The Dose-Response Effects of Consuming High Fructose Corn Syrup-Sweetened Beverages on Hepatic Lipid Content and Insulin Sensitivity in Young Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
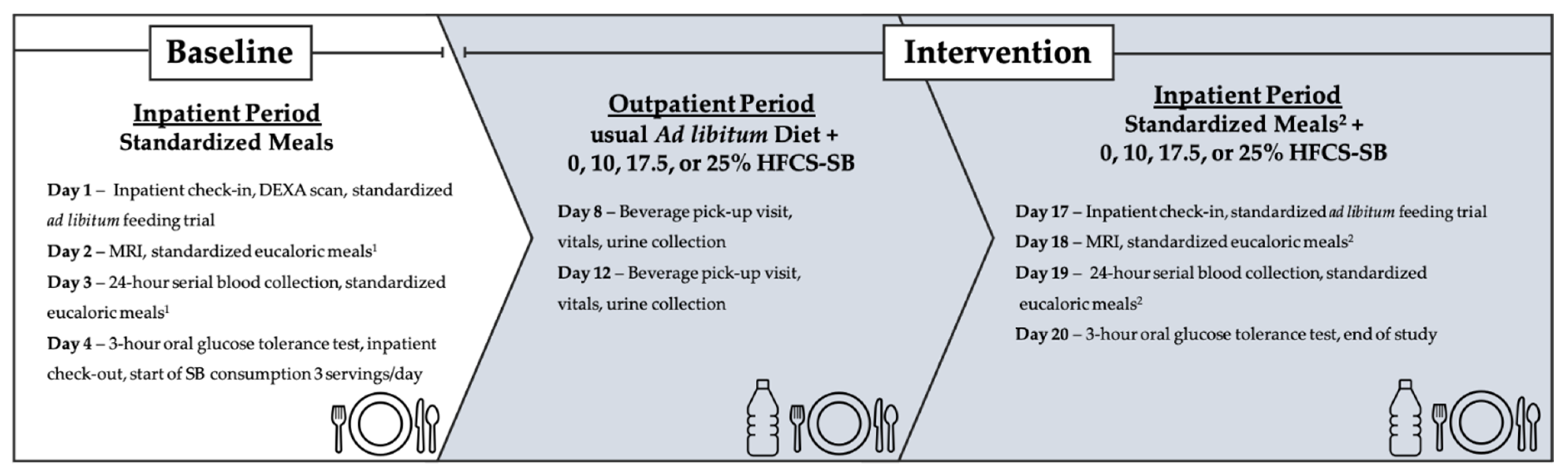
2.2. Dietary Protocol
2.2.1. Inpatient Meals
2.2.2. Study Beverages and Outpatient Meals
2.3. Hepatic Lipid Imaging
Hepatic Lipid Content Quantification
2.4. Insulin Sensitivity
2.5. Twenty-Four Hour Serial Blood Collection
2.6. Statistical Analyses
3. Results
3.1. Hepatic Lipid Content (MRI-PDFF)
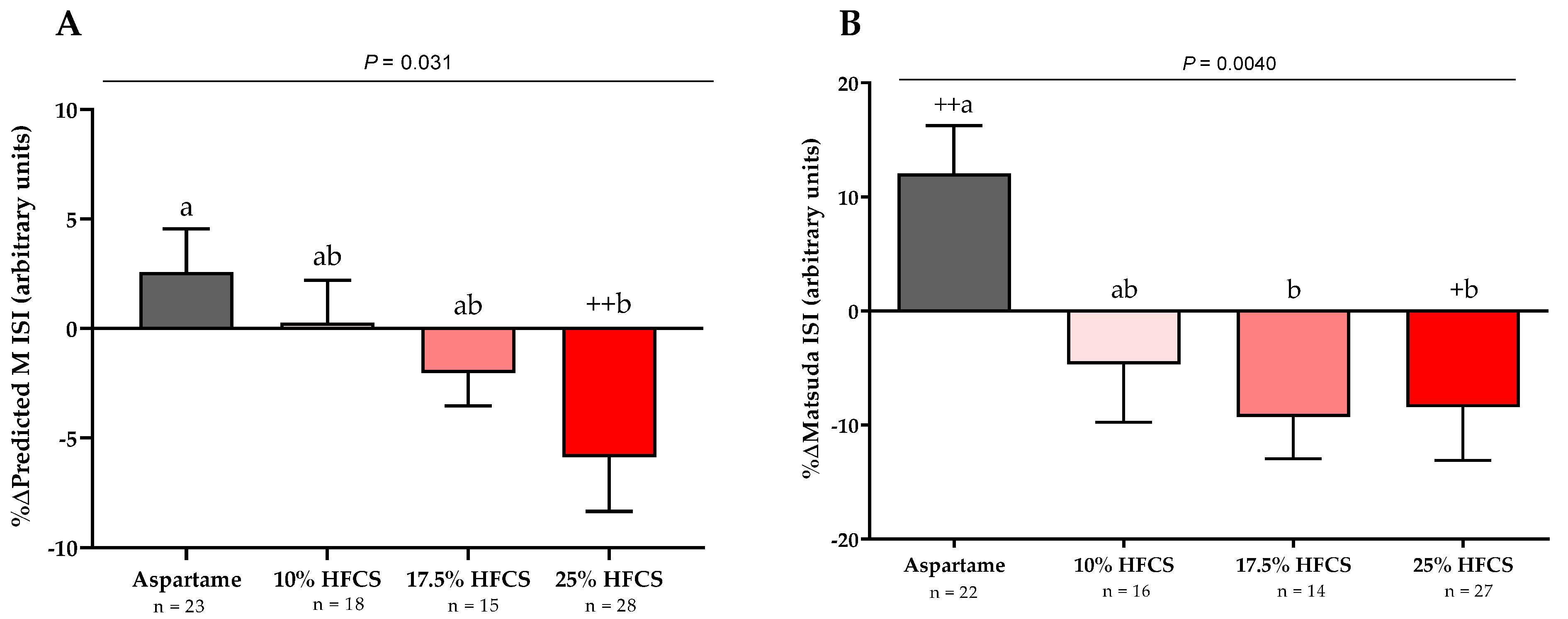
3.2. Insulin Sensitivity
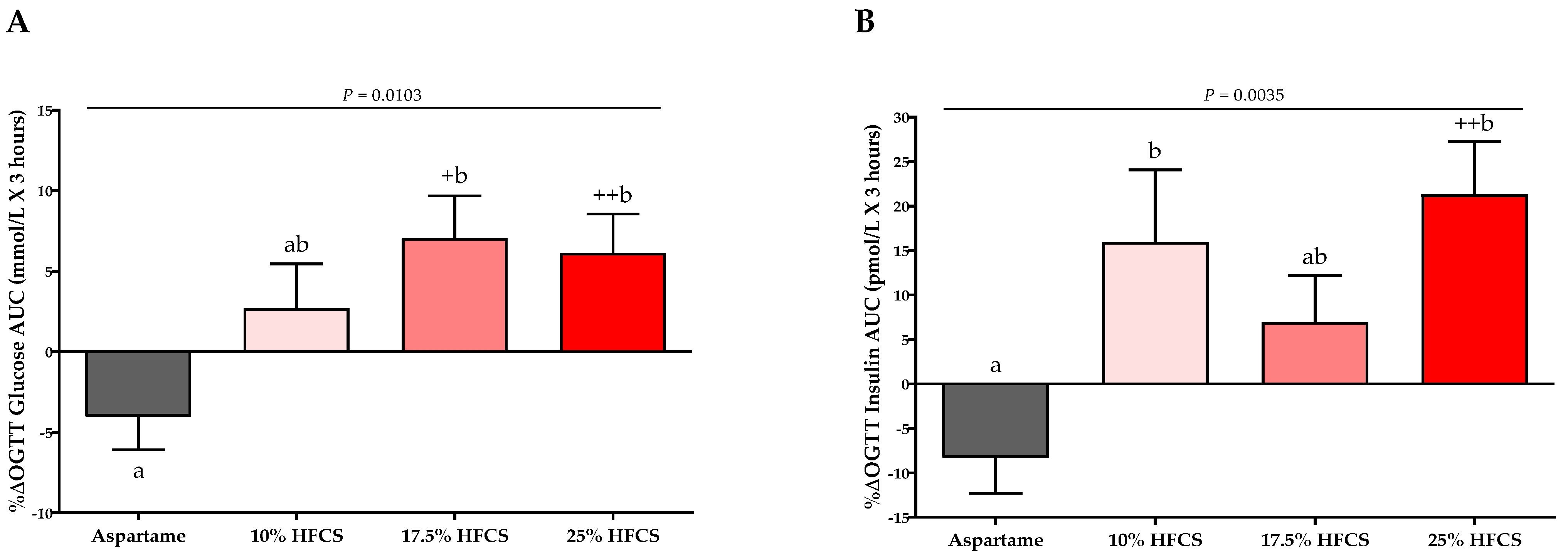
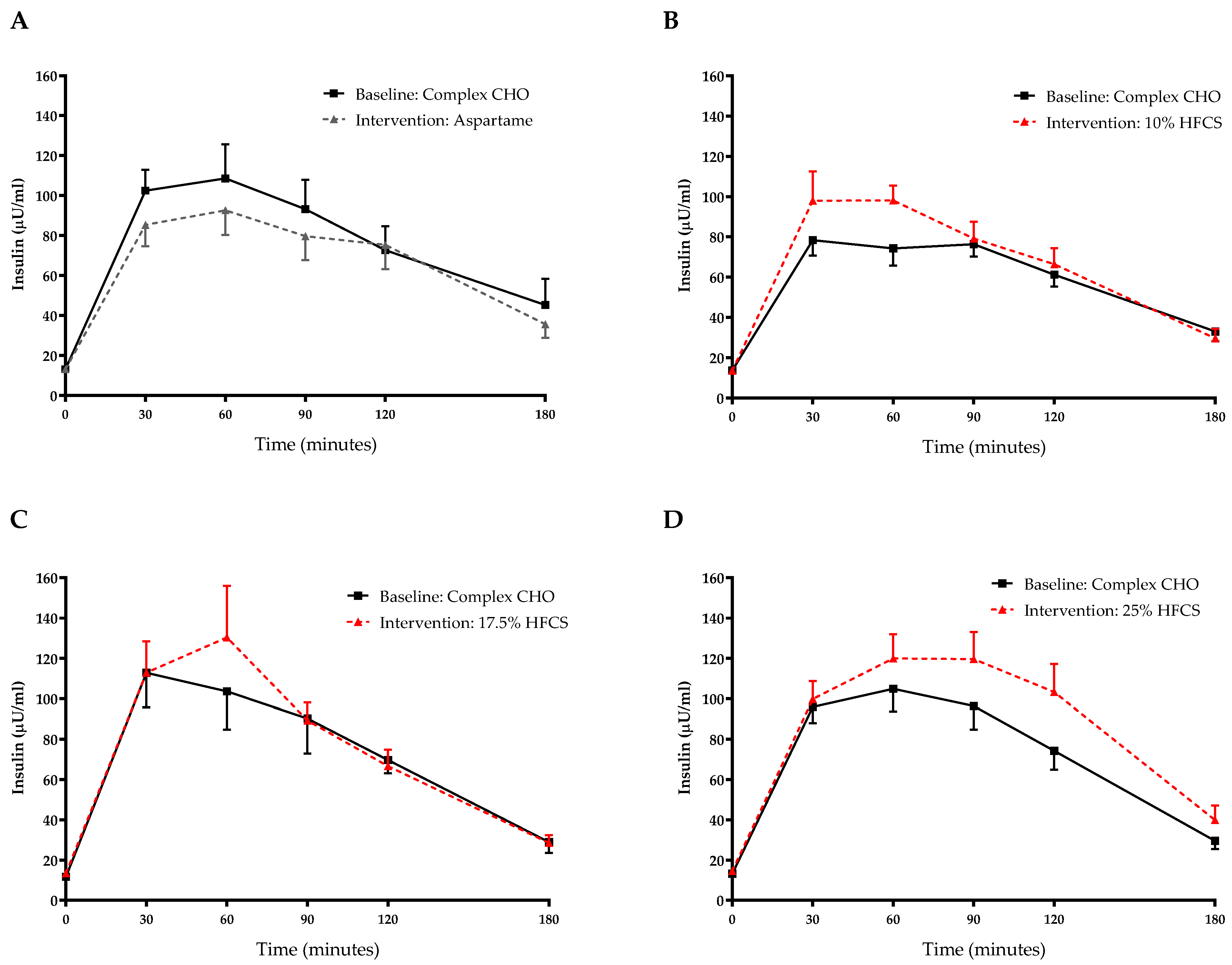
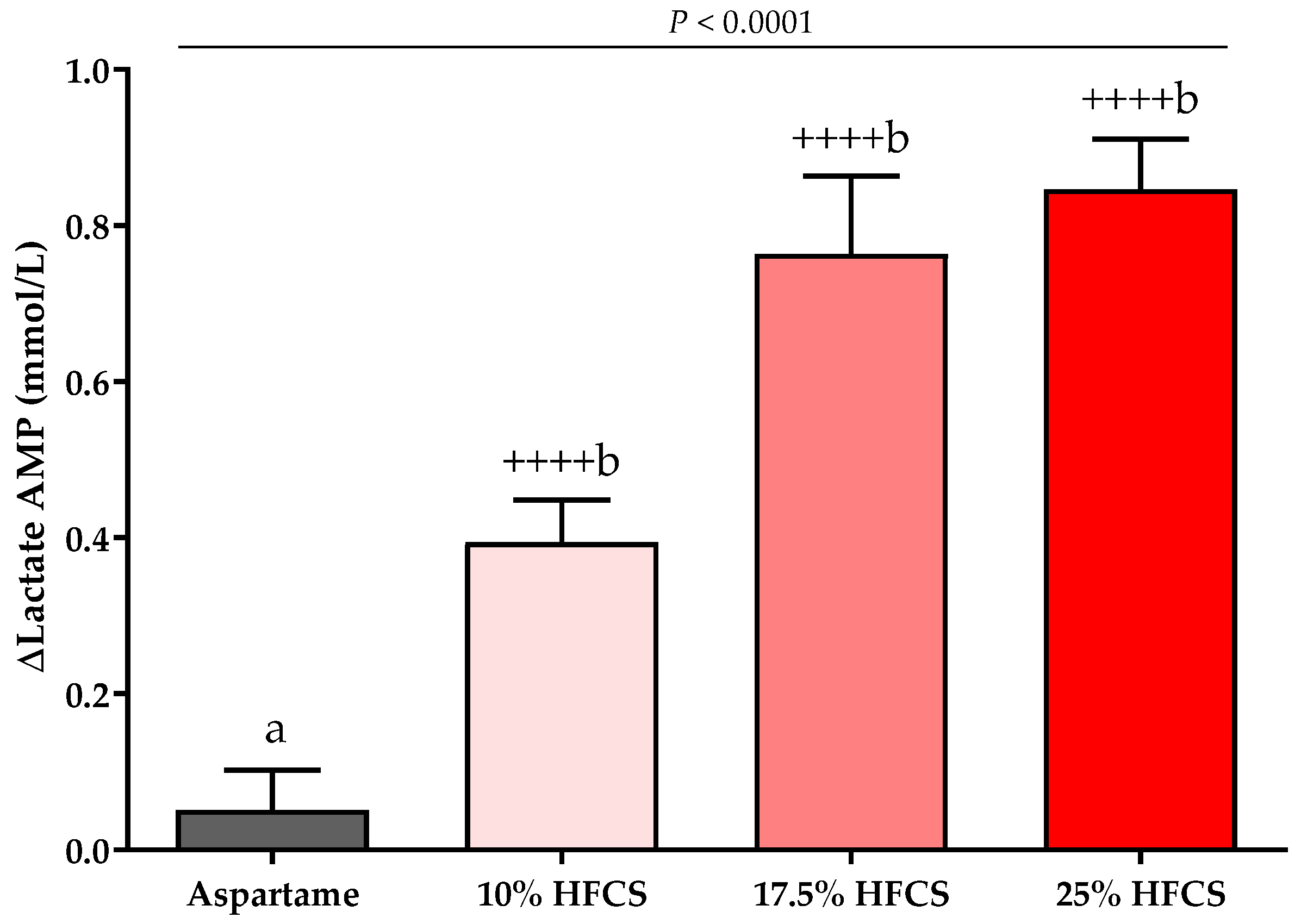
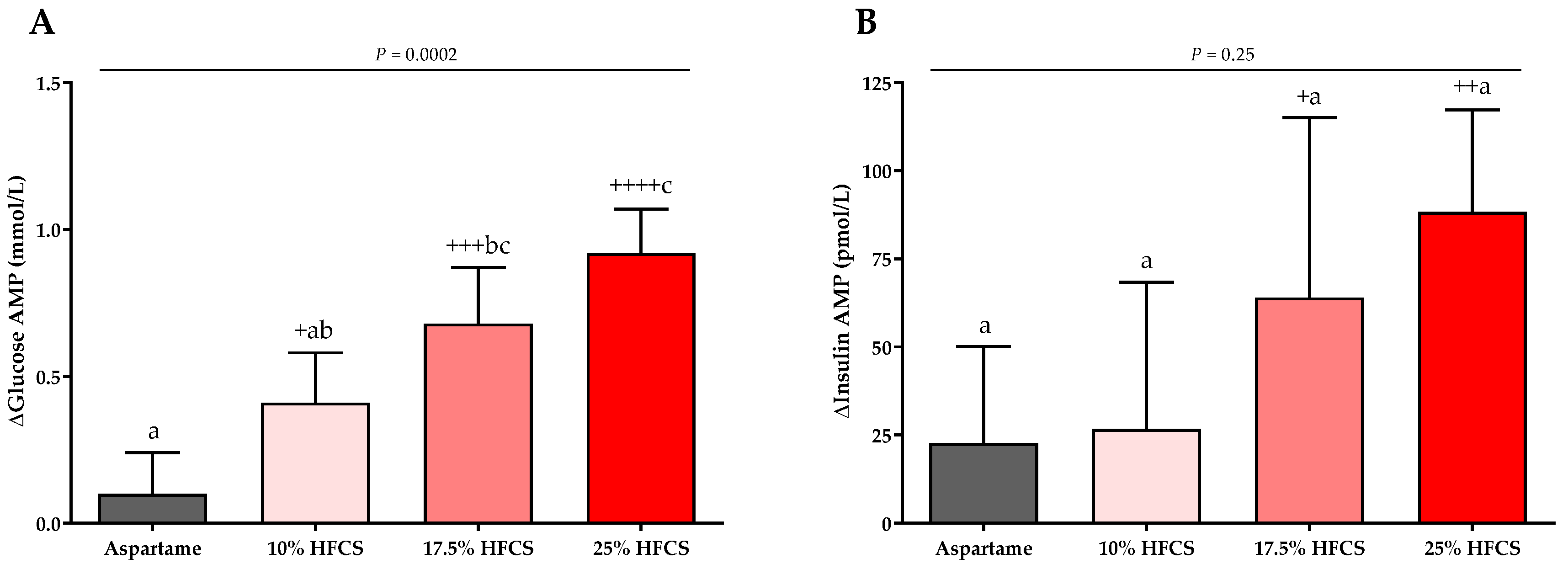
3.3. Post-Meal Lactate, Glucose and Insulin Concentrations
3.4. Mediation Analysis
3.4.1. Hepatic Lipid Content
3.4.2. Indices of Insulin Sensitivity
4. Discussion
Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Interntional Diabetes Federation. IDF Diabetes Atlas: 10th Edition. Available online: http://www.diabetesatlas.org (accessed on 15 November 2021).
