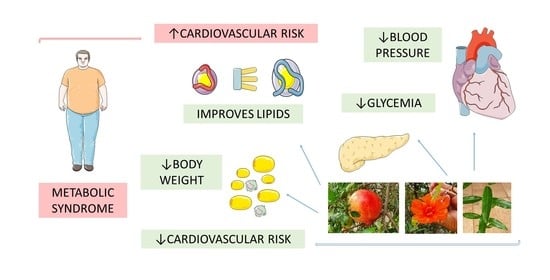
Pomegranate (Punica granatum L.) and Metabolic Syndrome Risk Factors and Outcomes: A Systematic Review of Clinical Studies
Abstract
:1. Introduction
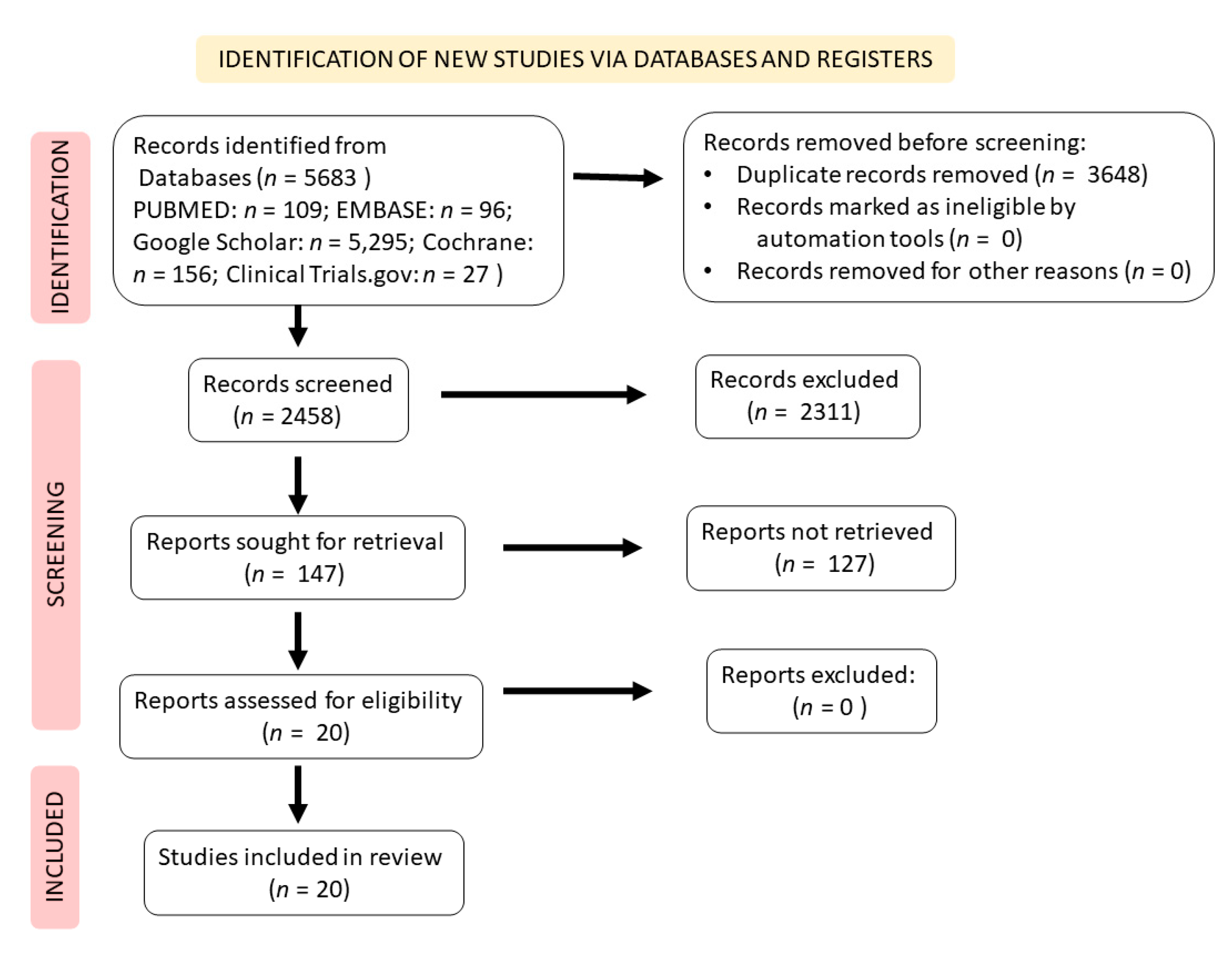
2. Literature Search Strategy and Study Selection
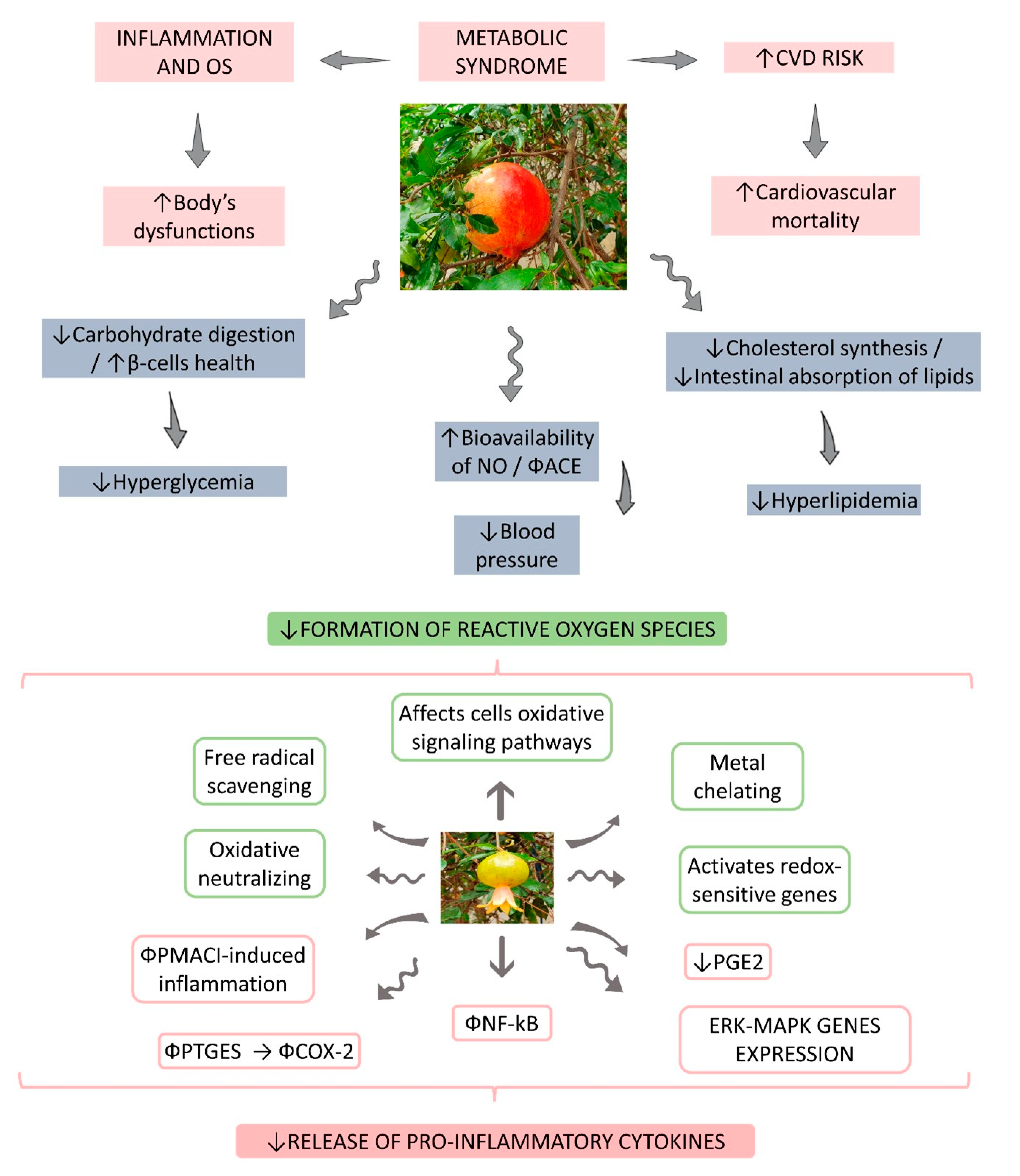
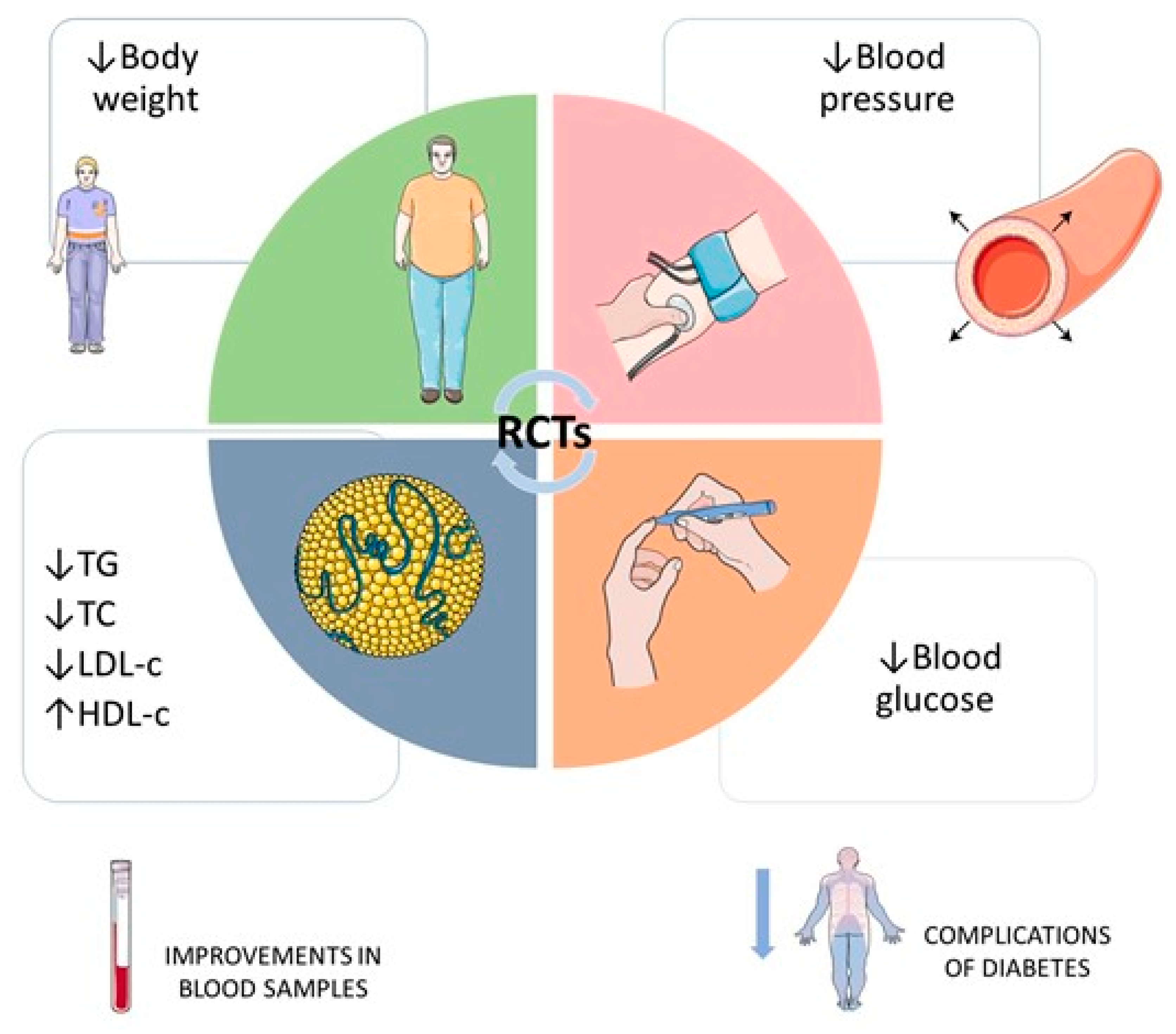
3. Result Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Šantić, Ž.; Pravdić, N.; Bevanda, M.; Galić, K. The historical use of medicinal plants in traditional and scientific medicine. Psychiatr. Danub. 2017, 29 (Suppl. 4), 787–792. [Google Scholar] [PubMed]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Les, F.; Prieto, J.M.; Arbonés-Mainar, J.M.; Valero, M.S.; López, V. Bioactive properties of commercialised pomegranate (Punica granatum) juice: Antioxidant, antiproliferative and enzyme inhibiting activities. Food Funct. 2015, 6, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Erkan, M.; Doğan, A. Pomegranate/Roma—Punica Granatum. In Exotic Fruits; Academic Press: London, UK, 2018; pp. 355–361. [Google Scholar] [CrossRef]
- Shaygannia, E.; Bahmani, M.; Zamanzad, B.; Rafieian-kopaei, M. A Review Study on Punica granatum L. J. Evid.-Based Complementary Altern. Med. 2015, 21, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.L.; Strandberg, K.R.; Croley, C.R.; Fraser, S.E.; Nagulapalli Venkata, K.C.; Fimognari, C.; Sethi, G.; Bishayee, A. Pomegranate bioactive constituents target multiple oncogenic and oncosuppressive signaling for cancer prevention and intervention. Semin. Cancer Biol. 2021, 73, 265–293. [Google Scholar] [CrossRef]
- Asgary, S.; Karimi, R.; Joshi, T.; Kilpatrick, K.L.; Moradi, S.; Samimi, Z.; Mohammadi, E.; Farzaei, M.H.; Bishayee, A. Effect of pomegranate juice on vascular adhesion factors: A systematic review and meta-analysis. Phytomedicine. 2021, 80, 153359. [Google Scholar] [CrossRef]
- Baliga, M.S.; Shivashankara, A.R.; Shetty, C.B.; Thilakchand, K.R.; Periera, N.; Palatty, P.L. Chapter 31—Antidiabetic Effects of Punica granatum L (Pomegranate): A Review. In Bioactive Food as Dietary Interventions for Diabetes; Watson, R.R., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 355–356. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Aiello, F.; Tenuta, M.C.; Leporini, M.; Falco, T.; Tundis, R. Chapter 3.46—Pomegranate (Punica granatum L.). In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Academic Press: London, UK, 2019; pp. 467–472. [Google Scholar]
- Barati Boldaji, R.; Akhlaghi, M.; Sagheb, M.M.; Esmaeilinezhad, Z. Pomegranate juice improves cardiometabolic risk factors, biomarkers of oxidative stress and inflammation in hemodialysis patients: A randomized crossover trial. J. Sci. Food Agric. 2020, 100, 846–854. [Google Scholar] [CrossRef]
- Khajebishak, Y.; Payahoo, L.; Alivand, M.; Hamishehkar, H.; Mobasseri, M.; Ebrahimzadeh, V.; Alipour, M.; Alipour, B. Effect of pomegranate seed oil supplementation on the GLUT-4 gene expression and glycemic control in obese people with type 2 diabetes: A randomized controlled clinical trial. J. Cell. Physiol. 2019, 234, 19621–19628. [Google Scholar] [CrossRef]
- Sohrab, G.; Roshan, H.; Ebrahimof, S.; Nikpayam, O.; Sotoudeh, G.; Siasi, F. Effects of pomegranate juice consumption on blood pressure and lipid profile in patients with type 2 diabetes: A single-blind randomized clinical trial. Clin. Nutr. ESPEN 2019, 29, 30–35. [Google Scholar] [CrossRef]
- Miguel, M.; Neves, M.; Antunes, M. Pomegranate (Punica granatum L.): A medicinal plant with myriad biologic properties: A short review. J. Med. Plants Res. Dec. Spec. Rev. 2010, 425, 2836–2847. [Google Scholar]
- Alexander, C.M.; Landsman, P.B.; Teutsch, S.M.; Haffner, S.M. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes 2003, 52, 1210–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbalho, S.M.; Bechara, M.D.; Quesada, K.; Gabaldi, M.R.; Goulart, R.D.A.; Tofano, R.J.; Gasparini, R.G. Metabolic syndrome, atherosclerosis and inflammation: An inseparable triad? J. Vasc. Bras 2015, 14, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Tofano, R.J.; Pescinni-Salzedas, L.M.; Chagas, E.F.B.; Detregiachi, C.R.P.; Guiguer, E.L.; Araujo, A.C.; Bechara, M.D.; Rubira, C.J.; Barbalho, S.M. Association of Metabolic Syndrome and Hyperferritinemia in Patients at Cardiovascular Risk. Diabetes. Metab. Syndr. Obes. 2020, 13, 3239–3248. [Google Scholar] [CrossRef] [PubMed]
