Essential Minerals and Metabolic Adaptation of Immune Cells
Abstract
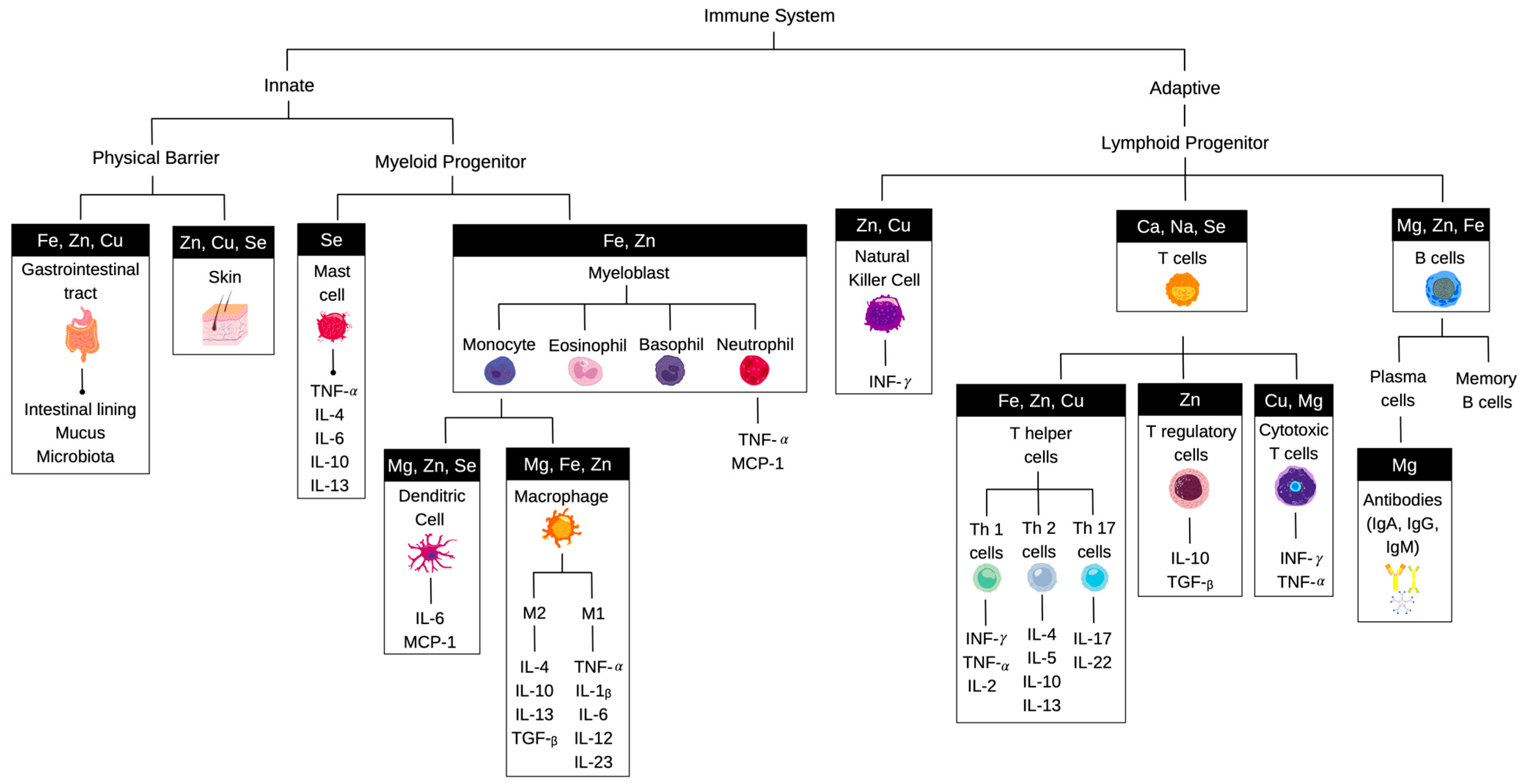
1. Introduction
2. Inflammation and Metabolic Dysfunction
2.1. Inflammation in Obesity
2.2. Inflammation in Diabetes
2.3. Inflammation in the Gastrointestinal Disorders
3. Immune Cell Metabolism and Metabolic Reprogramming
3.1. Inflamed Tissue Is a Metabolically Restrictive Environment
3.2. Metabolic Reprograming during Activation of Immune Cells
3.2.1. Neutrophils
3.2.2. Mast Cells
3.2.3. Macrophages
3.2.4. Dendritic Cells
3.2.5. NK Natural Killer Cells CD56+
3.2.6. B Cells CD19+
3.2.7. T Cells CD3+ CD8+ (Cytotoxic)
3.2.8. T Cells CD3+ CD4+ (Helper)
4. Influence of Essential Minerals on Immunological Outcomes
4.1. Calcium
4.2. Potassium
4.3. Magnesium
4.4. Iron
4.5. Other Major Minerals (Phosphorus, Sodium, Chloride, Sulfur)
4.6. Other Trace Minerals (Zinc, Copper, Selenium, Manganese)
5. Changes in the Mineral Content of Foods
5.1. Agrociltural Food Production
5.2. Bioavailability, Fortification, and Dietary Guidelines
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, M.C.; McCarthy, C.; Fearnbach, N.; Yang, S.; Shepherd, J.; Heymsfield, S.B. Emergence of the Obesity Epidemic: 6-Decade Visualization with Humanoid Avatars. Am. J. Clin. Nutr. 2022, 115, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Cantley, J.; Ashcroft, F.M. Q&A: Insulin Secretion and Type 2 Diabetes: Why Do β-Cells Fail? BMC Biol. 2015, 13, 33. [Google Scholar] [CrossRef]
- O’Hearn, M.; Lauren, B.N.; Wong, J.B.; Kim, D.D.; Mozaffarian, D. Trends and Disparities in Cardiometabolic Health Among U.S. Adults, 1999–2018. J. Am. Coll. Cardiol. 2022, 80, 138–151. [Google Scholar] [CrossRef]
- MacKenna, B.; Kennedy, N.A.; Mehrkar, A.; Rowan, A.; Galloway, J.; Matthewman, J.; Mansfield, K.E.; Bechman, K.; Yates, M.; Brown, J.; et al. Risk of Severe COVID-19 Outcomes Associated with Immune-Mediated Inflammatory Diseases and Immune-Modifying Therapies: A Nationwide Cohort Study in the OpenSAFELY Platform. Lancet Rheumatol. 2022, 4, e490–e506. [Google Scholar] [CrossRef]
- Boutari, C.; Mantzoros, C.S. A 2022 Update on the Epidemiology of Obesity and a Call to Action: As Its Twin COVID-19 Pandemic Appears to Be Receding, the Obesity and Dysmetabolism Pandemic Continues to Rage On. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef]
- Habbab, R.M.; Bhutta, Z.A. Prevalence and Social Determinants of Overweight and Obesity in Adolescents in Saudi Arabia: A Systematic Review. Clin. Obes. 2020, 10, e12400. [Google Scholar] [CrossRef]
- Oh, T.J.; Lee, H.; Cho, Y.M. East Asian Perspectives in Metabolic and Bariatric Surgery. J. Diabetes Investig. 2022, 13, 756–761. [Google Scholar] [CrossRef]
- Calle, M.C.; Fernandez, M.L. Inflammation and Type 2 Diabetes. Diabetes Metab. 2012, 38, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative Stress and Metabolic Disorders: Pathogenesis and Therapeutic Strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, O.; Polishchuk, I.; Gorchakova, N.; Zaychenko, H. Metabolic Syndrome and Lipid Metabolism Disorders: Molecular and Biochemical Aspects. Acta Fac. Med. Naissensis 2020, 37, 5–22. [Google Scholar] [CrossRef]
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of Obesity and Metabolic Syndrome on Immunity12. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Guilliams, M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity 2016, 44, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- De Fano, M.; Bartolini, D.; Tortoioli, C.; Vermigli, C.; Malara, M.; Galli, F.; Murdolo, G. Adipose Tissue Plasticity in Response to Pathophysiological Cues: A Connecting Link between Obesity and Its Associated Comorbidities. Int. J. Mol. Sci. 2022, 23, 5511. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Rathinasabapathy, T.; Sakthivel, L.P.; Komarnytsky, S. Plant-Based Support of Respiratory Health during Viral Outbreaks. J. Agric. Food Chem. 2022, 70, 2064–2076. [Google Scholar] [CrossRef]
- Soderholm, A.T.; Pedicord, V.A. Intestinal Epithelial Cells: At the Interface of the Microbiota and Mucosal Immunity. Immunology 2019, 158, 267–280. [Google Scholar] [CrossRef]