- Zimmet, P.; Alberti, K.G.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zou, B.; Yeo, Y.H.; Feng, Y.; Xie, X.; Lee, D.H.; Fujii, H.; Wu, Y.; Kam, L.Y.; Ji, F.; et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2019, 4, 389–398. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciardullo, S.; Perseghin, G. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population. Liver Int. 2021, 41, 1290–1293. [Google Scholar] [CrossRef]
- Caussy, C.; Aubin, A.; Loomba, R. The relationship between type 2 diabetes, NAFLD, and cardiovascular risk. Curr. Diabetes Rep. 2021, 21, 15. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Targher, G.; Day, C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 330–344. [Google Scholar] [CrossRef]
- Softic, S.; Stanhope, K.L.; Boucher, J.; Divanovic, S.; Lanaspa, M.A.; Johnson, R.J.; Kahn, C.R. Fructose and hepatic insulin resistance. Crit. Rev. Clin. Lab. Sci. 2020, 57, 308–322. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef] [PubMed]
- The USDA's Economic Research Service (ERS). Loss-Adjusted Food Availability Documentation. Available online: https://www.ers.usda.gov/data-products/food-availability-per-capita-data-system/loss-adjusted-food-availability-documentation/ (accessed on 20 January 2022).
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez Steele, E.; Baraldi, L.G.; Louzada, M.L.; Moubarac, J.C.; Mozaffarian, D.; Monteiro, C.A. Ultra-processed foods and added sugars in the US diet: Evidence from a nationally representative cross-sectional study. BMJ Open 2016, 6, e009892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neri, D.; Martinez-Steele, E.; Monteiro, C.A.; Levy, R.B. Consumption of ultra-processed foods and its association with added sugar content in the diets of US children, NHANES 2009–2014. Pediatr. Obes. 2019, 14, e12563. [Google Scholar] [CrossRef] [PubMed]
- Rosinger, A.; Herrick, K.; Gahche, J.; Park, S. Sugar-Sweetened Beverage Consumption among U.S. Adults, 2011–2014; NCHS data brief, no 270; National Center for Health Statistics: Hyattsville, MD, USA, 2017.
- Rosinger, A.; Herrick, K.; Gahche, J.; Park, S. Sugar-Sweetened Beverage Consumption among U.S. Youth, 2011–2014; NCHS data brief, no 271; National Center for Health Statistics: Hyattsville, MD, USA, 2017.