- Bovolini, A.; Garcia, J.; Andrade, M.A.; Duarte, J.A. Metabolic Syndrome Pathophysiology and Predisposing Factors. Int. J. Sports Med. 2021, 42, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Kahn, C.R.; Wang, G.; Lee, K.Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Investig. 2019, 129, 3990–4000. [Google Scholar] [CrossRef] [PubMed]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- Poon, V.T.; Kuk, J.L.; Ardern, C.I. Trajectories of metabolic syndrome development in young adults. PLoS ONE 2014, 9, e111647. [Google Scholar] [CrossRef]
- Saely, C.H.; Rein, P.; Drexel, H. The metabolic syndrome and risk of cardiovascular disease and diabetes: Experiences with the new diagnostic criteria from the International Diabetes Federation. Horm. Metab. Res. 2007, 39, 642–650. [Google Scholar] [CrossRef]
- Vishram, J.K. Prognostic interactions between cardiovascular risk factors. Dan. Med. J 2014, 61, B4892. [Google Scholar] [PubMed]
- Eghbali, S.; Askari, S.F.; Avan, R.; Sahebkar, A. Therapeutic Effects of Punica granatum (Pomegranate): An Updated Review of Clinical Trials. J. Nutr. Metab. 2021, 2021, 5297162. [Google Scholar] [CrossRef] [PubMed]
- Hosakatte, N.; Bapat, V. Bioactive Compounds in Underutilized Fruits and Nuts; Springer International Publishing: New York, NY, USA, 2020. [Google Scholar] [CrossRef]
- Konstantinidi, M.; Koutelidakis, A.E. Functional Foods and Bioactive Compounds: A Review of Its Possible Role on Weight Management and Obesity’s Metabolic Consequences. Medicines 2019, 6, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.-J.; Chen, L.-G.; Liang, W.-L.; Wang, C.-C. Anti-inflammatory effects of Punica granatum Linne in vitro and in vivo. Food Chem. 2010, 118, 315–322. [Google Scholar] [CrossRef]
- Prashanth, D.; Asha, M.K.; Amit, A. Antibacterial activity of Punica granatum. Fitoterapia 2001, 72, 171–173. [Google Scholar] [CrossRef]
- Hussein, L.; Gouda, M.; Buttar, H.S. Pomegranate, its Components, and Modern Deliverable Formulations as Potential Botanicals in the Prevention and Treatment of Various Cancers. Curr. Drug Deliv. 2021, 18, 1391–1405. [Google Scholar] [CrossRef]
- Morittu, V.M.; Mastellone, V.; Tundis, R.; Loizzo, M.R.; Tudisco, R.; Figoli, A.; Cassano, A.; Musco, N.; Britti, D.; Infascelli, F.; et al. Antioxidant, Biochemical, and In-Life Effects of Punica granatum L. Natural Juice vs. Clarified Juice by Polyvinylidene Fluoride Membrane. Foods 2020, 9, 242. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.; Zhang, W.; Li, J.; Du, L.; Lv, O.; Zhao, S.; Li, J. Beneficial Effects of Pomegranate on Lipid Metabolism in Metabolic Disorders. Mol. Nutr. Food Res. 2019, 63, e1800773. [Google Scholar] [CrossRef]
- Jafri, M.A.; Aslam, M.; Javed, K.; Singh, S. Effect of Punica granatum Linn. (flowers) on blood glucose level in normal and alloxan-induced diabetic rats. J. Ethnopharmacol. 2000, 70, 309–314. [Google Scholar] [CrossRef]
- Al-Muammar, M.N.; Khan, F. Obesity: The preventive role of the pomegranate (Punica granatum). Nutrition 2012, 28, 595–604. [Google Scholar] [CrossRef]
- Gharib, E.; Montasser Kouhsari, S. Study of the Antidiabetic Activity of Punica granatum L. Fruits Aqueous Extract on the Alloxan-Diabetic Wistar Rats. Iran. J. Pharm. Res. IJPR 2019, 18, 358–368. [Google Scholar] [PubMed]
- Fourati, M.; Smaoui, S.; Hlima, H.B.; Elhadef, K.; Braïek, O.B.; Ennouri, K.; Mtibaa, A.C.; Mellouli, L. Bioactive Compounds and Pharmacological Potential of Pomegranate (Punica granatum) Seeds–A Review. Plant Foods Hum. Nutr. 2020, 75, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Melgarejo-Sánchez, P.; Núñez-Gómez, D.; Martínez-Nicolás, J.J.; Hernández, F.; Legua, P.; Melgarejo, P. Pomegranate variety and pomegranate plant part, relevance from bioactive point of view: A review. Bioresour. Bioprocess. 2021, 8, 2. [Google Scholar] [CrossRef]