- Mariathasan, S.; Weiss, D.S.; Newton, K.; McBride, J.; O’Rourke, K.; Roose-Girma, M.; Lee, W.P.; Weinrauch, Y.; Monack, D.M.; Dixit, V.M. Cryopyrin Activates the Inflammasome in Response to Toxins and ATP. Nature 2006, 440, 228–232. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites Produced by Commensal Bacteria Promote Peripheral Regulatory T-Cell Generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Komarnytsky, S.; Retchin, S.; Vong, C.I.; Lila, M.A. Gains and Losses of Agricultural Food Production: Implications for the Twenty-First Century. Annu. Rev. Food Sci. Technol. 2022, 13, 239–261. [Google Scholar] [CrossRef]
- Mayer, A.-M.B.; Trenchard, L.; Rayns, F. Historical Changes in the Mineral Content of Fruit and Vegetables in the UK from 1940 to 2019: A Concern for Human Nutrition and Agriculture. Int. J. Food Sci. Nutr. 2022, 73, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, e816460. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.; Gilroy, D.W. Chronic Inflammation: A Failure of Resolution? Int. J. Exp. Pathol. 2007, 88, 85–94. [Google Scholar] [CrossRef]
- Nijhuis, J.; Rensen, S.S.; Slaats, Y.; van Dielen, F.M.H.; Buurman, W.A.; Greve, J.W.M. Neutrophil Activation in Morbid Obesity, Chronic Activation of Acute Inflammation. Obes. (Silver Spring) 2009, 17, 2014–2018. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and Metabolic Disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of Fats and Carbohydrate Intake with Cardiovascular Disease and Mortality in 18 Countries from Five Continents (PURE): A Prospective Cohort Study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Solon-Biet, S.; Wahl, D.; Cogger, V.C.; Willcox, B.J.; Willcox, D.C.; Raubenheimer, D.; Simpson, S.J. New Horizons: Dietary Protein, Ageing and the Okinawan Ratio. Age Ageing 2016, 45, 443–447. [Google Scholar] [CrossRef]
- Wali, J.A.; Raubenheimer, D.; Senior, A.M.; Le Couteur, D.G.; Simpson, S.J. Cardio-Metabolic Consequences of Dietary Carbohydrates: Reconciling Contradictions Using Nutritional Geometry. Cardiovasc. Res. 2021, 117, 386–401. [Google Scholar] [CrossRef]
- Kubota, N.; Kubota, T.; Kajiwara, E.; Iwamura, T.; Kumagai, H.; Watanabe, T.; Inoue, M.; Takamoto, I.; Sasako, T.; Kumagai, K.; et al. Differential Hepatic Distribution of Insulin Receptor Substrates Causes Selective Insulin Resistance in Diabetes and Obesity. Nat. Commun. 2016, 7, 12977. [Google Scholar] [CrossRef]
- Metz, H.E.; Kargl, J.; Busch, S.E.; Kim, K.-H.; Kurland, B.F.; Abberbock, S.R.; Randolph-Habecker, J.; Knoblaugh, S.E.; Kolls, J.K.; White, M.F.; et al. Insulin Receptor Substrate-1 Deficiency Drives a Proinflammatory Phenotype in KRAS Mutant Lung Adenocarcinoma. Proc. Natl. Acad. Sci. USA 2016, 113, 8795–8800. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Goeddel, D.V. TNF-R1 Signaling: A Beautiful Pathway. Science 2002, 296, 1634–1635. [Google Scholar] [CrossRef] [PubMed]
- Weyer, C.; Yudkin, J.S.; Stehouwer, C.D.A.; Schalkwijk, C.G.; Pratley, R.E.; Tataranni, P.A. Humoral Markers of Inflammation and Endothelial Dysfunction in Relation to Adiposity and in Vivo Insulin Action in Pima Indians. Atherosclerosis 2002, 161, 233–242. [Google Scholar] [CrossRef]
- McLaughlin, T.; Ackerman, S.E.; Shen, L.; Engleman, E. Role of Innate and Adaptive Immunity in Obesity-Associated Metabolic Disease. J. Clin. Investig. 2017, 127, 5–13. [Google Scholar] [CrossRef]
- Shao, B.-Z.; Xu, Z.-Q.; Han, B.-Z.; Su, D.-F.; Liu, C. NLRP3 Inflammasome and Its Inhibitors: A Review. Front. Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef]
- Tencerova, M.; Aouadi, M.; Vangala, P.; Nicoloro, S.M.; Yawe, J.C.; Cohen, J.L.; Shen, Y.; Garcia-Menendez, L.; Pedersen, D.J.; Gallagher-Dorval, K.; et al. Activated Kupffer Cells Inhibit Insulin Sensitivity in Obese Mice. FASEB J. 2015, 29, 2959–2969. [Google Scholar] [CrossRef] [PubMed]