- Ma, J.; Jacques, P.F.; Meigs, J.B.; Fox, C.S.; Rogers, G.T.; Smith, C.E.; Hruby, A.; Saltzman, E.; McKeown, N.M. Sugar-sweetened beverage but not diet soda consumption is positively associated with progression of insulin resistance and prediabetes. J. Nutr. 2016, 146, 2544–2550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, P.; Li, Q.; Zhao, Y.; Chen, Q.; Sun, X.; Liu, Y.; Li, H.; Wang, T.; Chen, X.; Zhou, Q.; et al. Sugar and artificially sweetened beverages and risk of obesity, type 2 diabetes mellitus, hypertension, and all-cause mortality: A dose-response meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2020, 35, 655–671. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Li, Z.; Lam, C.W.K.; Xiao, Y.; Wu, Q.; Zhang, W. Consumption of sugar-sweetened beverages has a dose-dependent effect on the risk of non-alcoholic fatty liver disease: An updated systematic review and dose-response meta-analysis. Int. J. Environ. Res. Public Health 2019, 16, 2192. [Google Scholar] [CrossRef] [Green Version]
- Sigala, D.M.; Hieronimus, B.; Medici, V.; Lee, V.; Nunez, M.V.; Bremer, A.A.; Cox, C.L.; Price, C.A.; Benyam, Y.; Chaudhari, A.J.; et al. Consuming sucrose- or HFCS-sweetened beverages increases hepatic lipid and decreases insulin sensitivity in adults. J. Clin. Endocrinol. Metab. 2021, 106, 3248–3264. [Google Scholar] [CrossRef]
- Maersk, M.; Belza, A.; Stodkilde-Jorgensen, H.; Ringgaard, S.; Chabanova, E.; Thomsen, H.; Pedersen, S.B.; Astrup, A.; Richelsen, B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: A 6-mo randomized intervention study. Am. J. Clin. Nutr. 2012, 95, 283–289. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Noworolski, S.M.; Wen, M.J.; Dyachenko, A.; Prior, J.L.; Weinberg, M.E.; Herraiz, L.A.; Tai, V.W.; Bergeron, N.; Bersot, T.P.; et al. Effect of a high-fructose weight-maintaining diet on lipogenesis and liver fat. J. Clin. Endocrinol. Metab. 2015, 100, 2434–2442. [Google Scholar] [CrossRef]
- Taskinen, M.R.; Soderlund, S.; Bogl, L.H.; Hakkarainen, A.; Matikainen, N.; Pietilainen, K.H.; Rasanen, S.; Lundbom, N.; Bjornson, E.; Eliasson, B.; et al. Adverse effects of fructose on cardiometabolic risk factors and hepatic lipid metabolism in subjects with abdominal obesity. J. Intern. Med. 2017, 282, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aeberli, I.; Hochuli, M.; Gerber, P.A.; Sze, L.; Murer, S.B.; Tappy, L.; Spinas, G.A.; Berneis, K. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: A randomized controlled trial. Diabetes Care 2013, 36, 150–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, V.; Despland, C.; Brandejsky, V.; Kreis, R.; Schneiter, P.; Chiolero, A.; Boesch, C.; Tappy, L. Sugar- and artificially sweetened beverages and intrahepatic fat: A randomized controlled trial. Obesity 2015, 23, 2335–2339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibarra-Reynoso, L.D.R.; Lopez-Lemus, H.L.; Garay-Sevilla, M.E.; Malacara, J.M. Effect of restriction of foods with high fructose corn syrup content on metabolic indices and fatty liver in obese children. Obes. Facts 2017, 10, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Schwimmer, J.B.; Ugalde-Nicalo, P.; Welsh, J.A.; Angeles, J.E.; Cordero, M.; Harlow, K.E.; Alazraki, A.; Durelle, J.; Knight-Scott, J.; Newton, K.P.; et al. Effect of a low free sugar diet vs. usual diet on nonalcoholic fatty liver disease in adolescent boys: A randomized clinical trial. JAMA 2019, 321, 256–265. [Google Scholar] [CrossRef] [Green Version]
- Simons, N.; Veeraiah, P.; Simons, P.; Schaper, N.C.; Kooi, M.E.; Schrauwen-Hinderling, V.B.; Feskens, E.J.M.; van der Ploeg, E.; Van den Eynde, M.D.G.; Schalkwijk, C.G.; et al. Effects of fructose restriction on liver steatosis (FRUITLESS); a double-blind randomized controlled trial. Am. J. Clin. Nutr. 2021, 113, 391–400. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Noworolski, S.M.; Erkin-Cakmak, A.; Korn, N.J.; Wen, M.J.; Tai, V.W.; Jones, G.M.; Palii, S.P.; Velasco-Alin, M.; Pan, K.; et al. Effects of dietary fructose restriction on liver fat, de novo lipogenesis, and insulin kinetics in children with obesity. Gastroenterology 2017, 153, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Ishimoto, T.; Lanaspa, M.A.; Le, M.T.; Garcia, G.E.; Diggle, C.P.; Maclean, P.S.; Jackman, M.R.; Asipu, A.; Roncal-Jimenez, C.A.; Kosugi, T.; et al. Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc. Natl. Acad. Sci. USA 2012, 109, 4320–4325. [Google Scholar] [CrossRef] [Green Version]
- Softic, S.; Gupta, M.K.; Wang, G.X.; Fujisaka, S.; O’Neill, B.T.; Rao, T.N.; Willoughby, J.; Harbison, C.; Fitzgerald, K.; Ilkayeva, O.; et al. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J. Clin. Investig. 2017, 127, 4059–4074. [Google Scholar] [CrossRef] [Green Version]
- Francey, C.; Cros, J.; Rosset, R.; Creze, C.; Rey, V.; Stefanoni, N.; Schneiter, P.; Tappy, L.; Seyssel, K. The extra-splanchnic fructose escape after ingestion of a fructose-glucose drink: An exploratory study in healthy humans using a dual fructose isotope method. Clin. Nutr. ESPEN 2019, 29, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Havel, P.J. Dietary fructose: Implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr. Rev. 2005, 63, 133–157. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, M.A.; Birnbaum, M.J. Molecular aspects of fructose metabolism and metabolic disease. Cell Metab. 2021, 33, 2329–2354. [Google Scholar] [CrossRef]
