Effect of Lycopene Intake on the Fasting Blood Glucose Level: A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Literature Search
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment
2.6. Statistical Analysis
2.7. Subgroup Analysis
3. Results
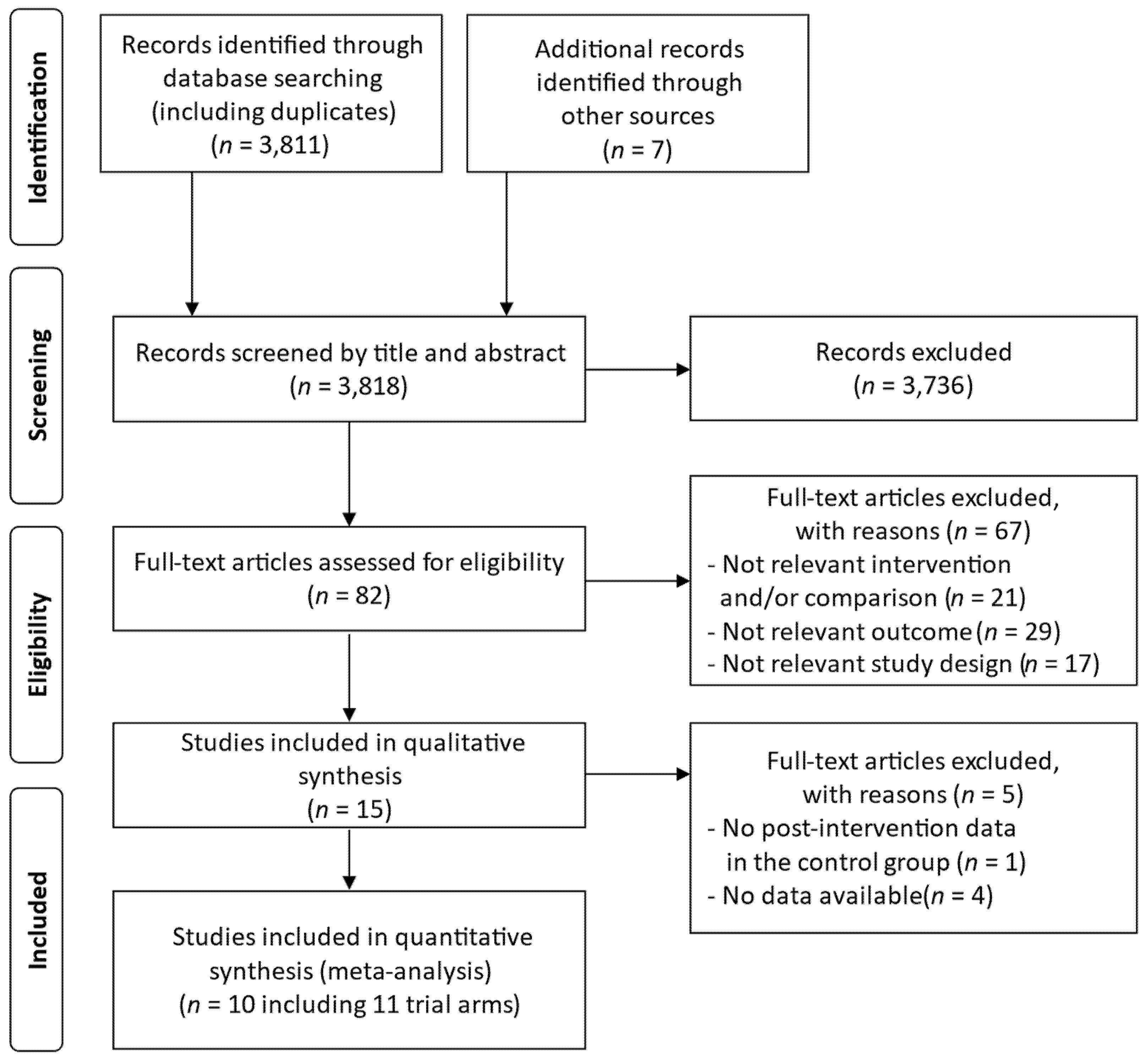
3.1. Search Results
3.2. Study Characteristics
3.3. Quality Assessment of the Studies