- Fink, L.N.; Oberbach, A.; Costford, S.R.; Chan, K.L.; Sams, A.; Blüher, M.; Klip, A. Expression of Anti-Inflammatory Macrophage Genes within Skeletal Muscle Correlates with Insulin Sensitivity in Human Obesity and Type 2 Diabetes. Diabetologia 2013, 56, 1623–1628. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Hu, Z.; Yang, S.; Sun, L.; Yu, Z.; Wang, G. Role of Adaptive and Innate Immunity in Type 2 Diabetes Mellitus. J. Diabetes Res. 2018, 2018, 7457269. [Google Scholar] [CrossRef]
- Martin, M.A.; Goya, L.; Ramos, S. Protective Effects of Tea, Red Wine and Cocoa in Diabetes. Evidences from Human Studies. Food Chem. Toxicol. 2017, 109, 302–314. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E. Mechanisms of Diabetic Complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Duncan, B.B.; Schmidt, M.I.; Pankow, J.S.; Ballantyne, C.M.; Couper, D.; Vigo, A.; Hoogeveen, R.; Folsom, A.R.; Heiss, G. Atherosclerosis Risk in Communities Study Low-Grade Systemic Inflammation and the Development of Type 2 Diabetes: The Atherosclerosis Risk in Communities Study. Diabetes 2003, 52, 1799–1805. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, V.; Uchida, T.; Yenush, L.; Davis, R.; White, M.F. The C-Jun NH(2)-Terminal Kinase Promotes Insulin Resistance during Association with Insulin Receptor Substrate-1 and Phosphorylation of Ser(307). J. Biol. Chem. 2000, 275, 9047–9054. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Fraterrigo, G.; Yoshino, J.; Luecking, C.; Kirbach, K.; Kelly, S.C.; de Las Fuentes, L.; He, S.; Okunade, A.L.; Patterson, B.W.; et al. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell Metab. 2016, 23, 591–601. [Google Scholar] [CrossRef]
- Williamson, R.T. On the Treatment of Glycosuria and Diabetes Mellitus with Sodium Salicylate. Br. Med. J. 1901, 1, 760–762. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Konstantopoulos, N.; Lee, J.; Hansen, L.; Li, Z.W.; Karin, M.; Shoelson, S.E. Reversal of Obesity- and Diet-Induced Insulin Resistance with Salicylates or Targeted Disruption of Ikkbeta. Science 2001, 293, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Hundal, R.S.; Petersen, K.F.; Mayerson, A.B.; Randhawa, P.S.; Inzucchi, S.; Shoelson, S.E.; Shulman, G.I. Mechanism by Which High-Dose Aspirin Improves Glucose Metabolism in Type 2 Diabetes. J. Clin. Investig. 2002, 109, 1321–1326. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Involvement of Gut Microbiota, Microbial Metabolites and Interaction with Polyphenol in Host Immunometabolism. Nutrients 2020, 12, E3054. [Google Scholar] [CrossRef]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in Intestinal Mucosal Defense and Inflammation: Learning From Clinical and Experimental Studies. Front. Immunol. 2020, 11, 2054. [Google Scholar] [CrossRef]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J. Immunol. Res. 2018, 2018, 2645465. [Google Scholar] [CrossRef]
- Hase, K.; Kawano, K.; Nochi, T.; Pontes, G.S.; Fukuda, S.; Ebisawa, M.; Kadokura, K.; Tobe, T.; Fujimura, Y.; Kawano, S.; et al. Uptake through Glycoprotein 2 of FimH(+) Bacteria by M Cells Initiates Mucosal Immune Response. Nature 2009, 462, 226–230. [Google Scholar] [CrossRef]
- Cornes, J.S. Number, Size, and Distribution of Peyer’s Patches in the Human Small Intestine: Part I The Development of Peyer’s Patches. Gut 1965, 6, 225–229. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut Microbiota, Metabolites and Host Immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Palatini, K.M.; Durand, P.-J.; Rathinasabapathy, T.; Esposito, D.; Komarnytsky, S. Bitter Receptors and Glucose Transporters Interact to Control Carbohydrate and Immune Responses in the Gut. FASEB J. 2016, 30, 682.6. [Google Scholar] [CrossRef]
- Nestares, T.; Martín-Masot, R.; Labella, A.; Aparicio, V.A.; Flor-Alemany, M.; López-Frías, M.; Maldonado, J. Is a Gluten-Free Diet Enough to Maintain Correct Micronutrients Status in Young Patients with Celiac Disease? Nutrients 2020, 12, E844. [Google Scholar] [CrossRef]
- Naik, A.; Venu, N. Nutrition Care in Adult Inflammatory Bowel Disease. Pract. Gastroenterol. 2012, 106, 18–27. [Google Scholar]
- Couper, C.; Doriot, A.; Siddiqui, M.T.R.; Steiger, E. Nutrition Management of the High-Output Fistulae. Nutr. Clin. Pr. 2021, 36, 282–296. [Google Scholar] [CrossRef]