- Hieronimus, B.; Stanhope, K.L. Dietary fructose and dyslipidemia: New mechanisms involving apolipoprotein CIII. Curr. Opin. Lipidol. 2020, 31, 20–26. [Google Scholar] [CrossRef]
- Cox, C.L.; Stanhope, K.L.; Schwarz, J.M.; Graham, J.L.; Hatcher, B.; Griffen, S.C.; Bremer, A.A.; Berglund, L.; McGahan, J.P.; Havel, P.J.; et al. Consumption of fructose-sweetened beverages for 10 weeks reduces net fat oxidation and energy expenditure in overweight/obese men and women. Eur. J. Clin. Nutr. 2012, 66, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Jornayvaz, F.R.; Shulman, G.I. Diacylglycerol activation of protein kinase Cepsilon and hepatic insulin resistance. Cell Metab. 2012, 15, 574–584. [Google Scholar] [CrossRef] [Green Version]
- Adiels, M.; Taskinen, M.R.; Packard, C.; Caslake, M.J.; Soro-Paavonen, A.; Westerbacka, J.; Vehkavaara, S.; Hakkinen, A.; Olofsson, S.O.; Yki-Jarvinen, H.; et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia 2006, 49, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Lewis, G.F.; Carpentier, A.; Adeli, K.; Giacca, A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr. Rev. 2002, 23, 201–229. [Google Scholar] [CrossRef]
- Boren, J.; Packard, C.J.; Taskinen, M.R. The roles of ApoC-III on the metabolism of triglyceride-rich lipoproteins in humans. Front. Endocrinol. 2020, 11, 474. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, J.R.; Langlet, F.; Kido, Y.; Accili, D. Pathogenesis of selective insulin resistance in isolated hepatocytes. J. Biol. Chem. 2015, 290, 13972–13980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krssak, M.; Falk Petersen, K.; Dresner, A.; DiPietro, L.; Vogel, S.M.; Rothman, D.L.; Roden, M.; Shulman, G.I. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: A 1H NMR spectroscopy study. Diabetologia 1999, 42, 113–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baena, M.; Sanguesa, G.; Davalos, A.; Latasa, M.J.; Sala-Vila, A.; Sanchez, R.M.; Roglans, N.; Laguna, J.C.; Alegret, M. Fructose, but not glucose, impairs insulin signaling in the three major insulin-sensitive tissues. Sci. Rep. 2016, 6, 26149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyssel, K.; Meugnier, E.; Le, K.A.; Durand, C.; Disse, E.; Blond, E.; Pays, L.; Nataf, S.; Brozek, J.; Vidal, H.; et al. Fructose overfeeding in first-degree relatives of type 2 diabetic patients impacts energy metabolism and mitochondrial functions in skeletal muscle. Mol. Nutr. Food Res. 2016, 60, 2691–2699. [Google Scholar] [CrossRef]
- Tappy, L. Fructose metabolism and noncommunicable diseases: Recent findings and new research perspectives. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 214–222. [Google Scholar] [CrossRef]
- Lombardi, A.M.; Fabris, R.; Bassetto, F.; Serra, R.; Leturque, A.; Federspil, G.; Girard, J.; Vettor, R. Hyperlactatemia reduces muscle glucose uptake and GLUT-4 mRNA while increasing (E1alpha)PDH gene expression in rat. Am. J. Physiol. 1999, 276, E922–E929. [Google Scholar] [CrossRef]
- King, C.; Lanaspa, M.A.; Jensen, T.; Tolan, D.R.; Sanchez-Lozada, L.G.; Johnson, R.J. Uric acid as a cause of the metabolic syndrome. In Uric Acid in Chronic Kidney Disease; Treviño-Becerra, A., Iseki, K., Eds.; Contributions to Nephrology; Karger: Basel, Switzerland, 2018; Volume 192, pp. 88–102. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Cicerchi, C.; Li, N.; Roncal-Jimenez, C.A.; Ishimoto, T.; Le, M.; Garcia, G.E.; Thomas, J.B.; Rivard, C.J.; et al. Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PLoS ONE 2012, 7, e47948. [Google Scholar] [CrossRef] [Green Version]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: Potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Shafiu, M.; Sundaram, S.; Le, M.; Ishimoto, T.; Sautin, Y.Y.; Lanaspa, M.A. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 2013, 62, 3307–3315. [Google Scholar] [CrossRef] [Green Version]
- Stanhope, K.L.; Medici, V.; Bremer, A.A.; Lee, V.; Lam, H.D.; Nunez, M.V.; Chen, G.X.; Keim, N.L.; Havel, P.J. A dose-response study of consuming high-fructose corn syrup-sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am. J. Clin. Nutr. 2015, 101, 1144–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigala, D.M.; Widaman, A.M.; Hieronimus, B.; Nunez, M.V.; Lee, V.; Benyam, Y.; Bremer, A.A.; Medici, V.; Havel, P.J.; Stanhope, K.L.; et al. Effects of consuming sugar-sweetened beverages for 2 weeks on 24-h circulating leptin profiles, ad libitum food intake and body weight in young adults. Nutrients 2020, 12, 3893. [Google Scholar] [CrossRef] [PubMed]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Hieronimus, B.; Medici, V.; Bremer, A.A.; Lee, V.; Nunez, M.V.; Sigala, D.M.; Keim, N.L.; Havel, P.J.; Stanhope, K.L. Synergistic effects of fructose and glucose on lipoprotein risk factors for cardiovascular disease in young adults. Metabolism 2020, 112, 154356. [Google Scholar] [CrossRef]
- Reeder, S.B.; Sirlin, C.B. Quantification of liver fat with magnetic resonance imaging. Magn. Reson. Imaging Clin. N. Am. 2010, 18, 337–357. [Google Scholar] [CrossRef] [Green Version]
- Yokoo, T.; Shiehmorteza, M.; Bydder, M.; Hamilton, G.; Kono, Y.; Kono, A.; Lavine, J.E.; Sirlin, C.B. Spectrally-Modeled Hepatic Fat Quantification by Multi-Echo Gradient-Recalled-Echo Magnetic Resonance Imaging at 3.0T. Proc. Intl. Soc. Mag. Reson. Med. 2009, 17, 209. [Google Scholar]
- Hamilton, G.; Yokoo, T.; Bydder, M.; Cruite, I.; Schroeder, M.E.; Sirlin, C.B.; Middleton, M.S. In vivo characterization of the liver fat (1)H MR spectrum. NMR Biomed. 2011, 24, 784–790. [Google Scholar] [CrossRef] [Green Version]