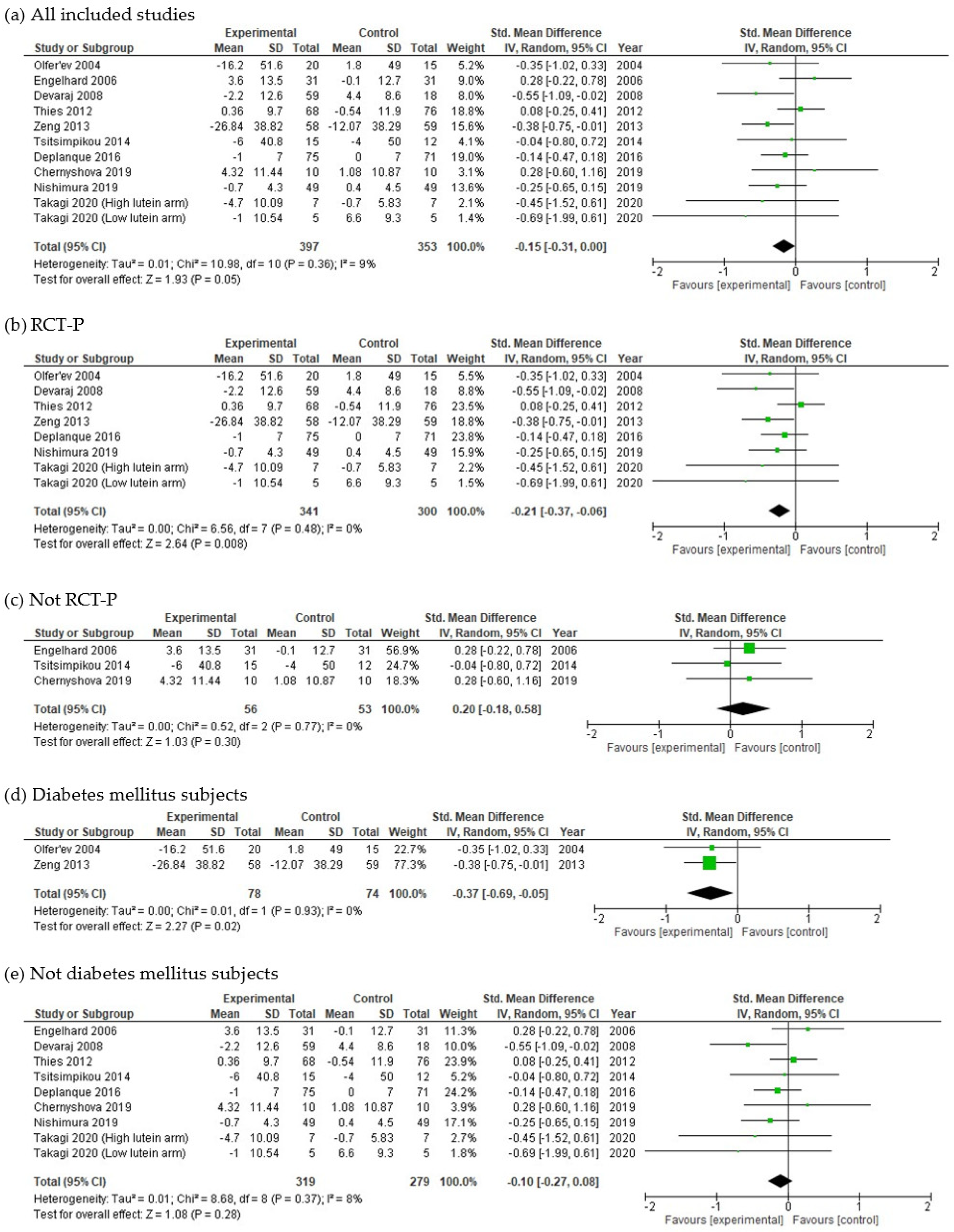
3.4. Meta-Analysis
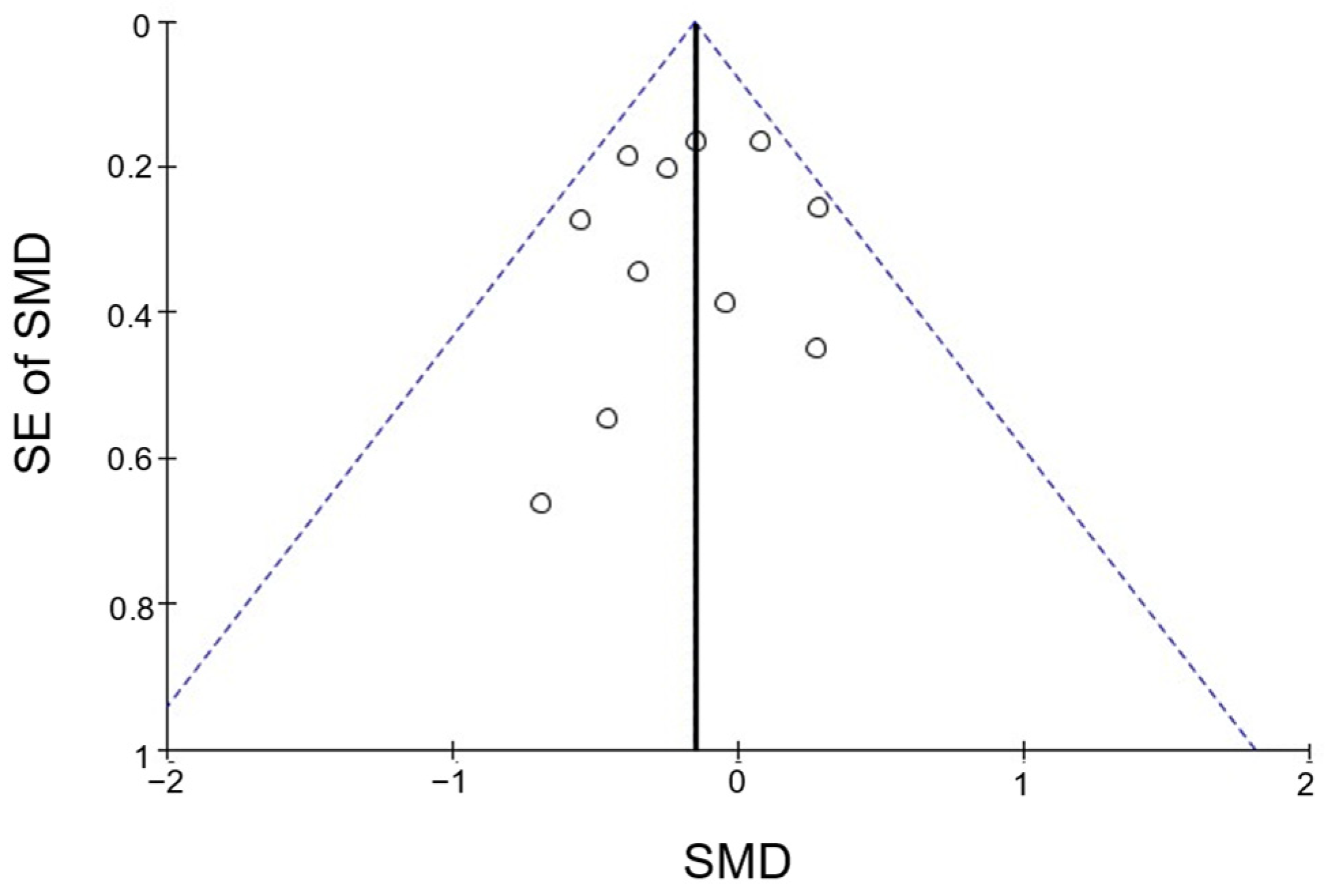
3.5. Publication Bias
4. Discussion
4.1. Effects of Lycopene on FBG
4.2. Effect Size and Possible Mechanisms
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bassi-Dibai, D.; Santos-de-Araújo, A.D.; Dibai-Filho, A.V.; de Azevedo, L.F.S.; Goulart, C.D.L.; Luz, G.C.P.; Burke, P.R.; Garcia-Araújo, A.S.; Borghi-Silva, A. Rehabilitation of individuals with diabetes mellitus: Focus on diabetic myopathy. Front. Endocrinol. 2022, 13, 869921. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Karuranga, S.; Fernandes, J.R.; Huang, Y.; Malanda, B. (Eds.) IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017; Available online: https://diabetesatlas.org/atlas/eighth-edition/ (accessed on 12 November 2022).