- Holmberg, F.E.O.; Pedersen, J.; Jørgensen, P.; Soendergaard, C.; Jensen, K.B.; Nielsen, O.H. Intestinal Barrier Integrity and Inflammatory Bowel Disease: Stem Cell-Based Approaches to Regenerate the Barrier. J. Tissue Eng. Regen Med. 2018, 12, 923–935. [Google Scholar] [CrossRef]
- Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Han, Y.; Wu, N.; Yi, P.; Tang, L.; Pan, Q.; Rao, S.; et al. The Cancer Metabolic Reprogramming and Immune Response. Mol. Cancer 2021, 20, 28. [Google Scholar] [CrossRef]
- Pearce, E.L.; Pearce, E.J. Metabolic Pathways in Immune Cell Activation and Quiescence. Immunity 2013, 38, 633–643. [Google Scholar] [CrossRef]
- Nielsen, S.T.; Janum, S.; Krogh-Madsen, R.; Solomon, T.P.; Møller, K. The Incretin Effect in Critically Ill Patients: A Case—Control Study. Crit. Care 2015, 19, 402. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.; Mendoza-Marí, Y.; Rodríguez-Rodríguez, N.; García del Barco Herrera, D.; García-Ojalvo, A.; Fernández-Mayola, M.; Guillén-Nieto, G.; Valdés-Sosa, P.A. Burn Injury Insulin Resistance and Central Nervous System Complications: A Review. Burn. Open 2020, 4, 41–52. [Google Scholar] [CrossRef]
- Sonagra, A.D.; Biradar, S.M.; Dattatreya, K.; Jayaprakash Murthy, D.S. Normal Pregnancy—A State of Insulin Resistance. J. Clin. Diagn Res. 2014, 8, CC01–CC03. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Jia, J.; Arif, A.; Ray, P.S.; Fox, P.L. The GAIT System: A Gatekeeper of Inflammatory Gene Expression. Trends Biochem. Sci. 2009, 34, 324–331. [Google Scholar] [CrossRef]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate Is an Inflammatory Signal That Induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zink, F.; Hezel, F.; Vogt, J.; Wachter, U.; Wepler, M.; Loconte, M.; Kranz, C.; Hellmann, A.; Mizaikoff, B.; et al. Metabolic Substrate Utilization in Stress-Induced Immune Cells. Intensive Care Med. Exp. 2020, 8, 28. [Google Scholar] [CrossRef]
- Mendoza, R.P.; Fudge, D.H.; Brown, J.M. Cellular Energetics of Mast Cell Development and Activation. Cells 2021, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Wollam, J.; Olefsky, J.M. An Integrated View of Immunometabolism. Cell 2018, 172, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.M.; Saltiel, A.R. Adapting to Obesity with Adipose Tissue Inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef]
- Castoldi, A.; Naffah de Souza, C.; Câmara, N.O.S.; Moraes-Vieira, P.M. The Macrophage Switch in Obesity Development. Front. Immunol. 2016, 6, 637. [Google Scholar] [CrossRef]
- O’Neill, L.A.J. A Broken Krebs Cycle in Macrophages. Immunity 2015, 42, 393–394. [Google Scholar] [CrossRef]
- Zigmond, E.; Bernshtein, B.; Friedlander, G.; Walker, C.R.; Yona, S.; Kim, K.-W.; Brenner, O.; Krauthgamer, R.; Varol, C.; Müller, W.; et al. Macrophage-Restricted Interleukin-10 Receptor Deficiency, but Not IL-10 Deficiency, Causes Severe Spontaneous Colitis. Immunity 2014, 40, 720–733. [Google Scholar] [CrossRef]
- Stefanovic-Racic, M.; Yang, X.; Turner, M.M.S.; Mantell, B.S.; Stolz, D.B.; Sumpter, T.L.; Sipula, I.J.; Dedousis, N.; Scott, D.K.; Morel, P.A.; et al. Dendritic Cells Promote Macrophage Infiltration and Comprise a Substantial Proportion of Obesity-Associated Increases in CD11c+ Cells in Adipose Tissue and Liver. Diabetes 2012, 61, 2330–2339. [Google Scholar] [CrossRef]
- Assmann, N.; O’Brien, K.L.; Donnelly, R.P.; Dyck, L.; Zaiatz-Bittencourt, V.; Loftus, R.M.; Heinrich, P.; Oefner, P.J.; Lynch, L.; Gardiner, C.M.; et al. Srebp-Controlled Glucose Metabolism Is Essential for NK Cell Functional Responses. Nat. Immunol. 2017, 18, 1197–1206. [Google Scholar] [CrossRef]
- Kane, H.; Lynch, L. Innate Immune Control of Adipose Tissue Homeostasis. Trends Immunol. 2019, 40, 857–872. [Google Scholar] [CrossRef]
- Golubovskaya, V.; Wu, L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers 2016, 8, 36. [Google Scholar] [CrossRef]
- Zhang, D.; Jin, W.; Wu, R.; Li, J.; Park, S.-A.; Tu, E.; Zanvit, P.; Xu, J.; Liu, O.; Cain, A.; et al. High Glucose Intake Exacerbates Autoimmunity through Reactive-Oxygen-Species-Mediated TGF-β Cytokine Activation. Immunity 2019, 51, 671–681.e5. [Google Scholar] [CrossRef]