- Tang, A.; Tan, J.; Sun, M.; Hamilton, G.; Bydder, M.; Wolfson, T.; Gamst, A.C.; Middleton, M.; Brunt, E.M.; Loomba, R.; et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 2013, 267, 422–431. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Tura, A.; Chemello, G.; Szendroedi, J.; Gobl, C.; Faerch, K.; Vrbikova, J.; Pacini, G.; Ferrannini, E.; Roden, M. Prediction of clamp-derived insulin sensitivity from the oral glucose insulin sensitivity index. Diabetologia 2018, 61, 1135–1141. [Google Scholar] [CrossRef] [Green Version]
- Mari, A.; Pacini, G.; Murphy, E.; Ludvik, B.; Nolan, J.J. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care 2001, 24, 539–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1989, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr. Opin. Cardiol. 2006, 21, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Grundy, S.M.; Wang, W.; Smith, S.C., Jr.; Vega, G.L.; Wu, Z.; Zeng, Z.; Wang, W.; Zhao, D. Ethnic-specific criteria for the metabolic syndrome: Evidence from China. Diabetes Care 2006, 29, 1414–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Judd, C.M.; Kenny, D.A. Process Analysis: Estimating Mediation Treatment Evaluations. Evaluation Rev. 1981, 5, 602–619. [Google Scholar] [CrossRef]
- Fairchild, A.J.; McDaniel, H.L. Best (but oft-forgotten) practices: Mediation analysis. Am. J. Clin. Nutr. 2017, 105, 1259–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn, C.R. Knockout mice challenge our concepts of glucose homeostasis and the pathogenesis of diabetes. Exp. Diabesity Res. 2003, 4, 169–182. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Park, S.Y.; Choi, C.S. Insulin resistance: From mechanisms to therapeutic strategies. Diabetes Metab. J. 2021. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Bondini, S.; Younossi, Z.M. Non-alcoholic fatty liver disease and hepatitis C infection. Minerva Gastroenterol. Dietol. 2006, 52, 135–143. [Google Scholar]
- Day, C.P. Non-alcoholic fatty liver disease: Current concepts and management strategies. Clin. Med. 2006, 6, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Boslaugh, S. Encyclopedia of Epidemiology: Dose-Response Relationship; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2008. [Google Scholar]
- Rothman, K.J.; Greenland, S. Causation and causal inference in epidemiology. Am. J. Public Health 2005, 95 (Suppl. S1), S144–S150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.; Zhu, Y.; Malik, V.; Li, X.; Peng, X.; Zhang, F.F.; Shan, Z.; Liu, L. Intake of sugar-sweetened and low-calorie sweetened beverages and risk of cardiovascular disease: A meta-analysis and systematic review. Adv. Nutr 2021, 12, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Black, R.N.; Spence, M.; McMahon, R.O.; Cuskelly, G.J.; Ennis, C.N.; McCance, D.R.; Young, I.S.; Bell, P.M.; Hunter, S.J. Effect of eucaloric high- and low-sucrose diets with identical macronutrient profile on insulin resistance and vascular risk: A randomized controlled trial. Diabetes 2006, 55, 3566–3572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, V.; Micha, R.; Choi, E.; Karageorgou, D.; Webb, P.; Mozaffarian, D. Evaluation of the quality of evidence of the association of foods and nutrients with cardiovascular disease and diabetes: A systematic review. JAMA Netw. Open 2022, 5, e2146705. [Google Scholar] [CrossRef]
- Kazierad, D.J.; Chidsey, K.; Somayaji, V.R.; Bergman, A.J.; Birnbaum, M.J.; Calle, R.A. Inhibition of ketohexokinase in adults with NAFLD reduces liver fat and inflammatory markers: A randomized phase 2 trial. Clin. Adv. 2021, 2, 800–813. [Google Scholar] [CrossRef]
- Kahn, R.; Sievenpiper, J.L. Dietary sugar and body weight: Have we reached a crisis in the epidemic of obesity and diabetes? We have, but the pox on sugar is overwrought and overworked. Diabetes Care 2014, 37, 957–962. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Human fatty liver disease: Old questions and new insights. Science 2011, 332, 1519–1523. [Google Scholar] [CrossRef] [Green Version]
- Ramms, B.; Gordts, P. Apolipoprotein C-III in triglyceride-rich lipoprotein metabolism. Curr. Opin. Lipidol. 2018, 29, 171–179. [Google Scholar] [CrossRef]
- Paiva, A.A.; Raposo, H.F.; Wanschel, A.C.; Nardelli, T.R.; Oliveira, H.C. Apolipoprotein CIII Overexpression-Induced Hypertriglyceridemia Increases Nonalcoholic Fatty Liver Disease in Association with Inflammation and Cell Death. Oxid. Med. Cell. Longev. 2017, 2017, 1838679. [Google Scholar] [CrossRef]
- Choi, C.S.; Kim, Y.B.; Lee, F.N.; Zabolotny, J.M.; Kahn, B.B.; Youn, J.H. Lactate induces insulin resistance in skeletal muscle by suppressing glycolysis and impairing insulin signaling. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E233–E240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botteri, G.; Montori, M.; Guma, A.; Pizarro, J.; Cedo, L.; Escola-Gil, J.C.; Li, D.; Barroso, E.; Palomer, X.; Kohan, A.B.; et al. VLDL and apolipoprotein CIII induce ER stress and inflammation and attenuate insulin signalling via Toll-like receptor 2 in mouse skeletal muscle cells. Diabetologia 2017, 60, 2262–2273. [Google Scholar] [CrossRef] [PubMed]
- Christopoulou, E.; Tsimihodimos, V.; Filippatos, T.; Elisaf, M. Apolipoprotein CIII and diabetes. Is there a link? Diabetes Metab. Res. Rev. 2019, 35, e3118. [Google Scholar] [CrossRef] [PubMed]