- Sreekumar, S.; Sithul, H.; Muraleedharan, P.; Azeez, J.M.; Sreeharshan, S. Pomegranate Fruit as a Rich Source of Biologically Active Compounds. BioMed Res. Int. 2014, 2014, 686921. [Google Scholar] [CrossRef]
- Vučić, V.; Grabež, M.; Trchounian, A.; Arsić, A. Composition and Potential Health Benefits of Pomegranate: A Review. Curr. Pharm. Des. 2019, 25, 1817–1827. [Google Scholar] [CrossRef]
- Walia, A.; Gupta, A.; Sharma, V. Role of Bioactive Compounds in Human Health. Acta Sci. Med. Sci. 2019, 3, 25–33. [Google Scholar]
- Abdelkarim, G.; Benaicha, S.; Elmajdoub, N.; Bellaoui, M.; Hamal, A. What is a bioactive compound? A combined definition for a preliminary consensus. Int. J. Nutr. Food Sci. 2014, 3, 174–179. [Google Scholar]
- Biesalski, H.K.; Dragsted, L.O.; Elmadfa, I.; Grossklaus, R.; Müller, M.; Schrenk, D.; Walter, P.; Weber, P. Bioactive compounds: Definition and assessment of activity. Nutrition 2009, 25, 1202–1205. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Wall-Medrano, A.; González-Aguilar, G.A.; López-Díaz, J.A.; Álvarez-Parrilla, E.; de la Rosa, L.A.; Ramos-Jimenez, A. [Hydrolyzable tannins; biochemistry, nutritional & analytical aspects and health effects]. Nutr. Hosp. 2014, 31, 55–66. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharm. 2021, 133, 110985. [Google Scholar] [CrossRef] [PubMed]
- BenSaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Complement. Altern. Med. 2017, 17, 47. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Singh, A.; Mishra, A. Gallic acid: Molecular rival of cancer. Environ. Toxicol. Pharmacol. 2013, 35, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.F.; Xie, W.D.; Zhang, Z.; Xing, D.M.; Ding, Y.; Wang, W.; Ma, C.; Du, L.J. Bioactive compounds from the seeds of Punica granatum (pomegranate). J. Nat. Prod. 2004, 67, 2096–2098. [Google Scholar] [CrossRef]
- Zarfeshany, A.; Asgary, S.; Javanmard, S.H. Potent health effects of pomegranate. Adv. Biomed. Res. 2014, 3, 100. [Google Scholar] [CrossRef]
- Ibrahim, A.; Awad, S.; El-Sayed, M. Impact of pomegranate peel as prebiotic in bio-yoghurt. Br. Food J. 2020, 122, 2911–2926. [Google Scholar] [CrossRef]
- Gülçin, İ.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, H.S. Inhibitory Effect of Tannic Acid Extracted from Grape Seeds and Pomegranate Peels on Some Microorganisms. Mesop. J. Agric. 2008, 36, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Al-Harbi, S.A.; Abdulrahman, A.O.; Zamzami, M.A.; Khan, M.I. Urolithins: The Gut Based Polyphenol Metabolites of Ellagitannins in Cancer Prevention, a Review. Front. Nutr. 2021, 8, 647582. [Google Scholar] [CrossRef]
- Toney, A.M.; Fox, D.; Chaidez, V.; Ramer-Tait, A.E.; Chung, S. Immunomodulatory Role of Urolithin A on Metabolic Diseases. Biomedicines 2021, 9, 192. [Google Scholar] [CrossRef]
- Djedjibegovic, J.; Marjanovic, A.; Panieri, E.; Saso, L. Ellagic Acid-Derived Urolithins as Modulators of Oxidative Stress. Oxidative Med. Cell. Longev. 2020, 2020, 5194508. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman, A.O.; Kuerban, A.; Alshehri, Z.A.; Abdulaal, W.H.; Khan, J.A.; Khan, M.I. Urolithins Attenuate Multiple Symptoms of Obesity in Rats Fed on a High-Fat Diet. Diabetes. Metab. Syndr. Obes. 2020, 13, 3337–3348. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, D.; Andreux, P.A.; Valdés, P.; Singh, A.; Rinsch, C.; Auwerx, J. Impact of the Natural Compound Urolithin A on Health, Disease, and Aging. Trends. Mol. Med. 2021, 27, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Bassiri-Jahromi, S. Punica granatum (Pomegranate) activity in health promotion and cancer prevention. Oncol. Rev. 2018, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Suman, M.; Bhatnagar, P. A review on proactive pomegranate one of the healthiest foods. Int J Chem Stud. 2019, 7, 89–194. [Google Scholar]
- Medjakovic, S.; Jungbauer, A. Pomegranate: A fruit that ameliorates metabolic syndrome. Food Funct. 2013, 4, 19–39. [Google Scholar] [CrossRef]
- Jandari, S.; Hatami, E.; Ziaei, R.; Ghavami, A.; Yamchi, A.M. The effect of pomegranate (Punica granatum) supplementation on metabolic status in patients with type 2 diabetes: A systematic review and meta-analysis. Complementary Ther. Med. 2020, 52, 102478. [Google Scholar] [CrossRef]