- Simpson, S.J.; Raubenheimer, D. Perspective: Tricks of the Trade. Nature 2014, 508, S66. [Google Scholar] [CrossRef]
- Solon-Biet, S.M.; McMahon, A.C.; Ballard, J.W.O.; Ruohonen, K.; Wu, L.E.; Cogger, V.C.; Warren, A.; Huang, X.; Pichaud, N.; Melvin, R.G.; et al. The Ratio of Macronutrients, Not Caloric Intake, Dictates Cardiometabolic Health, Aging, and Longevity in Ad Libitum-Fed Mice. Cell Metab. 2014, 19, 418–430. [Google Scholar] [CrossRef]
- Sørensen, A.; Mayntz, D.; Raubenheimer, D.; Simpson, S.J. Protein-Leverage in Mice: The Geometry of Macronutrient Balancing and Consequences for Fat Deposition. Obes. (Silver Spring) 2008, 16, 566–571. [Google Scholar] [CrossRef]
- Simpson, S.J.; Le Couteur, D.G.; Raubenheimer, D. Putting the Balance Back in Diet. Cell 2015, 161, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Gharibzahedi, S.M.T.; Jafari, S.M. The Importance of Minerals in Human Nutrition: Bioavailability, Food Fortification, Processing Effects and Nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Lukaski, H.C. Vitamin and Mineral Status: Effects on Physical Performance. Nutrition 2004, 20, 632–644. [Google Scholar] [CrossRef]
- Fulgoni, V.L.; Keast, D.R.; Bailey, R.L.; Dwyer, J. Foods, Fortificants, and Supplements: Where Do Americans Get Their Nutrients? J. Nutr. 2011, 141, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; McBurney, M.; Fulgoni, V.L. Multivitamin/Mineral Supplement Contribution to Micronutrient Intakes in the United States, 2007–2010. J. Am. Coll. Nutr. 2014, 33, 94–102. [Google Scholar] [CrossRef]
- Drake, V. Micronutrient Inadequacies in the US Population: An Overview 2017. Available online: https://lpi.oregonstate.edu/mic/micronutrient-inadequacies/overview (accessed on 8 June 2022).
- Zheng, J.; Zeng, X.; Wang, S. Calcium Ion as Cellular Messenger. Sci. China Life Sci. 2015, 58, 1–5. [Google Scholar] [CrossRef]
- Milner, J.A.; McDonald, S.S.; Anderson, D.E.; Greenwald, P. Molecular Targets for Nutrients Involved with Cancer Prevention. Nutr. Cancer 2001, 41, 1–16. [Google Scholar] [CrossRef]
- Jia, W.; He, M.; He, Y.-W.; Mcleod, I. Autophagy, a Novel Pathway to Regulate Calcium Mobilization in T Lymphocytes. Front. Immunol. 2013, 4, 179. [Google Scholar] [CrossRef]
- Khalili, H.; Malik, S.; Ananthakrishnan, A.N.; Garber, J.J.; Higuchi, L.M.; Joshi, A.; Peloquin, J.; Richter, J.M.; Stewart, K.O.; Curhan, G.C.; et al. Identification and Characterization of a Novel Association between Dietary Potassium and Risk of Crohn’s Disease and Ulcerative Colitis. Front. Immunol. 2016, 7, 554. [Google Scholar] [CrossRef]
- Arlehamn, C.S.L.; Pétrilli, V.; Gross, O.; Tschopp, J.; Evans, T.J. The Role of Potassium in Inflammasome Activation by Bacteria. J. Biol. Chem. 2010, 285, 10508–10518. [Google Scholar] [CrossRef]
- Hill, A.F.; Polvino, W.J.; Wilson, D.B. The Significance of Glucose, Insulin and Potassium for Immunology and Oncology: A New Model of Immunity. J. Immune Based Vaccines 2005, 3, 5. [Google Scholar] [CrossRef][Green Version]
- Swaminathan, R. Magnesium Metabolism and Its Disorders. Clin. Biochem. Rev. 2003, 24, 47–66. [Google Scholar]
- Tam, M.; Gómez, S.; González-Gross, M.; Marcos, A. Possible Roles of Magnesium on the Immune System. Eur. J. Clin. Nutr. 2003, 57, 1193–1197. [Google Scholar] [CrossRef]
- von Drygalski, A.; Adamson, J.W. Iron Metabolism in Man. JPEN J. Parenter Enter. Nutr. 2013, 37, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Cassat, J.E.; Skaar, E.P. Iron in Infection and Immunity. Cell Host Microbe 2013, 13, 509–519. [Google Scholar] [CrossRef]
- Torti, S.V.; Torti, F.M. Iron: The Cancer Connection. Mol. Asp. Med. 2020, 75, 100860. [Google Scholar] [CrossRef] [PubMed]
- Heintzman, D.R.; Fisher, E.L.; Rathmell, J.C. Microenvironmental Influences on T Cell Immunity in Cancer and Inflammation. Cell Mol. Immunol. 2022, 19, 316–326. [Google Scholar] [CrossRef]
- Ni, S.; Yuan, Y.; Kuang, Y.; Li, X. Iron Metabolism and Immune Regulation. Front. Immunol. 2022, 13, 816282. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, C.; Wu, Q.; An, P.; Huang, L.; Wang, J.; Chen, C.; Chen, X.; Zhang, F.; Ma, L.; et al. Iron-Dependent Histone 3 Lysine 9 Demethylation Controls B Cell Proliferation and Humoral Immune Responses. Nat. Commun. 2019, 10, 2935. [Google Scholar] [CrossRef]