- Turner, R.C.; Cull, C.A.; Frighi, V.; Holman, R.R. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: Progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA 1999, 281, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef]
- Salmerón, J.; Ascherio, A.; Rimm, E.B.; Colditz, G.A.; Spiegelman, D.; Jenkins, D.J.; Stampfer, M.J.; Wing, A.L.; Willett, W.C. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 1997, 20, 545–550. [Google Scholar] [CrossRef]
- Ojo, O.; Ojo, O.O.; Adebowale, F.; Wang, X. The effect of dietary glycaemic index on glycaemia in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2018, 10, 373. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Lee, D.; Ahmed, A.; Cheung, A.; Khan, T.A.; Mejia, S.B.; Mirrahimi, A.; Jenkins, D.J.A.; Livesey, G.; Wolever, T.M.S.; et al. Effect of low glycaemic index or load dietary patterns on glycaemic control and cardiometabolic risk factors in diabetes: Systematic review and meta-analysis of randomised controlled trials. BMJ 2021, 374, n1651. [Google Scholar] [CrossRef]
- Abdali, D.; Samson, S.E.; Grover, A.K. How effective are antioxidant supplements in obesity and diabetes? Med. Princ. Pract. 2015, 24, 201–215. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S.H. Mechanism of generation of oxidative stress and pathophysiology of type 2 diabetes mellitus: How are they interlinked? J. Cell. Biochem. 2017, 118, 3577–3585. [Google Scholar] [CrossRef]
- Mancini, F.R.; Affret, A.; Dow, C.; Balkau, B.; Bonnet, F.; Boutron-Ruault, M.C.; Fagherazzi, G. Dietary antioxidant capacity and risk of type 2 diabetes in the large prospective E3N-EPIC cohort. Diabetologia 2018, 61, 308–316. [Google Scholar] [CrossRef]
- Golmohammadi, M.; Ayremlou, P.; Zarrin, R. Higher oxidative balance score is associated with better glycemic control among Iranian adults with type-2 diabetes. Int. J. Vitam. Nutr. Res. 2021, 91, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fan, Y.; Zhang, X.; Hou, W.; Tang, Z. Fruit and vegetable intake and risk of type 2 diabetes mellitus: Meta-analysis of prospective cohort studies. BMJ Open 2014, 4, e005497. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Fang, J.; Gao, Z.; Zhang, C.; Xie, S. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: A meta-analysis. J. Diabetes Investig. 2016, 7, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Kapała, A.; Szlendak, M.; Motacka, E. The anti-cancer activity of lycopene: A systematic review of human and animal studies. Nutrients 2022, 14, 5152. [Google Scholar] [CrossRef] [PubMed]
- Przybylska, S.; Tokarczyk, G. Lycopene in the prevention of cardiovascular diseases. Int. J. Mol. Sci. 2022, 23, 1957. [Google Scholar] [CrossRef] [PubMed]
- Leh, H.E.; Lee, L.K. Lycopene: A potent antioxidant for the amelioration of type II diabetes mellitus. Molecules 2022, 27, 2335. [Google Scholar] [CrossRef]
- Stahl, W.; Heinrich, U.; Aust, O.; Tronnier, H.; Sies, H. Lycopene-rich products and dietary photoprotection. Photochem. Photobiol. Sci. 2006, 5, 238–242. [Google Scholar] [CrossRef]
- Costa-Rodrigues, J.; Fernandes, M.H.; Pinho, O.; Monteiro, P.R.R. Modulation of human osteoclastogenesis and osteoblastogenesis by lycopene. J. Nutr. Biochem. 2018, 57, 26–34. [Google Scholar] [CrossRef]
- Quansah, D.Y.; Ha, K.; Jun, S.; Kim, S.A.; Shin, S.; Wie, G.A.; Joung, H. Associations of dietary antioxidants and risk of type 2 diabetes: Data from the 2007-2012 Korea national health and nutrition examination survey. Molecules 2017, 22, 1664. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, Z.; Tong, W.; Yang, K.; Guo, K.; Liu, G.; Pan, A. Dietary intake and circulating concentrations of carotenoids and risk of type 2 diabetes: A dose-response meta-analysis of prospective observational studies. Adv. Nutr. 2021, 12, 1723–1733. [Google Scholar] [CrossRef]
- Leh, H.E.; Sopian, M.M.; Bakar, M.H.A.; Lee, L.K. The role of lycopene for the amelioration of glycaemic status and peripheral antioxidant capacity among the type II diabetes mellitus patients: A case–control study. Ann. Med. 2021, 53, 1059–1065. [Google Scholar] [CrossRef]
- Mummidi, S.; Farook, V.S.; Reddivari, L.; Hernandez-Ruiz, J.; Diaz-Badillo, A.; Fowler, S.P.; Resendez, R.G.; Akhtar, F.; Lehman, D.M.; Jenkinson, C.P.; et al. Serum carotenoids and pediatric metabolic index predict insulin sensitivity in Mexican American children. Sci. Rep. 2021, 11, 871. [Google Scholar] [CrossRef]
- Li, H.; Chen, A.; Zhao, L.; Bhagavathula, A.S.; Amirthalingam, P.; Rahmani, J.; Salehisahlabadi, A.; Abdulazeem, H.M.; Adebayo, O.; Yin, X. Effect of tomato consumption on fasting blood glucose and lipid profiles: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2020, 34, 1956–1965. [Google Scholar] [CrossRef]
- Tierney, A.C.; Rumble, C.E.; Billings, L.M.; George, E.S. Effect of dietary and supplemental lycopene on cardiovascular risk factors: A systematic review and meta-analysis. Adv. Nutr. 2020, 11, 1453–1488. [Google Scholar] [CrossRef]
- Valero, M.A.; Vidal, A.; Burgos, R.; Calvo, F.L.; Martínez, C.; Luengo, L.M.; Cuerda, C. Meta-analysis on the role of lycopene in type 2 diabetes mellitus. Nutr. Hosp. 2011, 26, 1236–1241. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Inoue, T.; Yoshida, K.; Sasaki, E.; Aizawa, K.; Kamioka, H. Effects of lycopene intake on HDL-cholesterol and triglyceride levels: A systematic review with meta-analysis. J. Food Sci. 2021, 86, 3285–3302. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0; The Cochrane Collaboration: London, UK, 2021; Available online: https://training.cochrane.org/handbook/archive/v5.1/ (accessed on 12 November 2022).