- Akaberi, M.; Boghrati, Z.; Sahebkar, A.; Emami, S.A. Therapeutic Potential of Pomegranate in Metabolic Disorders. Adv. Exp. Med. Biol. 2021, 1328, 421–440. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clin. Res. Ed.) 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 205–228. [Google Scholar]
- Asgary, S.; Sahebkar, A.; Afshani, M.R.; Keshvari, M.; Haghjooyjavanmard, S.; Rafieian-Kopaei, M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytother. Res. 2014, 28, 193–199. [Google Scholar] [CrossRef]
- Abedini, M.; Ghasemi-Tehrani, H.; Tarrahi, M.J.; Amani, R. The effect of concentrated pomegranate juice consumption on risk factors of cardiovascular diseases in women with polycystic ovary syndrome: A randomized controlled trial. Phytother. Res. 2020, 35, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilinezhad, Z.; Barati-Boldaji, R.; Brett, N.R.; de Zepetnek, J.O.T.; Bellissimo, N.; Babajafari, S.; Sohrabi, Z. The effect of synbiotics pomegranate juice on cardiovascular risk factors in PCOS patients: A randomized, triple-blinded, controlled trial. J. Endocrinol. Investig. 2020, 43, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, B.; Saedisomeolia, A.; Wood, L.G.; Yaseri, M.; Tavasoli, S. Effects of pomegranate extract supplementation on inflammation in overweight and obese individuals: A randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2016, 22, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Sohrab, G.; Nasrollahzadeh, J.; Zand, H.; Amiri, Z.; Tohidi, M.; Kimiagar, M. Effects of pomegranate juice consumption on inflammatory markers in patients with type 2 diabetes: A randomized, placebo-controlled trial. J. Res. Med. Sci. 2014, 19, 215–220. [Google Scholar] [PubMed]
- Wu, P.T.; Fitschen, P.J.; Kistler, B.M.; Jeong, J.H.; Chung, H.R.; Aviram, M.; Phillips, S.A.; Fernhall, B.; Wilund, K.R. Effects of Pomegranate Extract Supplementation on Cardiovascular Risk Factors and Physical Function in Hemodialysis Patients. J. Med. Food 2015, 18, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Sumner, M.D.; Elliott-Eller, M.; Weidner, G.; Daubenmier, J.J.; Chew, M.H.; Marlin, R.; Raisin, C.J.; Ornish, D. Effects of pomegranate juice consumption on myocardial perfusion in patients with coronary heart disease. Am. J. Cardiol. 2005, 96, 810–814. [Google Scholar] [CrossRef]
- Aviram, M.; Rosenblat, M.; Gaitini, D.; Nitecki, S.; Hoffman, A.; Dornfeld, L.; Volkova, N.; Presser, D.; Attias, J.; Liker, H.; et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin. Nutr. 2004, 23, 423–433. [Google Scholar] [CrossRef]
- Rivara, M.B.; Mehrotra, R.; Linke, L.; Ruzinski, J.; Ikizler, T.A.; Himmelfarb, J. A pilot randomized crossover trial assessing the safety and short-term effects of pomegranate supplementation in hemodialysis patients. J. Ren. Nutr. 2015, 25, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Stockton, A.; Farhat, G.; McDougall, G.J.; Al-Dujaili, E.A.S. Effect of pomegranate extract on blood pressure and anthropometry in adults: A double-blind placebo-controlled randomised clinical trial. J. Nutr. Sci. 2017, 6, e39. [Google Scholar] [CrossRef] [Green Version]
- Lynn, A.; Hamadeh, H.; Leung, W.C.; Russell, J.M.; Barker, M.E. Effects of pomegranate juice supplementation on pulse wave velocity and blood pressure in healthy young and middle-aged men and women. Plant Foods Hum. Nutr. 2012, 67, 309–314. [Google Scholar] [CrossRef]
- Tsang, C.; Smail, N.F.; Almoosawi, S.; Davidson, I.; Al-Dujaili, E.A. Intake of polyphenol-rich pomegranate pure juice influences urinary glucocorticoids, blood pressure and homeostasis model assessment of insulin resistance in human volunteers. J. Nutr. Sci. 2012, 1, e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Ortiz, M.; Martínez-Abundis, E.; Espinel-Bermúdez, M.C.; Pérez-Rubio, K.G. Effect of pomegranate juice on insulin secretion and sensitivity in patients with obesity. Ann. Nutr. Metab. 2011, 58, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Fuster-Muñoz, E.; Roche, E.; Funes, L.; Martínez-Peinado, P.; Sempere, J.M.; Vicente-Salar, N. Effects of pomegranate juice in circulating parameters, cytokines, and oxidative stress markers in endurance-based athletes: A randomized controlled trial. Nutrition 2016, 32, 539–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manthou, E.; Georgakouli, K.; Deli, C.K.; Sotiropoulos, A.; Fatouros, I.G.; Kouretas, D.; Haroutounian, S.; Matthaiou, C.; Koutedakis, Y.; Jamurtas, A.Z. Effect of pomegranate juice consumption on biochemical parameters and complete blood count. Exp. Ther. Med. 2017, 14, 1756–1762. [Google Scholar] [CrossRef] [Green Version]