- Jobin, K.; Müller, D.N.; Jantsch, J.; Kurts, C. Sodium and Its Manifold Impact on Our Immune System. Trends Immunol. 2021, 42, 469–479. [Google Scholar] [CrossRef]
- Wang, G. Chloride Flux in Phagocytes. Immunol. Rev. 2016, 273, 219–231. [Google Scholar] [CrossRef]
- Nimni, M.E.; Han, B.; Cordoba, F. Are We Getting Enough Sulfur in Our Diet? Nutr. Metab. 2007, 4, 24. [Google Scholar] [CrossRef]
- Dilek, N.; Papapetropoulos, A.; Toliver-Kinsky, T.; Szabo, C. Hydrogen Sulfide: An Endogenous Regulator of the Immune System. Pharmacol. Res. 2020, 161, 105119. [Google Scholar] [CrossRef]
- Rahman, M.A.; Cumming, B.M.; Addicott, K.W.; Pacl, H.T.; Russell, S.L.; Nargan, K.; Naidoo, T.; Ramdial, P.K.; Adamson, J.H.; Wang, R.; et al. Hydrogen Sulfide Dysregulates the Immune Response by Suppressing Central Carbon Metabolism to Promote Tuberculosis. Proc. Natl. Acad. Sci. USA 2020, 117, 6663–6674. [Google Scholar] [CrossRef]
- El-Khodor, B.F.; James, K.; Chang, Q.; Zhang, W.; Loiselle, Y.R.; Panda, C.; Hanania, T. Elevation of Brain Magnesium with Swiss Chard and Buckwheat Extracts in an Animal Model of Reduced Magnesium Dietary Intake. Nutr. Neurosci. 2021, 25, 2638–2649. [Google Scholar] [CrossRef]
- Visser, M.; Van Zyl, T.; Hanekom, S.M.; Baumgartner, J.; Van der Hoeven, M.; Taljaard-Krugell, C.; Smuts, C.M.; Faber, M. Nutrient Density, but Not Cost of Diet, Is Associated with Anemia and Iron Deficiency in School-Age Children in South Africa. Nutrition 2021, 84, 111096. [Google Scholar] [CrossRef]
- Hopkins, H.T.; Murphy, E.W.; Smith, D.P. Minerals and Proximate Composition of Organ Meats. J. Am. Diet. Assoc. 1961, 38, 344–349. [Google Scholar] [CrossRef]
- Sanna, A.; Firinu, D.; Zavattari, P.; Valera, P. Zinc Status and Autoimmunity: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 68. [Google Scholar] [CrossRef]
- Fallah, A.; Mohammad-Hasani, A.; Colagar, A.H. Zinc Is an Essential Element for Male Fertility: A Review of Zn Roles in Men’s Health, Germination, Sperm Quality, and Fertilization. J. Reprod. Infertil. 2018, 19, 69–81. [Google Scholar]
- Haase, H.; Rink, L. Multiple Impacts of Zinc on Immune Function. Metallomics 2014, 6, 1175–1180. [Google Scholar] [CrossRef]
- Bonaventura, P.; Lamboux, A.; Albarède, F.; Miossec, P. A Feedback Loop between Inflammation and Zn Uptake. PLoS ONE 2016, 11, e0147146. [Google Scholar] [CrossRef]
- Collins, J.F.; Klevay, L.M. Copper. Adv. Nutr. 2011, 2, 520–522. [Google Scholar] [CrossRef]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.-F.; Margaritis, I. Dietary Copper and Human Health: Current Evidence and Unresolved Issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace Elements in Human Physiology and Pathology. Copper. Biomed. Pharm. 2003, 57, 386–398. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S. Current Knowledge on the Importance of Selenium in Food for Living Organisms: A Review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef]
- Aschner, M.; Erikson, K. Manganese. Adv. Nutr. 2017, 8, 520–521. [Google Scholar] [CrossRef]
- Skaar, E.P.; Raffatellu, M. Metals in Infectious Diseases and Nutritional Immunity. Metallomics 2015, 7, 926–928. [Google Scholar] [CrossRef]
- Weiss, G.; Carver, P.L. Role of Divalent Metals in Infectious Disease Susceptibility and Outcome. Clin. Microbiol. Infect. 2018, 24, 16–23. [Google Scholar] [CrossRef]
- Cunrath, O.; Bumann, D. Host Resistance Factor SLC11A1 Restricts Salmonella Growth through Magnesium Deprivation. Science 2019, 366, 995–999. [Google Scholar] [CrossRef]
- Tako, E. Dietary Trace Minerals. Nutrients 2019, 11, 2823. [Google Scholar] [CrossRef]
- Harlan, J.R.; de Wet, J.M.J.; Price, E.G. Comparative Evolution of Cereals. Evolution 1973, 27, 311–325. [Google Scholar] [CrossRef]
