassossy, H.M.; Mohamed, G.A.; El-Halawany, A.M.; Alshali, K.Z.; Banjar, Z.M. Mangostanaxanthones III and IV: Advanced glycation end-product inhibitors from the pericarp of Garcinia mangostana. J. Nat. Med. 2017, 71, 216–226. [Google Scholar] [CrossRef]
- Balunas, M.J.; Su, B.; Brueggemeier, R.W.; Kinghorn, A.D. Xanthones from the botanical dietary supplement mangosteen (Garcinia mangostana) with aromatase inhibitory activity. J. Nat. Prod. 2008, 71, 1161–1166. [Google Scholar] [CrossRef]
- Hao, J.; Sun, W.; Xu, H. Pathogenesis of Concanavalin A induced autoimmune hepatitis in mice. Int. Immunopharmacol. 2022, 102, 108411. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, H.; Hou, T. Concanavalin A-induced autoimmune hepatitis model in mice: Mechanisms and future outlook. Open Life Sci. 2022, 17, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Liu, M.; Weng, S.Y.; Li, J.J.; Xie, C.; He, H.L.; Guan, W.; Yuan, Y.S.; Gao, J. Immune mechanisms of Concanavalin A model of autoimmune hepatitis. World J Gastroenterol. 2012, 18, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liu, Y.; Liu, X.; Gao, S.; Sun, X. Glycyrrhizic acid ammonium salt alleviates Concanavalin A-induced immunological liver injury in mice through the regulation of the balance of immune cells and the inhibition of hepatocyte apoptosis. Biomed. Pharmacother. 2019, 120, 109481. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, P.; Wang, Y.; Wei, S.; Zhang, L.; Wang, J.; Lu, X.; Zhou, H.; Li, R.; Wen, J.; et al. Hepatoprotective effect of San-Cao granule on Con A-induced liver injury in mice and mechanisms of action exploration. Front. Pharmacol. 2018, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mao, Y. Eugenol attenuates concanavalin A-induced hepatitis through modulation of cytokine levels and inhibition of mitochondrial oxidative stress. Arch. Biol. Sci. 2019, 71, 339–346. [Google Scholar] [CrossRef]
- Mounieb, F.; Ramadan, L.; Akool, E.S.; Balah, A. Propolis alleviates concanavalin A-induced hepatitis by modulating cytokine secretion and inhibition of reactive oxygen species. N-S Arch. Pharmacol. 2017, 390, 1105–1115. [Google Scholar] [CrossRef]
- Bai, K.; Hong, B.; He, J.; Huang, W. Antioxidant Capacity and Hepatoprotective Role of Chitosan-Stabilized Selenium Nanoparticles in Concanavalin A-Induced Liver Injury in Mice. Nutrients 2020, 12, 857. [Google Scholar] [CrossRef]
- Luo, Q.; Zhu, L.; Ding, J.; Zhuang, X.; Xu, L.; Chen, F. Protective effect of galangin in Concanavalin A-induced hepatitis in mice. Drug Des Devel Ther. 2015, 9, 2983–2992. [Google Scholar]
- Ma, B.; Mao, Y.; Chang, L.; Dai, T.; Xin, X.; Ma, F.; Wang, Z.; Shen, Z.; Mei, Q.; Zhu, Y. S-Propargyl-cysteine prevents concanavalin A-induced immunological liver injury in mice. Pharm Biol. 2022, 60, 1169–1176. [Google Scholar] [CrossRef]
- Das, M.; Sabio, G.; Jiang, F.; Rincon, M.; Flavell, R.A.; Davis, R.J. Induction of hepatitis by JNK-mediated expression of TNF-alpha. Cell 2009, 136, 249–260. [Google Scholar] [CrossRef]
- Heymann, F.; Hamesch, K.; Weiskirchen, R.; Tacke, F. The concanavalin A mbodel of acute hepatitis in mice. Lab Anim. 2015, 49, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Wang, J.; Yu, F.; Cheng, J.; Li, H.; Guo, C.; Fan, X. Ghrelin reduces liver impairment in a model of concanavalin A-induced acute hepatitis in mice. Drug Des. Devel. Ther. 2015, 9, 5385–5396. [Google Scholar] [CrossRef] [PubMed]


| Gene (Mouse) | Accession | Sequence (5′-3′) | PCR Product Size (bp) |
|---|---|---|---|
| Nrf2 | NM_010902 | F: TGAAGCTCAGCTCGCATTGA R: TGCTCCAGCTCGACAATGTT | 108 |
| HO-1 | NM_010442 | F: GAAATCATCCCTTGCACGCC | 122 |
| R: CCTGAGAGGTCACCCAGGTA | |||
| GCLc | NM_010295 | F: GCTTTGGGTCGCAAGTAGGA | 181 |
| R: GCGTCCCGTCCGTTCC | |||
| NF-ĸB | NM_008689 | F: CCACTGTCAACAGATGGCCC | 158 |
| R: TTGCAAATTTTGACCTGTGGGT | |||
| IL-6 | NM_031168 | F: CCCCAATTTCCAATGCTCTCC | 141 |
| R: CGCACTAGGTTTGCCGAGTA | |||
| IL-1β | NM_008361 | F: TGCCACCTTTTGACAGTGATG | 138 |
| R: TGATGTGCTGCTGCGAGATT | |||
| TNF-α | AY423855 | F: TCCCAAATGGCCTCCCTCTC | 98 |
| R: TACGACGTGGGCTACAGGCT | |||
| β-actin | NM_007393 | F: CTGAGCTGCGTTTTACACCC | 200 |
| R: CGCCTTCACCGTTCCAGTTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, G.A.; Ibrahim, S.R.M.; Hareeri, R.H.; Binmahfouz, L.S.; Bagher, A.M.; Abdallah, H.M.; Elsaed, W.M.; El-Agamy, D.S. Garcinone E Mitigates Oxidative Inflammatory Response and Protects against Experimental Autoimmune Hepatitis via Modulation of Nrf2/HO-1, NF-κB and TNF-α/JNK Axis. Nutrients 2023, 15, 16. https://doi.org/10.3390/nu15010016
Mohamed GA, Ibrahim SRM, Hareeri RH, Binmahfouz LS, Bagher AM, Abdallah HM, Elsaed WM, El-Agamy DS. Garcinone E Mitigates Oxidative Inflammatory Response and Protects against Experimental Autoimmune Hepatitis via Modulation of Nrf2/HO-1, NF-κB and TNF-α/JNK Axis. Nutrients. 2023; 15(1):16. https://doi.org/10.3390/nu15010016
Chicago/Turabian StyleMohamed, Gamal A., Sabrin R. M. Ibrahim, Rawan H. Hareeri, Lenah S. Binmahfouz, Amina M. Bagher, Hossam M. Abdallah, Wael M. Elsaed, and Dina S. El-Agamy. 2023. "Garcinone E Mitigates Oxidative Inflammatory Response and Protects against Experimental Autoimmune Hepatitis via Modulation of Nrf2/HO-1, NF-κB and TNF-α/JNK Axis" Nutrients 15, no. 1: 16. https://doi.org/10.3390/nu15010016
APA StyleMohamed, G. A., Ibrahim, S. R. M., Hareeri, R. H., Binmahfouz, L. S., Bagher, A. M., Abdallah, H. M., Elsaed, W. M., & El-Agamy, D. S. (2023). Garcinone E Mitigates Oxidative Inflammatory Response and Protects against Experimental Autoimmune Hepatitis via Modulation of Nrf2/HO-1, NF-κB and TNF-α/JNK Axis. Nutrients, 15(1), 16. https://doi.org/10.3390/nu15010016