- Kojadinovic, M.I.; Arsic, A.C.; Debeljak-Martacic, J.D.; Konic-Ristic, A.I.; Kardum, N.D.; Popovic, T.B.; Glibetic, M.D. Consumption of pomegranate juice decreases blood lipid peroxidation and levels of arachidonic acid in women with metabolic syndrome. J. Sci. Food Agric. 2017, 97, 1798–1804. [Google Scholar] [CrossRef]
- Shema-Didi, L.; Kristal, B.; Sela, S.; Geron, R.; Ore, L. Does Pomegranate intake attenuate cardiovascular risk factors in hemodialysis patients? Nutr. J. 2014, 13, 18. [Google Scholar] [CrossRef] [Green Version]
- Park, J.E.; Kim, J.Y.; Kim, J.; Kim, Y.J.; Kim, M.J.; Kwon, S.W.; Kwon, O. Pomegranate vinegar beverage reduces visceral fat accumulation in association with AMPK activation in overweight women: A double-blind, randomized, and placebo-controlled trial. J. Funct. Foods 2014, 8, 274–281. [Google Scholar] [CrossRef]
- Kandylis, P.; Kokkinomagoulos, E. Food Applications and Potential Health Benefits of Pomegranate and its Derivatives. Foods 2020, 9, 122. [Google Scholar] [CrossRef] [Green Version]
| Bioactive Compounds | Plant Parts | Molecular Structures | Effects | References |
|---|---|---|---|---|
| Gallic acid | Peel, juice, flower, seeds, and fruit | 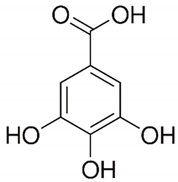 | Antidiabetic, anti-inflammatory, and antioxidant | [36,37,38,39,40,41,42,43,44] |
| Ellagic acid | Peel, juice, fruit, flower, and seeds | 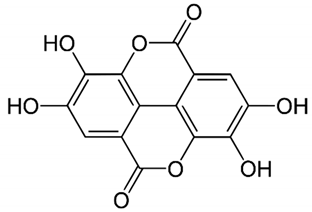 | Antidiabetic, anti-obesity, anti-inflammatory, and antioxidant | [32,34,36,37,38,39,40,45,46,47,48] |
| Quercetin | Peel and seeds | 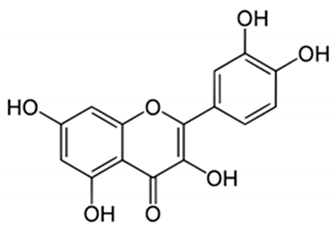 | Antidiabetic and anti-inflammatory | [8,36,37,38,39,40] |
| Punicalin | Peel, juice, and fruit | 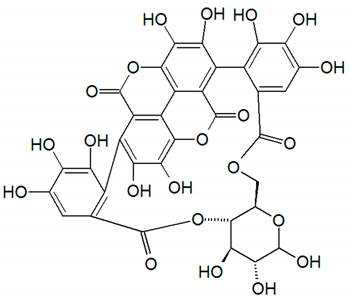 | Antidiabetic and antioxidant | [36,37,38,39,40,48,49] |
| Epicatechin | Peel | 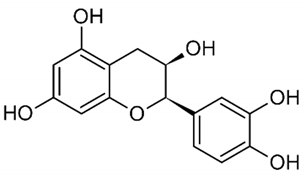 | Anti-inflammatory | [36,37,39,48] |
| Tannic acid | Peel | 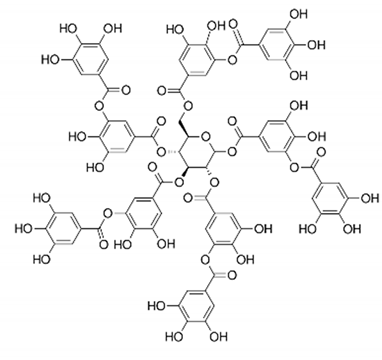 | Anti-obesity and antioxidant | [34,50,51,52] |
| Punicalagin | Peel, flower, seeds, juice, and fruit |  | Antidiabetic, antioxidant, and anti-inflammatory | [8,32,36,37,38,39,46,49] |
| Urolithin A | Polyphenol ellagitannin–gut microbial-derived metabolite | 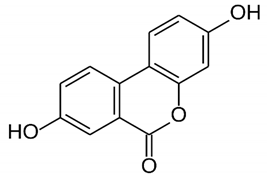 | Anti-obesity and anti-inflammatory | [53,54,55,56,57] |
| Urolithin B | Polyphenol ellagitannin–gut microbial-derived metabolite | 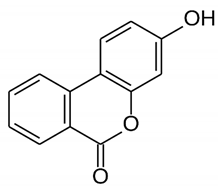 | Anti-obesity and antioxidant | [53,55,56] |
| Anthocyanins | Fruit | 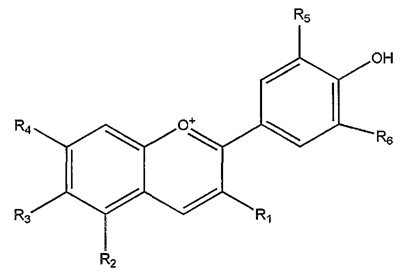 | Antioxidant | [38,49,58,59] |
| Type of the Study | Country of the Study | Interventions | Lipid Profile | Body Weightor Obesity | Diabetes or IR | Blood Pressure | Adverse Effects | Reference |
|---|---|---|---|---|---|---|---|---|
| Randomized clinical trial with 42 women participants (18–40 years) diagnosed with PCOS. | Iran | Participants were randomized into two groups: intervention (n = 21, 45 mL/day of concentrated pomegranate juice added to 180 mL of water for 8 weeks) and control (n = 21). | Reduction of TG (p < 0.03) and increase of HDL-c (p < 0.01). No significant changes in the control group. | Intervention BMI: 29.65 ± 0.70 to 29.30 ± 0.72 (p = 0.02) Control BMI: 31.86 ± 1.15 to 31.89 ± 1.17 | Not significant | Reduction of SBP and DBP (p < 0.001) in the intervention group. Control: no significant changes. | There were no adverse effects. | Abedini et al., 2020 [66] |