- Bravo, S.; Lowndes, J.; Sinnett, S.; Yu, Z.; Rippe, J. Consumption of sucrose and high-fructose corn syrup does not increase liver fat or ectopic fat deposition in muscles. Appl. Physiol. Nutr. Metab. 2013, 38, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Lowndes, J.; Rippe, J. High-fructose corn syrup and sucrose have equivalent effects on energy-regulating hormones at normal human consumption levels. Nutr. Res. 2013, 33, 1043–1052. [Google Scholar] [CrossRef] [Green Version]
- Stanhope, K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef]
- Nestle, M. Corporate Funding of Food and Nutrition Research: Science or Marketing? JAMA Intern. Med. 2016, 176, 13–14. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Department of Agriculture: Washington, DC, USA, 2020.
- Han, E.; Powell, L.M. Consumption patterns of sugar-sweetened beverages in the United States. J. Acad. Nutr. Diet. 2013, 113, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Caussy, C.; Reeder, S.B.; Sirlin, C.B.; Loomba, R. Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology 2018, 68, 763–772. [Google Scholar] [CrossRef] [Green Version]


| Parameters | Aspartame | 10% HFCS | 17.5% HFCS | 25% HFCS |
|---|---|---|---|---|
| Age (years) | 25.4 ± 6.2 1 | 27.7 ± 6.0 | 24.1 ± 5.0 | 26.8 ± 6.6 |
| Sex (M/F) | 11/12 | 9/9 | 7/9 | 15/13 |
| Weight (kg) | 71.8 ± 10.6 | 70.9 ± 10.3 | 69.9 ± 14.3 | 72.9 ± 14.5 |
| BMI (kg/m2) | 24.8 ± 3.3 | 24.9 ± 3.8 | 24.2 ± 3.3 | 24.9 ± 4.0 |
| Waist circumference (cm) | 75.2 ± 6.4 | 76.2 ± 9.3 | 73.3 ± 7.7 | 77.0 ± 10.1 |
| Body fat (%) | 27.1 ± 9.8 | 26.8 ± 9.8 | 25.9 ± 9.9 | 26.0 ± 9.7 |
| Energy requirement (kcal/d) | 2354 ± 322 | 2323 ± 247 | 2326 ± 375 | 2390 ± 350 |
| Systolic blood pressure (mmHg) | 112.4 ± 11.5 | 114.9 ± 9.8 | 114.9 ± 8.4 | 117.1 ± 10.0 |
| Diastolic blood pressure (mmHg) | 69.2 ± 8.6 | 72.8 ± 8.2 | 71.3 ± 4.7 | 72.7 ± 7.2 |
| FST triglyceride (mmol/L) | 1.1 ± 0.6 | 1.4 ± 0.8 | 1.2 ± 0.6 | 1.2 ± 0.6 |
| FST total cholesterol (mmol/L) | 3.9 ± 0.7 | 4.2 ± 0.7 | 4.3 ± 0.9 | 4.1 ± 0.9 |
| FST HDL-cholesterol (mmol/L) | 1.02 ± 0.19 | 1.14 ± 0.29 | 1.20 ± 0.24 | 1.18 ± 0.35 |
| FST glucose (mmol/L) | 5.02 ± 0.37 | 4.96 ± 0.31 | 4.98 ± 0.34 | 5.03 ± 0.35 |
| FST insulin (pmol/L) | 88.2 ± 37.5 | 83.3 ± 23.6 | 79.2 ± 20.8 | 90.3 ± 37.5 |
| MSRF | 1.2 ± 1.0 | 0.9 ± 0.8 | 0.7 ± 1.0 | 1.1 ± 1.0 |
| FST AST (U/L) | 23.1 ± 8.4 | 25.3 ± 8.5 | 22.1 ± 5.2 | 22.1 ± 5.6 |
| FST ALT (U/L) | 23.4 ± 20.1 | 22.2 ± 5.9 | 20.6 ± 8.1 | 21.5 ± 8.9 |
| Outcome | Aspartame (n = 23) | 10% HFCS (n = 18) | 17.5% HFCS (n = 16) | 25% HFCS (n = 28) | Effect of | p-Value |
|---|---|---|---|---|---|---|
| Hepatic Lipid Content (MRI-PDFF, %) | Dose 2 | 0.016 | ||||
| Baseline | 1.6 ± 0.8 1 | 1.2 ± 0.2 | 1.4 ± 0.3 | 2.3 ± 0.8 | Sex 3 | 0.13 |
| Intervention | 1.4 ± 0.7 | 1.3 ± 0.2 | 1.6 ± 0.3 | 2.8 ± 0.9 | MSRF 4 | 0.033 |
| Predicted M ISI (arbitrary units) | Dose | 0.0072 | ||||
| Baseline | 1.52 ± 0.06 | 1.54 ± 0.06 | 1.60 ± 0.06 | 1.52 ± 0.05 | Sex | 0.44 |
| Intervention | 1.55 ± 0.05 | 1.54 ± 0.05 | 1.56 ± 0.06 | 1.45 ± 0.07 | MSRF | 0.33 |
| Matsuda ISI (arbitrary units) | Dose | 0.0087 | ||||
| Baseline | 3.6 ± 0.3 | 3.7 ± 0.2 | 3.5 ± 0.2 | 3.3 ± 0.2 | Sex | 0.67 |
| Intervention | 3.9 ± 0.3 | 3.5 ± 0.3 | 3.2 ± 0.3 | 3.0 ± 0.3 | MSRF | 0.59 |
| OGTT Glucose AUC (mmol/L × 3 h) | Dose | 0.0004 | ||||
| Baseline | 20.9 ± 0.7 | 19.2 ± 0.8 | 19.1 ± 1.0 | 21.1 ± 0.7 | Sex | 0.75 |
| Intervention | 19.9 ± 0.7 | 19.7 ± 0.9 | 20.3 ± 1.2 | 22.5 ± 0.9 | MSRF | 0.99 |
| OGTT Insulin AUC (pmol/L × 3 h) | Dose | 0.0004 | ||||
| Baseline | 1392 ± 184 | 1033 ± 65 | 1292 ± 162 | 1335 ± 106 | Sex | 0.89 |
| Intervention | 1229 ± 151 | 1188 ± 97 | 1376 ± 165 | 1625 ± 154 | MSRF | 0.31 |
| HOMA-IR (arbitrary units) | Dose | 0.11 | ||||
| Baseline | 3.0 ± 0.2 | 3.0 ± 0.3 | 2.5 ± 0.2 | 3.0 ± 0.2 | Sex | 0.97 |
| Intervention | 2.9 ± 0.2 | 2.9 ± 0.3 | 2.8 ± 0.2 | 3.3 ± 0.3 | MSRF | 0.0087 |
| Lactate AMP (mmol/L) | Dose | <0.0001 | ||||
| Baseline | 0.68 ± 0.06 | 0.62 ± 0.06 | 0.69 ± 0.06 | 0.73 ± 0.06 | Sex | 0.66 |
| Intervention | 0.73 ± 0.04 | 1.01 ± 0.07 | 1.50 ± 0.11 | 1.60 ± 0.09 | MSRF | 0.61 |
| Glucose AMP (mmol/L) | Dose | <0.0001 | ||||
| Baseline | 1.77 ± 0.19 | 1.88 ± 0.18 | 1.90 ± 0.22 | 2.04 ± 0.18 | Sex | 0.17 |
| Intervention | 1.87 ± 0.19 | 2.30 ± 0.16 | 2.63 ± 0.18 | 2.96 ± 0.21 | MSRF | 0.69 |
| Insulin AMP (pmol/L) | Dose | 0.095 | ||||
| Baseline | 544.5. ± 84.0 | 424.3 ± 46.5 | 525.0 ± 62.5 | 506.9 ± 45.1 | Sex | 0.80 |
| Intervention | 567.4 ± 75.7 | 450.7 ± 35.4 | 588.9 ± 54.2 | 595.9 ± 48.6 | MSRF | 0.19 |
| Body Weight (kg) | Dose | 0.017 | ||||
| Baseline | 71.8 ± 2.2 | 70.9 ± 2.4 | 69.9 ± 3.6 | 72.9 ± 2.7 | Sex | 0.079 |
| Intervention | 71.7 ± 2.2 | 70.9 ± 2.4 | 70.2 ± 3.7 | 73.7 ± 2.8 | MSRF | 0.15 |
| HFCS Dose | Hepatic Lipid Content | Predicted M ISI | Matsuda ISI | Glucose OGTT AUC | Insulin OGTT AUC | Uric Acid 24 h AUC | Post-Meal Lactate AMP | Post-Meal Glucose AMP | apoCIII PP | TG 24 h AUC | TG PP | Body Weight | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HFCS dose | R | 1.000 | 0.256 | −0.285 | −0.238 | 0.372 | 0.371 | 0.608 | 0.632 | 0.406 | 0.385 | 0.319 | 0.521 | 0.303 |
| p-value | 0.034 | 0.018 | 0.049 | 0.002 | 0.002 | <0.0001 | <0.0001 | 0.001 | 0.001 | 0.008 | <0.0001 | 0.011 | ||
| Hepatic Lipid Content | R | 0.256 | 1.000 | −0.236 | −0.232 | 0.148 | 0.263 | 0.308 | 0.119 | 0.276 | 0.547 | 0.440 | 0.337 | 0.290 |
| p-value | 0.034 | 0.051 | 0.056 | 0.226 | 0.029 | 0.010 | 0.330 | 0.022 | <0.0001 | 0.000 | 0.005 | 0.016 | ||
| Predicted M ISI | R | −0.285 | −0.236 | 1.000 | 0.672 | −0.638 | −0.582 | −0.191 | −0.192 | −0.057 | −0.382 | −0.192 | −0.215 | −0.152 |