- Furlan, A.D.; Malmivaara, A.; Chou, R.; Maher, C.G.; Deyo, R.A.; Schoene, M.; Bronfort, G.; van Tulder, M.W.; Editorial Board of the Cochrane Back, Neck Group. 2015 updated method guideline for systematic reviews in the cochrane back and neck group. Spine 2015, 40, 1660–1673. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Upritchard, J.E.; Sutherland, W.H.F.; Mann, J.I. Effect of supplementation with tomato juice, vitamin E, and vitamin C on LDL oxidation and products of inflammatory activity in type 2 diabetes. Diabetes Care 2000, 23, 733–738. [Google Scholar] [CrossRef]
- Olfer’ev, A.M.; Il’ina, M.V.; Berzak, N.V.; Stetsenko, A.V.; Olfer’ev, M.A.; Chudakova, I.A.; Kapitanov, A.B.; Shamarin, V.M. Effect of lycopene on blood lipoproteids in women with type 2 diabetes mellitus in postmenopause. Vopr. Pitan. 2004, 73, 19–23. [Google Scholar] [PubMed]
- Engelhard, Y.N.; Gazer, B.; Paran, E. Natural antioxidants from tomato extract reduce blood pressure in patients with grade-1 hypertension: A double-blind, placebo-controlled pilot study. Am. Heart J. 2006, 151, 100. [Google Scholar] [CrossRef] [PubMed]
- Neyestani, T.R.; Shariat-Zadeh, N.; Gharavi, A.; Kalayi, A.; Khalaji, H. The opposite associations of lycopene and body fat mass with humoral immunity in type 2 diabetes mellitus: A possible role in atherogenesis. Iran. J. Allergy Asthma Immunol. 2007, 6, 79–87. [Google Scholar]
- Devaraj, S.; Mathur, S.; Basu, A.; Aung, H.H.; Vasu, V.T.; Meyers, S.; Jialal, I. A dose-response study on the effects of purified lycopene supplementation on biomarkers of oxidative stress. J. Am. Coll. Nutr. 2008, 27, 267–273. [Google Scholar] [CrossRef]
- Kim, J.Y.; Paik, J.K.; Kim, O.Y.; Park, H.W.; Lee, J.H.; Jang, Y.; Lee, J.H. Effects of lycopene supplementation on oxidative stress and markers of endothelial function in healthy men. Atherosclerosis 2011, 215, 189–195. [Google Scholar] [CrossRef]
- Thies, F.; Masson, L.F.; Rudd, A.; Vaughan, N.; Tsang, C.; Brittenden, J.; Simpson, W.G.; Duthie, S.; Horgan, G.W.; Duthie, G. Effect of a tomato-rich diet on markers of cardiovascular disease risk in moderately overweight, disease-free, middle-aged adults: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1013–1022. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, H.; Li, J.; Liu, Y.; Wang, T.; Mu, G. Effects of lycopene on cytokines and carotid intima-media thickness in type 2 diabetic patients. Acta Nutr. Sin. 2013, 35, 268–272. [Google Scholar]
- Samaras, A.; Tsarouhas, K.; Paschalidis, E.; Giamouzis, G.; Triposkiadis, F.; Tsitsimpikou, C.; Becker, A.T.; Goutzourelas, N.; Kouretas, D. Effect of a special carbohydrate-protein bar and tomato juice supplementation on oxidative stress markers and vascular endothelial dynamics in ultra-marathon runners. Food Chem. Toxicol. 2014, 69, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Tsitsimpikou, C.; Tsarouhas, K.; Kioukia-Fougia, N.; Skondra, C.; Fragkiadaki, P.; Papalexis, P.; Stamatopoulos, P.; Kaplanis, I.; Hayes, A.W.; Tsatsakis, A.; et al. Dietary supplementation with tomato-juice in patients with metabolic syndrome: A suggestion to alleviate detrimental clinical factors. Food Chem. Toxicol. 2014, 74, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Deplanque, X.; Muscente-Paque, D.; Chappuis, E. Proprietary tomato extract improves metabolic response to high-fat meal in healthy normal weight subjects. Food Nutr. Res. 2016, 60, 32537. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chernyshova, M.P.; Pristenskiy, D.V.; Lozbiakova, M.V.; Chalyk, N.E.; Bandaletova, T.Y.; Petyaev, I.M. Systemic and skin-targeting beneficial effects of lycopene-enriched ice cream: A pilot study. J. Dairy Sci. 2019, 102, 14–25. [Google Scholar] [CrossRef]