| Randomized, triple-blind, placebo-controlled trial with 92 women participants (15–48 years) diagnosed with PCOS. | Iran | Participants received symbiotic pomegranate juice (300 mL/day with symbiotic organisms/8 week) or pomegranate juice (300 mL/day)/8 weeks) or symbiotic beverage (water with symbiotic organisms/8 weeks) or placebo. | SPJ: reduction of LDL-c and increase of HDL-c (p < 0.01); PJ: increase of HDL-c (p < 0.01); SB: decrease of TC and LDL-c (p < 0.01). | Not reported | Not reported | Reduction of SBP and DBP (p < 0.001) in the intervention group. Control: no significant changes. | Any adverse effects were encountered. | Esmaeilinezhad et al., 2020 [67] |
| Randomized crossover trial with 41 hemodialysis participants (47.8 ± 13.3 years, 25♂, 16♀). | Iran | Participants received pomegranate juice (100 mL right after hemodialysis sections 3 x/week) or placebo. This study had a washout design (4-week period), and the study’s intervention took 20 weeks. | Reduction of TG (p < 0.001) and increase of HDL-c (p < 0.001). No significant changes in the control group. | Not significant | Not reported | Reduction of SBP and DBP (p < 0.001) in the intervention group. Control: no significant changes. | Stomach discomfort was reported by 1 dropped-out participant of the pomegranate juice. | Barati Boldaji et al., 2020 [10] |
| Randomized, single-blind, placebo-controlled clinical trial with 60 participants (40–65 years; 30♀, 30♂) diagnosed with T2DM. | Iran | Subjects were randomly separated into control (n = 30) and treatment (n = 30, 200 mL of pomegranate juice daily/6 weeks). | Not significant. | Not significant | Not reported | Reduction of SBP and DBP (p < 0.001) in the intervention group. Control: no significant changes. | Not reported. | Sohrab et al., 2019 [12] |
| Randomized study with 23 women (aged 40–60 years) diagnosed with MetS. | Serbia | Participants received 300 mL of polyphenolic-rich pomegranate juice daily/6 weeks) or placebo. | Not significant. | Not significant | Not significant | Not significant. | Not reported. | Kojadinovic et al., 2017 [80] |
| Randomized, double-blind, placebo-controlled clinical trial with 53 participants (18–65 years, 40♀, 13♂). | United Kingdom | Participants received pomegranate extract capsule (210 mg of punicalagins, 328 mg of polyphenols, and 0.37 mg of anthocyanins)/day/8 weeks) or placebo. | Not reported. | Not significant | Not reported | Reduction of DBP (p < 0.05) in the intervention group. Control: no significant changes. | Not reported. | Stockton et al., 2017 [74] |
| Randomized and controlled clinical trial with 10 healthy participants (5♂ and 5♀). | Greece | Participants received 500 mL of pomegranate juice daily for 14 days or placebo. | Not significant. | Not significant | Not significant | Not significant. | Not reported. | Manthou et al., 2017 [79] |
| Randomized, double-blind, placebo-controlled trial with 42 (30–60 years) overweight and obese participants. | Iran | Participants received 1000 mg of pomegranate extract containing 40% of ellagic acid daily for 30 days) or placebo. | Intervention: reduction of TC (p < 0.003) and LDL-c (p < 0.009); increase of HDL-c (p < 0.001). | Not significant | Intervention and control: reduction of GLU (p < 0.001) and IR (p < 0.001) | Not reported. | One individual in the placebo group presented stomach cramps. | Hosseini et al., 2016 [68] |
| Randomized, double-blind, parallel-group, multicenter, controlled trial with 31 male athletes. | Spain | Participants received placebo or pomegranate juice (200 mL/day) or diluted pomegranate juice (200 mL/day dilute in the same amount of water)/21 day. | PJ and diluted PJ: increase of HDL-c: (p < 0.05). | Not significant | Not significant | Not reported. | Not reported. | Fuster-Muñoz et al., 2016 [78] |
| Prospective, randomized, crossover clinical trial with 24 participants (13♀, 11♂, 61 ± 14 years) diagnosed with ESRN receiving hemodialysis thrice weekly. | USA | Participants received pomegranate juice (100 mL/day/4 weeks) or pomegranate extract (1050 mg/day/4 weeks). After 4 weeks, there was a washout period of 4 weeks and after the intervention was inverted, the groups received the alternative intervention for more 4 weeks. | Not significant. | Not reported | Not reported | Not significant. | No direct study-related adverse effects were reported. | Rivara et al., 2015 [73] |