| p-value | 0.018 | 0.051 | <0.0001 | <0.0001 | <0.0001 | 0.115 | 0.114 | 0.643 | 0.001 | 0.115 | 0.076 | 0.213 | ||
| Matsuda ISI | R | −0.238 | −0.232 | 0.672 | 1.000 | −0.502 | −0.690 | −0.096 | −0.293 | −0.123 | −0.233 | −0.016 | −0.127 | 0.163 |
| p-value | 0.049 | 0.056 | <0.0001 | <0.0001 | <0.0001 | 0.435 | 0.015 | 0.314 | 0.054 | 0.898 | 0.299 | 0.182 | ||
| Glucose OGTT AUC | R | 0.372 | 0.148 | −0.638 | −0.502 | 1.000 | 0.597 | 0.193 | 0.295 | 0.249 | 0.163 | 0.126 | 0.168 | −0.013 |
| p-value | 0.002 | 0.226 | <0.0001 | <0.0001 | <0.0001 | 0.113 | 0.014 | 0.039 | 0.181 | 0.302 | 0.168 | 0.916 | ||
| Insulin OGTT AUC | R | 0.371 | 0.263 | −0.582 | −0.690 | 0.597 | 1.000 | 0.157 | 0.305 | 0.161 | 0.181 | 0.055 | 0.196 | −0.055 |
| p-value | 0.002 | 0.029 | <0.0001 | <0.0001 | <0.0001 | 0.199 | 0.011 | 0.187 | 0.136 | 0.652 | 0.107 | 0.656 | ||
| Uric acid 24 h AUC | R | 0.608 | 0.308 | −0.191 | −0.096 | 0.193 | 0.157 | 1.000 | 0.304 | 0.314 | 0.296 | 0.166 | 0.259 | 0.347 |
| p-value | <0.0001 | 0.010 | 0.115 | 0.435 | 0.113 | 0.199 | 0.011 | 0.009 | 0.014 | 0.172 | 0.032 | 0.003 | ||
| Post-meal Lactate AMP | R | 0.632 | 0.119 | −0.192 | −0.293 | 0.295 | 0.305 | 0.304 | 1.000 | 0.464 | 0.230 | 0.124 | 0.266 | 0.020 |
| p-value | <0.0001 | 0.330 | 0.114 | 0.015 | 0.014 | 0.011 | 0.011 | <0.0001 | 0.058 | 0.311 | 0.027 | 0.868 | ||
| Post-meal Glucose AMP | R | 0.406 | 0.276 | −0.057 | −0.123 | 0.249 | 0.161 | 0.314 | 0.464 | 1.000 | 0.191 | 0.261 | 0.301 | 0.059 |
| p-value | 0.001 | 0.022 | 0.643 | 0.314 | 0.039 | 0.187 | 0.009 | <0.0001 | 0.116 | 0.030 | 0.012 | 0.628 | ||
| apoCIII PP | R | 0.385 | 0.547 | −0.382 | −0.233 | 0.163 | 0.181 | 0.296 | 0.230 | 0.191 | 1.000 | 0.723 | 0.640 | 0.252 |
| p-value | 0.001 | <0.0001 | 0.001 | 0.054 | 0.181 | 0.136 | 0.014 | 0.058 | 0.116 | <0.0001 | <0.0001 | 0.037 | ||
| TG 24 h AUC | R | 0.319 | 0.440 | −0.192 | −0.016 | 0.126 | 0.055 | 0.166 | 0.124 | 0.261 | 0.723 | 1.000 | 0.772 | 0.285 |
| p-value | 0.008 | 0.000 | 0.115 | 0.898 | 0.302 | 0.652 | 0.172 | 0.311 | 0.030 | <0.0001 | <0.0001 | 0.018 | ||
| TG PP | R | 0.521 | 0.337 | −0.215 | −0.127 | 0.168 | 0.196 | 0.259 | 0.266 | 0.301 | 0.640 | 0.772 | 1.000 | 0.184 |
| p-value | <0.0001 | 0.005 | 0.076 | 0.299 | 0.168 | 0.107 | 0.032 | 0.027 | 0.012 | <0.0001 | <0.0001 | 0.130 | ||
| Body weight | R | 0.303 | 0.290 | −0.152 | 0.163 | −0.013 | −0.055 | 0.347 | 0.020 | 0.059 | 0.252 | 0.285 | 0.184 | 1.000 |
| p-value | 0.011 | 0.016 | 0.213 | 0.182 | 0.916 | 0.656 | 0.003 | 0.868 | 0.628 | 0.037 | 0.018 | 0.130 |
| Outcome (Absolute Change) | R for Effect of HFCS Dose w/o Mediator | Mediator (Absolute Change) | R for Effect of HFCS Dose w/Mediator | Attenuation (%) |
|---|---|---|---|---|
| OGTT Insulin | 0.371 | Hepatic Lipid Content | 0.314 | 15 |
| Hepatic Lipid Content | 0.256 | OGTT Insulin | 0.166 | 35 |
| Postprandial apoCIII | 0.385 | Hepatic Lipid Content | 0.25 | 35 |
| Hepatic Lipid Content | 0.259 | Postprandial apoCIII | 0.048 | 81 |
| Uric acid 24h AUC | 0.608 | Hepatic Lipid Content | 0.536 | 12 |
| Hepatic Lipid Content | 0.256 | Uric acid 24h AUC | 0.084 | 67 |
| Post-meal glucose AMP | 0.406 | Hepatic Lipid Content | 0.338 | 17 |
| Hepatic Lipid Content | 0.256 | Post-meal glucose AMP | 0.153 | 40 |
| Body weight | 0.303 | Hepatic Lipid Content | 0.23 | 24 |
| Hepatic Lipid Content | 0.256 | Body weight | 0.171 | 33 |
| Predicted M ISI | −0.285 | Postprandial apoCIII | −0.149 | 48 |
| Postprandial apoCIII | 0.385 | Predicted M ISI | 0.284 | 26 |
| Matsuda | −0.238 | Post-meal lactate AMP | −0.068 | 71 |
| Post-meal lactate AMP | 0.632 | Matsuda | 0.576 | 9 |
| OGTT Insulin | 0.371 | Post-meal lactate AMP | 0.23 | 38 |
| Post-meal lactate AMP | 0.632 | OGTT Insulin | 0.56 | 12 |
| OGTT Glucose | 0.372 | Post-meal lactate AMP | 0.238 | 36 |
| Post-meal lactate AMP | 0.632 | OGTT Insulin | 0.556 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sigala, D.M.; Hieronimus, B.; Medici, V.; Lee, V.; Nunez, M.V.; Bremer, A.A.; Cox, C.L.; Price, C.A.; Benyam, Y.; Abdelhafez, Y.; et al. The Dose-Response Effects of Consuming High Fructose Corn Syrup-Sweetened Beverages on Hepatic Lipid Content and Insulin Sensitivity in Young Adults. Nutrients 2022, 14, 1648. https://doi.org/10.3390/nu14081648
Sigala DM, Hieronimus B, Medici V, Lee V, Nunez MV, Bremer AA, Cox CL, Price CA, Benyam Y, Abdelhafez Y, et al. The Dose-Response Effects of Consuming High Fructose Corn Syrup-Sweetened Beverages on Hepatic Lipid Content and Insulin Sensitivity in Young Adults. Nutrients. 2022; 14(8):1648. https://doi.org/10.3390/nu14081648
Chicago/Turabian StyleSigala, Desiree M., Bettina Hieronimus, Valentina Medici, Vivien Lee, Marinelle V. Nunez, Andrew A. Bremer, Chad L. Cox, Candice A. Price, Yanet Benyam, Yasser Abdelhafez, and et al. 2022. "The Dose-Response Effects of Consuming High Fructose Corn Syrup-Sweetened Beverages on Hepatic Lipid Content and Insulin Sensitivity in Young Adults" Nutrients 14, no. 8: 1648. https://doi.org/10.3390/nu14081648
APA StyleSigala, D. M., Hieronimus, B., Medici, V., Lee, V., Nunez, M. V., Bremer, A. A., Cox, C. L., Price, C. A., Benyam, Y., Abdelhafez, Y., McGahan, J. P., Keim, N. L., Goran, M. I., Pacini, G., Tura, A., Sirlin, C. B., Chaudhari, A. J., Havel, P. J., & Stanhope, K. L. (2022). The Dose-Response Effects of Consuming High Fructose Corn Syrup-Sweetened Beverages on Hepatic Lipid Content and Insulin Sensitivity in Young Adults. Nutrients, 14(8), 1648. https://doi.org/10.3390/nu14081648