- Nishimura, M.; Tominaga, N.; Ishikawa-Takano, Y.; Maeda-Yamamoto, M.; Nishihira, J. Effect of 12-week daily intake of the high-lycopene tomato (Solanum Lycopersicum), a variety named “PR-7”, on lipid metabolism: A randomized, double-blind, placebo-controlled, parallel-group study. Nutrients 2019, 11, 1177. [Google Scholar] [CrossRef]
- Wiese, M.; Bashmakov, Y.; Chalyk, N.; Nielsen, D.S.; Krych, Ł.; Kot, W.; Klochkov, V.; Pristensky, D.; Bandaletova, T.; Chernyshova, M.; et al. Prebiotic effect of lycopene and dark chocolate on gut microbiome with systemic changes in liver metabolism, skeletal muscles and skin in moderately obese persons. BioMed Res. Int. 2019, 2019, 4625279. [Google Scholar] [CrossRef]
- Takagi, T.; Hayashi, R.; Nakai, Y.; Okada, S.; Miyashita, R.; Yamada, M.; Mihara, Y.; Mizushima, K.; Morita, M.; Uchiyama, K.; et al. Dietary intake of carotenoid-rich vegetables reduces visceral adiposity in obese Japanese men—A randomized, double-blind trial. Nutrients 2020, 12, 2342. [Google Scholar] [CrossRef]
- Michaličková, D.; Belović, M.; Ilić, N.; Kotur-Stevuljević, J.; Slanař, O.; Šobajić, S. Comparison of polyphenol-enriched tomato juice and standard tomato juice for cardiovascular benefits in subjects with stage 1 hypertension: A randomized controlled study. Plant Foods Hum. Nutr. 2019, 74, 122–127. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Kapoor, M.C. Types of studies and research design. Indian J. Anaesth. 2016, 60, 626–630. [Google Scholar] [CrossRef]
- Gao, Q.; Zhong, C.; Zhou, X.; Chen, R.; Xiong, T.; Hong, M.; Li, Q.; Kong, M.; Han, W.; Sun, G.; et al. The association between intake of dietary lycopene and other carotenoids and gestational diabetes mellitus risk during mid-trimester: A cross-sectional study. Br. J. Nutr. 2019, 121, 1405–1412. [Google Scholar] [CrossRef]
- Shabani, E.; Sayemiri, K.; Mohammadpour, M. The effect of garlic on lipid profile and glucose parameters in diabetic patients: A systematic review and meta-analysis. Prim. Care Diabetes 2019, 13, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Suksomboon, N.; Poolsup, N.; Punthanitisarn, S. Effect of Aloe vera on glycaemic control in prediabetes and type 2 diabetes: A systematic review and meta-analysis. J. Clin. Pharm. Ther. 2016, 41, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Tominaga, N.; Wakagi, M.; Ishikawa-Takano, Y. Consumption of lycopene-rich tomatoes improved glucose homeostasis in rats via an increase in leptin levels. J. Nat. Med. 2020, 74, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zheng, Z.; Jiang, Z. Effects of lycopene on metabolism of glycolipid in type 2 diabetic rats. Biomed. Pharmacother. 2019, 109, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Yin, Y.; Lu, R.; Jiang, Z. Lycopene ameliorated oxidative stress and inflammation in type 2 diabetic rats. J. Food Sci. 2019, 84, 1194–1200. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; PRISMA-S Group. PRISMA-S: An extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, Q.; Vist, G.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