- Cordain, L.; Watkins, B.A.; Florant, G.L.; Kelher, M.; Rogers, L.; Li, Y. Fatty Acid Analysis of Wild Ruminant Tissues: Evolutionary Implications for Reducing Diet-Related Chronic Disease. Eur. J. Clin. Nutr. 2002, 56, 181–191. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and Evolution of the Western Diet: Health Implications for the 21st Century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- van Heerden, S.M.; Morey, L. Nutrient Content of South African C2 Beef Offal. Food Meas. 2014, 8, 249–258. [Google Scholar] [CrossRef]
- Bester, M.; Schönfeldt, H.C.; Pretorius, B.; Hall, N. The Nutrient Content of Selected South African Lamb and Mutton Organ Meats (Offal). Food Chem. 2018, 238, 3–8. [Google Scholar] [CrossRef]
- Lewis, J.; Buss, D.H. Trace Nutrients. 5. Minerals and Vitamins in the British Household Food Supply. Br. J. Nutr. 1988, 60, 413–424. [Google Scholar] [CrossRef]
- Ferguson, E.L.; Watson, L.; Berger, J.; Chea, M.; Chittchang, U.; Fahmida, U.; Khov, K.; Kounnavong, S.; Le, B.M.; Rojroongwasinkul, N.; et al. Realistic Food-Based Approaches Alone May Not Ensure Dietary Adequacy for Women and Young Children in South-East Asia. Matern Child. Health J. 2019, 23, 55–66. [Google Scholar] [CrossRef]
- Sazawal, S.; Black, R.E.; Ramsan, M.; Chwaya, H.M.; Stoltzfus, R.J.; Dutta, A.; Dhingra, U.; Kabole, I.; Deb, S.; Othman, M.K.; et al. Effects of Routine Prophylactic Supplementation with Iron and Folic Acid on Admission to Hospital and Mortality in Preschool Children in a High Malaria Transmission Setting: Community-Based, Randomised, Placebo-Controlled Trial. Lancet 2006, 367, 133–143. [Google Scholar] [CrossRef]
- Soofi, S.; Cousens, S.; Iqbal, S.P.; Akhund, T.; Khan, J.; Ahmed, I.; Zaidi, A.K.M.; Bhutta, Z.A. Effect of Provision of Daily Zinc and Iron with Several Micronutrients on Growth and Morbidity among Young Children in Pakistan: A Cluster-Randomised Trial. Lancet 2013, 382, 29–40. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; et al. Vitamin, Mineral, and Multivitamin Supplementation to Prevent Cardiovascular Disease and Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2022, 327, 2326–2333. [Google Scholar] [CrossRef]
- O’Connor, E.A.; Evans, C.V.; Ivlev, I.; Rushkin, M.C.; Thomas, R.G.; Martin, A.; Lin, J.S. Vitamin and Mineral Supplements for the Primary Prevention of Cardiovascular Disease and Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2022, 327, 2334–2347. [Google Scholar] [CrossRef]
- Gibson, R.S.; Perlas, L.; Hotz, C. Improving the Bioavailability of Nutrients in Plant Foods at the Household Level. Proc. Nutr. Soc. 2006, 65, 160–168. [Google Scholar] [CrossRef]
- Bielik, V.; Kolisek, M. Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome. Int. J. Mol. Sci. 2021, 22, 6803. [Google Scholar] [CrossRef] [PubMed]
- LaChance, L.R.; Ramsey, D. Antidepressant Foods: An Evidence-Based Nutrient Profiling System for Depression. World J. Psychiatry 2018, 8, 97–104. [Google Scholar] [CrossRef]
- DeSalvo, K.B.; Olson, R.; Casavale, K.O. Dietary Guidelines for Americans. JAMA 2016, 315, 457–458. [Google Scholar] [CrossRef]
- Patry, M.; Teinturier, R.; Goehrig, D.; Zetu, C.; Ripoche, D.; Kim, I.-S.; Bertolino, P.; Hennino, A. Βig-H3 Represses T-Cell Activation in Type 1 Diabetes. Diabetes 2015, 64, 4212–4219. [Google Scholar] [CrossRef]
- Talbot, N.A.; Wheeler-Jones, C.P.; Cleasby, M.E. Palmitoleic Acid Prevents Palmitic Acid-Induced Macrophage Activation and Consequent P38 MAPK-Mediated Skeletal Muscle Insulin Resistance. Mol. Cell. Endocrinol. 2014, 393, 129–142. [Google Scholar] [CrossRef]
- Jha, A.K.; Huang, S.C.-C.; Sergushichev, A.; Lampropoulou, V.; Ivanova, Y.; Loginicheva, E.; Chmielewski, K.; Stewart, K.M.; Ashall, J.; Everts, B.; et al. Network Integration of Parallel Metabolic and Transcriptional Data Reveals Metabolic Modules That Regulate Macrophage Polarization. Immunity 2015, 42, 419–430. [Google Scholar] [CrossRef]
- Tourkochristou, E.; Triantos, C.; Mouzaki, A. The Influence of Nutritional Factors on Immunological Outcomes. Front. Immunol. 2021, 12, 665968. [Google Scholar] [CrossRef]
| Minerals, mg | Mean Daily Intake from Naturally Occurring Foods, US Adults Ages ≥ 19 1 | Mean Daily Intake from All Foods Including Enriched and Fortified, US Population Ages ≥ 4 2 | RDA, Reference Daily Allowance for Ages ≥ 4 2 | EAR, % Less than Estimated Average Requirement 2 |