| Randomized, placebo-controlled, double-blind with 27 participants that need to pass through hemodialysis. | USA | Participants were randomly assigned into two groups: pomegranate (n = 13, 1000 mg capsule of pomegranate’s purified polyphenol extract 7 days/week for 6 months) and placebo (n = 14). | Not significant. | Not significant | Not reported | Reduction of SBP and DBP (p < 0.05) in the intervention group. Control: no significant changes. | NR here, but the authors suggested no GI adverse effects. | Wu et al., 2015 [70] |
| Randomized, double-blind, placebo-controlled clinical trial with 101 hemodialysis participants (66.5 ± 11.8 years, 54.5%♂ and 45.5♀). | Israel | Participants received 0.7 mmol of polyphenols in form of 100 cc of pomegranate juice, 3 × weeks/1 year or placebo. The interventions were all made during the first hour of dialysis sections. | Reduction of TG (p < 0.05) and increase of HDL-c (p < 0.05). No significant changes in the control group. | Not reported | Not reported | Reduction of SBP, DBP, and PP (p < 0.05)Control: no significant changes. | NR here, but the authors suggested no GI adverse effects. | Shema-Didi et al., 2014 [81] |
| Randomized and single-blind clinical trial with 21 participants (30–67 years, 15♀, 6♂) diagnosed with hypertension. | Iran | Participants were randomly separated into intervention group (150 mL daily of pomegranate juice for 2 weeks) and control. | Not significant. | Not reported | Not significant | Reduction of SBP and DBP (p < 0.05) in the intervention group. Control: no significant changes. | Not reported. | Asgary et al., 2014 [65] |
| Randomized, double-blind, placebo-controlled clinical trial with 44 participants (40–60 years, 23♂, 21♀) diagnosed with T2DM. | Iran | Participants were assigned into two groups: intervention (250 mL daily of pomegranate juice containing 1946 mg/L of polyphenols for 12 weeks) and control. | Not significant. | Not significant | Not significant | Not reported. | There were no adverse effects. | Sohrab et al., 2014 [69] |
| Randomized, double-blind, placebo-controlled clinical trial with 77 overweight women (20–65 years, BMI of 25–35 kg/m2). | South Korea | Participants received 200 mL/day of pomegranate vinegar containing 1.5 g of acetic acid added to 700 μg of ellagic acid for 8 weeks) or placebo. | Not significant | Not significant | Not significant | Not reported. | Not reported. | Park et al., 2014 [82] |
| Randomized, placebo-controlled, crossover clinical trial with 28 participants (16♀, 12♂). | United Kingdom | Participants received 500 mL of pomegranate juice with 1685 mg/L of polyphenols or placebo/4 weeks. After a 1-week washout period, groups were changed to the alternative experiment (placebo group was transformed in intervention and vice-versa)/4 weeks. | Not significant. | Not significant | InterventionHOMA-IR: 2.216 ± 1.43 to 1.825 ± 1.12 (p = 0.028) ControlHOMA-IR: 2.245 ± 0.23 to 2.226 ± 0.12 (not significant) | Reduction of SBP and DBP (p < 0.031) in the intervention group. Control: no significant changes. | NR here. | Tsang et al., 2012 [76] |
| Randomized, placebo-controlled, parallel-group, open-label clinical trial with 48 healthy participants (30–50 years, 32♀, ♂16 male). | United Kingdom | The groups were intervention (n = 24, 330 mL/day of pomegranate juice for 4 weeks) and control (n = 24). | Not reported | Not significant | Not reported | Reduction of SBP and DBP (p < 0.001) in the intervention group. Control: no significant changes. | n = 2 problems in consumption of the juice. | Lynn et al., 2012 [75] |
| Randomized, double-blind, placebo-controlled clinical trial with 20 obese participants (25–55 years). | Mexico | The obese patients were randomly assigned into two groups: intervention (120 mL of pomegranate juice daily for 1 month, n = 10) and placebo (n = 10). | Not significant | InterventionFM (%): 41.3 ± 6.2 to 39.9 ± 6.5 (p = 0.010) Control FM (%): 36.3 ± 7.7 to 37.4 ± 7.8 | Not significant | Not reported. | There were no adverse effects. | González-Ortiz et al., 2011 [77] |