| Selected Studies | Sources of Risk of Bias * | Total Number of “−” | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (A) | (B) | (C) | (D) | (E) | (F) | (G) | (H) | (I) | (J) | (K) | (L) | (M) | ||
| Upritchard 2000 [35] | + | + | − | − | + | − | − | − | − | + | − | + | + | 7 |
| Olfer’ev 2004 [36] | − | − | + | − | + | − | − | − | + | + | − | − | + | 8 |
| Engelhard 2006 [37] | − | − | + | − | + | + | − | − | + | + | − | − | + | 7 |
| Neyestani 2007 [38] | − | − | + | + | − | + | + | − | + | + | − | − | + | 6 |
| Devaraj 2008 [39] | − | − | + | − | + | − | − | − | + | + | − | − | + | 8 |
| Kim 2011 [40] | − | − | + | − | + | − | − | − | + | + | − | − | + | 8 |
| Thies 2012 [41] | − | − | − | − | + | − | − | + | + | + | − | − | + | 8 |
| Zeng 2013 [42] | − | − | − | − | + | − | − | − | + | + | − | + | + | 8 |
| Samaras 2014 [43] | − | − | − | − | + | + | + | − | − | + | − | − | − | 9 |
| Tsitsimpikou 2014 [44] | − | − | − | − | + | + | + | − | + | + | − | − | + | 7 |
| Deplanque 2016 [45] | − | + | + | + | + | + | − | − | + | + | − | + | − | 5 |
| Chernyshova 2019 [46] | − | − | − | − | + | + | + | − | − | + | − | − | + | 8 |
| Nishimura 2019 [47] | + | + | + | + | + | − | − | + | + | + | + | + | + | 2 |
| Wiese 2019 [48] | − | − | + | − | + | + | + | − | − | + | − | − | + | 7 |
| Takagi 2020 [49] | − | − | + | − | + | − | − | − | − | + | − | + | + | 8 |
| Sample Size, Sex | Participant, Age (Years) | Sample Size, Sex | Intervention/Control | Lycopene Dosage Per Day | Duration (Intake Period) | Outcome (Blood Biomarkers of Glucose Metabolism) | Study Design |
|---|---|---|---|---|---|---|---|
| Olfer’ev 2004 [36], Russia | Type 2 diabetic postmenopausal women, mean age 66.4 | I: 20 (all F) C: 15 (all F) | I: Tomato extract capsule (3/day) C: Placebo capsule (3/day) | I: 30 mg C: 0 mg | 12 weeks | FBG | RCT-P |
| Engelhard 2006 [37], Israel | Grade-1 hypertensive subjects, age range 30–73 | I: 31 (13 F/18 M) C: 31 (13 F/18 M) | I: Tomato extract capsule (1/day) C: Placebo capsule (1/day) | I: 15 mg C: 0 mg | 8 weeks | FBG | non-RCT-C |
| Devaraj 2008 [39], USA | Healthy subjects, age range ≥40 | I1: 21 (17 F/4 M) I2: 17 (13 F/4 M) I3: 21 (14 F/7 M) C: 18 (14 F/4 M) | I1: Lycopene capsule (1/day) I2: Lycopene capsule (1/day) I3: Lycopene capsule (1/day) C: Placebo capsule (1/day) | I1: 6.5 mg I2: 15 mg I3: 30 mg C: 0 mg | 8 weeks | FBG | RCT-P |
| Thies 2012 [41], UK | Moderate overweight subjects, age range 40–65 | I1: 68 (40 F/28 M) I2: 81 (46 F/35 M) C: 76 (46 F/30 M) | I1: Low-tomato diet and tomato extract capsule (1/day) I2: High-tomato diet C: Low-tomato diet | I1: 10 mg I2: 32–50 mg C: 0.3 mg | 12 weeks | FBG Insulin HOMA-IR QUICKI | RCT-P |
| Zeng 2013 [42], China | Type 2 diabetic patients, age range ≥60 | I: 58 C: 59 | I: Lycopene capsule (4/day) C: Placebo capsule (4/day) | I: 30 mg C: 0 mg | 6 months | FBG PBG HbA1c | RCT-P |
| Tsitsimpikou 2014 [44], Greece | Metabolic syndrome subjects, mean age 54.9 | I: 15 (2 F/13 M) C: 12 (1 F/11 M) | I: Tomato juice C: None | I: NA C: 0 mg | 2 months | FBG Insulin FIRI | non-RCT-P |
| Deplanque 2016 [45], France | Healthy subjects, mean age 34.9 | I: 75 C: 70 | I: Tomato extract capsule (1/day) C: Placebo capsule (1/day) | I: 15 mg C: 0 mg | 2 weeks | FBG | RCT-P |
| Chernyshova 2019 [46], Russia | Healthy subjects, mean age 33.4 | I: 10 (5 F/5 M) C: 10 (5 F/5 M) | I: Lycopene-enriched ice cream (50 g/day) C: Ice cream (50 g/day) | I: 7 mg C: 0 mg | 4 weeks | FBG | RCT-C |