|---|---|---|---|---|
| Calcium, Ca | 856 mg | 987 mg | 1300 mg | −24.1% |
| Phosphorus, P | 1308 mg | 1350 mg | 1250 mg | fair |
| Sodium, Na | 3361 mg | 3433 mg | 1500 mg 3 | excess |
| Potassium, K | 2695 mg | 2595 mg | 4700 mg | −44.8% |
| Magnesium, Mg | 278 mg | 286 mg | 420 mg | −31.9% |
| Iron, Fe | 10.3 mg | 15.1 mg | 8–18 mg | −16.1% |
| Zinc, Zn | 11.2 mg | 11.7 mg | 8–11 mg | fair |
| Copper, Cu | 1.3 mg | 1.3 mg | 0.9 mg | fair |
| Selenium, Se | 0.1 mg | 0.1 mg | 0.055 mg | fair |
| Manganese, Mn | n/a | 2.1–4.1 mg | 1.8–2.3 mg 3 | fair |
| Animal Foods | Refined | Plant Foods [127] | Lamb Organ Meats [126] | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minerals, mg | Milk 1 | Meat | Fish | Egg | Sug 2 | Fats | Veg 3 | Fruit | Bread | Flo 4 | Cer 5 | Int 6 | Lung | Heart | Liver | Sto 7 | Kid 8 | Spleen |
| Calcium, Ca | - 9 | - | - | - | - | - | - | - | - | - | - | 18.6 | 8.9 | 5.1 | 5.0 | 52.7 | 9.4 | 7.6 |
| Phosphorus, P | 406 | 215 | 41 | 91 | 5 | 1 | 140 | 24 | 149 | 59 | 80 | 124 | 271 | 195 | 423 | 170 | 330 | 406 |
| Sodium, Na | - | - | - | - | - | - | - | - | - | - | - | 38 | 160 | 101 | 71 | 80 | 234 | 112 |
| Potassium, K | 529 | 305 | 61 | 62 | 3 | 9 | 893 | 194 | 175 | 81 | 98 | 75 | 298 | 261 | 315 | 155 | 310 | 409 |
| Magnesium, Mg | 44 | 21 | 5 | 5 | 1 | 1 | 46 | 14 | 46 | 12 | 22 | 21.9 | 22.2 | 29.0 | 28.3 | 25.3 | 30.6 | 30.8 |
| Iron, Fe | - | - | - | - | - | - | - | - | - | - | - | 1.4 | 8.4 | 3.8 | 6.1 | 4.9 | 4.4 | 22.8 |
| Zinc, Zn | 1.73 | 3.01 | 0.14 | 0.60 | 0.05 | 0.06 | 0.86 | 0.12 | 1.17 | 0.36 | 0.58 | 2.6 | 2.6 | 2.5 | 4.2 | 3.9 | 3.7 | 3.6 |
| Copper, Cu | 0.022 | 0.328 | 0.028 | 0.031 | 0.012 | 0.017 | 0.214 | 0.056 | 0.221 | 0.089 | 0.094 | - | - | - | - | - | - | - |
| Selenium, Se | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Manganese, Mn | 0.03 | 0.10 | 0.01 | 0.01 | 0.01 | 0.01 | 0.54 | 0.16 | 1.06 | 0.26 | 0.36 | 0.01 | 0 | 0.04 | 0.27 | 0.19 | 0.05 | 0 |
| Minerals | Absorption 1 Bioavailability | Ion Channels and Carrier Proteins | Main Transport Proteins | Normal Value Range (Serum) 2 |
|---|---|---|---|---|
| Calcium, Ca | Duodenum 21–45% | TRPV6/5 influx NCX1/PMCA1b efflux | Albumin Calbindin | 9.0–10.5 mg/dL 2.2–2.6 mmol/L |
| Phosphorus, P | Jejunum 40–70% | SLC34A1/2/3 influx Npt2 NaPi cotransporters | Free Albumin | 2.8–4.5 mg/dL 0.97–1.45 mmol/L |
| Sodium, Na | Ileum/Colon 90% | NHE3 Na+/H+ exchange Na, K-ATPase efflux | Free | – 136–145 mmol/L |
| Potassium, K | Ileum/Colon 90% | Na, K-ATPase influx NKCC co-transporters | Free | – 3.5–5.1 mmol/L |
| Magnesium, Mg | Duodenum 40–60% | TRPM6/7 influx CNNM4/SLC41A1 efflux | Free Albumin | 1.5–2.4 mg/dL 0.62–0.99 mmol/L |
| Iron, Fe | Duodenum 14–18% | HCP1/DMT1 influx FPN1 efflux | Ferritin Transferrin | 50–150 μg/dL 9.2–27.5 μmol/L |
| Zinc, Zn | Jejunum 26–34% | SLC39A4/ZIP influx ZnT1/SLC30A1 efflux | Albumin | 66–110 μg/dL 10.1–16.8 μmol/L |
| Copper, Cu | Jejunum 30–40% | SLC31A1/2 influx ATP7A efflux | Ceruloplasmin | 7.0–15.5 μg/dL 11–24.2 μmol/L |
| Selenium, Se | Jejunum 69–86% | B(0)AT1/rBAT influx SLC26 family influx | Selenoproteins Selenosugars | 11–16.5 μg/dL 0.6–1.5 μmol/L |
| Manganese, Mn | Jejunum 3–4% | DMT1/TRPM7 influx SLC30A10 efflux | Ceruloplasmin Albumin | 30–90 ng/dL 5.5–16.4 nmol/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, M.; Gutierrez, J.; Komarnytsky, S. Essential Minerals and Metabolic Adaptation of Immune Cells. Nutrients 2023, 15, 123. https://doi.org/10.3390/nu15010123
Alghamdi M, Gutierrez J, Komarnytsky S. Essential Minerals and Metabolic Adaptation of Immune Cells. Nutrients. 2023; 15(1):123. https://doi.org/10.3390/nu15010123
Chicago/Turabian StyleAlghamdi, Malak, Janelle Gutierrez, and Slavko Komarnytsky. 2023. "Essential Minerals and Metabolic Adaptation of Immune Cells" Nutrients 15, no. 1: 123. https://doi.org/10.3390/nu15010123
APA StyleAlghamdi, M., Gutierrez, J., & Komarnytsky, S. (2023). Essential Minerals and Metabolic Adaptation of Immune Cells. Nutrients, 15(1), 123. https://doi.org/10.3390/nu15010123