| Randomized, double-blind, placebo-controlled study with 45 participants (5♀, 40♂) diagnosed with ischemic coronary heart disease and myocardial ischemia. | USA | Participants were randomly assigned into 2 groups: intervention (240 mL/day of pomegranate juice for 3 months) and control. | Not significant | Not significant | Not significant | Not significant. | Not reported. | Sumner et al., 2005 [71] |
| Randomized clinical trial with 19 participants (65–75 years, 5♀, 14♀) diagnosed with asymptomatic severe carotid artery stenosis. | USA | Participants received pomegranate juice (50 mL/day of pomegranate with 1.5 mmoles of polyphenols for 1 year) or placebo. After this period, 5 participants of the pomegranate intervention agreed to continue the study for up to 3 years. | Not significant | Not significant | Not significant | Reduction of SBP and DBP (p < 0.05) in the intervention group (1 y). Control: no significant changes. | Not reported. | Aviram et al., 2004 [72] |
| Study | Question Focus | Appropriate Randomization | Allocation Blinding | Double-Blind | Losses (<20%) | Prognostic and Demographic Characteristics | Outcomes | Intention to Treat Analysis | Sample Calculation | Adequate Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| Abedini et al., 2020 [66] | Yes | No | No | No | Yes | Yes | Yes | No | Yes | Yes |
| Esmaeilinezhad et al., 2020 [67] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Barati Boldaji et al., 2020 [10] | Yes | Nr | No | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Sohrab et al., 2019 [12] | Yes | Yes | No | No | Yes | Yes | Yes | No | Nr | Yes |
| Kojadinovic et al., 2017 [80] | Yes | Nr | No | No | Yes | Yes | Yes | Yes | Nr | Yes |
| Stockton et al., 2017 [74] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Manthou et al., 2017 [79] | Yes | Nr | No | No | Yes | Yes | Yes | Yes | Yes | No |
| Hosseini et al., 2016 [68] | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Fuster-Muñoz et al., 2016 [78] | Yes | Nr | Yes | Yes | No | Yes | Yes | No | No | No |
| Rivara et al., 2015 [73] | Yes | Nr | No | No | Yes | Yes | Yes | Yes | Nr | Yes |
| Wu et al., 2015 [70] | Yes | Nr | Yes | No | Yes | Yes | Yes | No | Nr | Yes |
| Shema-Didi et al., 2014 [81] | Yes | Nr | Yes | Yes | No | Yes | Yes | No | Nr | Yes |
| Asgary et al., 2014 [65] | Yes | Nr | No | No | Yes | Yes | Yes | Yes | Nr | No |
| Sohrab et al., 2014 [69] | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Park et al., 2014 [82] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Tsang et al., 2012 [76] | Yes | Nr | No | No | Yes | Yes | Yes | Yes | Nr | Yes |
| Lynn et al., 2012 [75] | Yes | No | No | No | Yes | Yes | Yes | No | Yes | Yes |
| González-Ortiz et al., 2011 [77] | Yes | Nr | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Sumner et al., 2005 [71] | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Nr | Yes |
| Aviram et al., 2004 [72] | Yes | Nr | No | No | Yes * | No | Yes | Yes * | Nr | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurindo, L.F.; Barbalho, S.M.; Marquess, A.R.; Grecco, A.I.d.S.; Goulart, R.d.A.; Tofano, R.J.; Bishayee, A. Pomegranate (Punica granatum L.) and Metabolic Syndrome Risk Factors and Outcomes: A Systematic Review of Clinical Studies. Nutrients 2022, 14, 1665. https://doi.org/10.3390/nu14081665
Laurindo LF, Barbalho SM, Marquess AR, Grecco AIdS, Goulart RdA, Tofano RJ, Bishayee A. Pomegranate (Punica granatum L.) and Metabolic Syndrome Risk Factors and Outcomes: A Systematic Review of Clinical Studies. Nutrients. 2022; 14(8):1665. https://doi.org/10.3390/nu14081665
Chicago/Turabian StyleLaurindo, Lucas Fornari, Sandra Maria Barbalho, Alexis R. Marquess, Annik Ianara de Souza Grecco, Ricardo de Alvares Goulart, Ricardo José Tofano, and Anupam Bishayee. 2022. "Pomegranate (Punica granatum L.) and Metabolic Syndrome Risk Factors and Outcomes: A Systematic Review of Clinical Studies" Nutrients 14, no. 8: 1665. https://doi.org/10.3390/nu14081665
APA StyleLaurindo, L. F., Barbalho, S. M., Marquess, A. R., Grecco, A. I. d. S., Goulart, R. d. A., Tofano, R. J., & Bishayee, A. (2022). Pomegranate (Punica granatum L.) and Metabolic Syndrome Risk Factors and Outcomes: A Systematic Review of Clinical Studies. Nutrients, 14(8), 1665. https://doi.org/10.3390/nu14081665