| Nishimura 2019 [47], Japan | Healthy subjects, age range 30–70 | I: 49 C: 49 | I: Semidried high-lycopene tomato (50 g/day) C: Semidried lycopene-free tomato (50 g/day) | I: 22.0–27.8 mg C: 0 mg | 12 weeks | FBG HbA1c HOMA-IR | RCT-P |
| Takagi 2020 [49], Japan | Obese men, age range 40–65 | I1: 7 (all M) I2: 5 (all M) C1: 7 (all M) C2: 5 (all M) | I1: Carrot and kale juice (high lycopene + high lutein) (200 mL/day) I2: Carrot and cabbage juice (high lycopene + low lutein) (200 mL/day) C1: Carrot and kale juice (low lycopene + high lutein) (200 mL/day) C2: Carrot and cabbage juice (low lycopene + low lutein) (200 mL/day) | I1: 7.56 mg I2: 8.6 mg C1: 0 mg C2: 0 mg | 8 weeks | FBG | RCT-P |
| Sample Size, Sex | Participant, Age (Years) | Sample Size, Sex | Intervention/Control | Lycopene Dosage Per Day | Duration (Intake Period) | Outcome (Blood Biomarkers of Glucose Metabolism) | Study Design | Reason for Exclusion |
|---|---|---|---|---|---|---|---|---|
| Upritchard 2000 [35], New Zealand | Type 2 diabetic patients, mean age 59 | I1: 15 (5 F/10 M) I2: 12 (6 F/6 M) I3: 12 (6 F/6 M) C: 13 (3 F/10 M) | I1: Tomato juice (500 mL/day) I2: Vitamin E (800 U/day) I3: Vitamin C (500 mg/day) C: Placebo capsule (1/day) | I1: NA I2: 0 mg I3: 0 mg C: 0 mg | 4 weeks | FBG HbA1c | RCT-P | No data available |
| Neyestani 2007 [38], Iran | Type 2 diabetic patients, mean age 54 | I: 16 (9 F/7 M) C: 19 (10 F/9 M) | I: Lycopene supplement C: Placebo supplement | I: 10 mg C: 0 mg | 8 weeks | FBG HbA1c | non-RCT-P | No data available |
| Kim 2011 [40], Korea | Healthy subjects, mean age 34.3 | I1: 41 (all M) I2: 37 (all M) C: 38 (all M) | I1: Tomato extract capsule (1/day) I2: Tomato extract capsule (1/day) C: Placebo capsule (1/day) | I1: 6 mg I2: 15 mg C: 0 mg | 8 weeks | FBG | RCT-P | No data available |
| Samaras 2014 [43], Greece | Ultra-marathon runners, mean age 44.9 | I1: 15 (2 F/13 M) I2: 16 (2 F/14 M) C: 12 (all M) | I1: Tomato juice I2: Protein bar C: Carbohydrate supplementation beverage | I1: NA I2: NA C: NA | 2 months | FBG | non-RCT-P | No post-intervention data in the control group |
| Wiese 2019 [48], Russia | Moderate obese subjects, mean age 55 | I1: 6 (3 F/3 M) I2: 6 (3 F/3 M) C1: 6 (3 F/3 M) C2: 6 (3 F/3 M) | I1: Lycopene-enriched dark chocolate (10 g/day) I2: Lycopene capsule (1/day) C1: Dark chocolate (10 g/day) C2: Lycopene capsule (1/day) | I1: 7 mg I2: 30 mg C1: 0 mg C2: 7 mg | 1 month | FBG | RCT-P | No data available |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, T.; Yoshida, K.; Sasaki, E.; Aizawa, K.; Kamioka, H. Effect of Lycopene Intake on the Fasting Blood Glucose Level: A Systematic Review with Meta-Analysis. Nutrients 2023, 15, 122. https://doi.org/10.3390/nu15010122
Inoue T, Yoshida K, Sasaki E, Aizawa K, Kamioka H. Effect of Lycopene Intake on the Fasting Blood Glucose Level: A Systematic Review with Meta-Analysis. Nutrients. 2023; 15(1):122. https://doi.org/10.3390/nu15010122
Chicago/Turabian StyleInoue, Takuro, Kazutaka Yoshida, Erika Sasaki, Koichi Aizawa, and Hiroharu Kamioka. 2023. "Effect of Lycopene Intake on the Fasting Blood Glucose Level: A Systematic Review with Meta-Analysis" Nutrients 15, no. 1: 122. https://doi.org/10.3390/nu15010122
APA StyleInoue, T., Yoshida, K., Sasaki, E., Aizawa, K., & Kamioka, H. (2023). Effect of Lycopene Intake on the Fasting Blood Glucose Level: A Systematic Review with Meta-Analysis. Nutrients, 15(1), 122. https://doi.org/10.3390/nu15010122







