Exploring the Gut Microbiome in Myasthenia Gravis
Abstract
1. Introduction
2. Myasthenia Gravis—The Pathogenesis, Risk Factors, and Clinical Manifestations
2.1. Pathogenesis of MG
2.2. Infections as a Risk Factor for MG
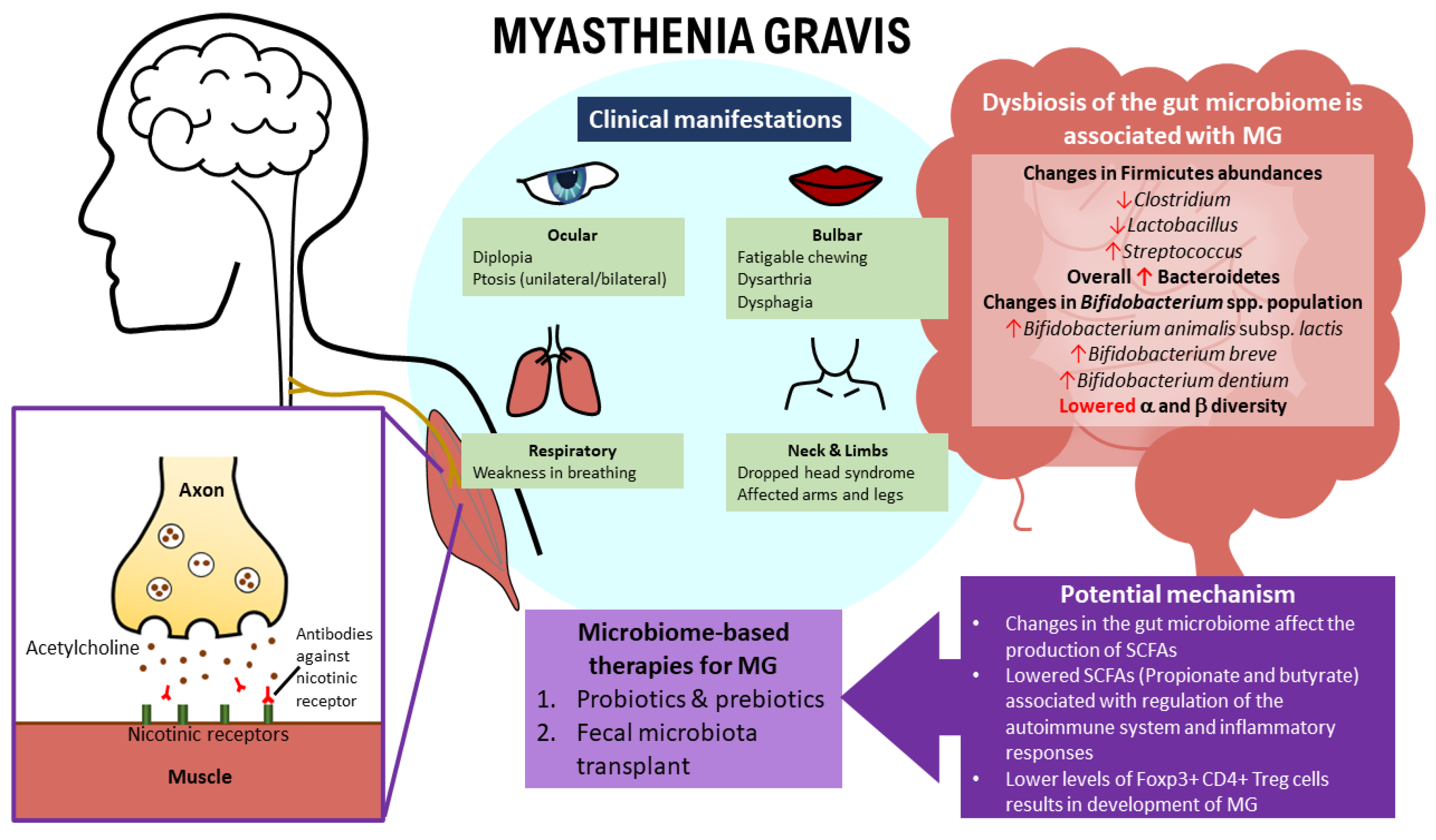
2.3. Clinical Manifestations of MG
3. Human Gut Microbiome and Its Relation with Human Wellbeing
3.1. The Relationship between MG and the Gut Microbiome
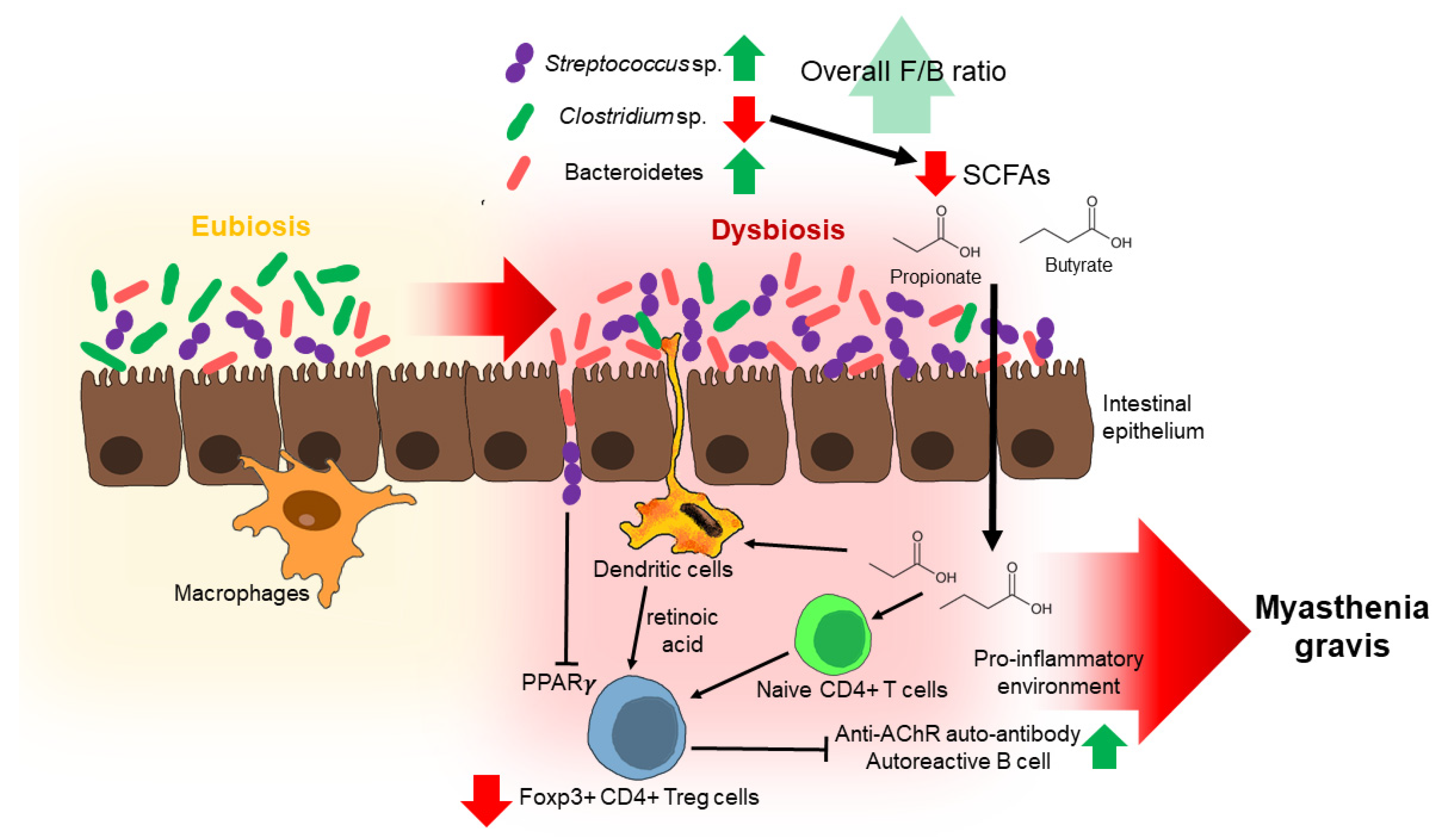
3.2. Gut Microbiota Composition between HCs and MG Patients
Possible Mechanisms by Which Some Gut Microbiota May Contribute to MG Development
4. The Relationship between Gut Microbiome Dysbiosis and Biomarkers in MG Patients
5. Alterations in Fecal Metabolome of MG Patients
6. Link between Gut Microbial OTUs with Metabolites and Some Clinical Characteristics of MG
7. Insights and Future Perspectives on the Treatment of MG Based on Gut Microbiome Modulation
7.1. Probiotics
7.2. Prebiotics
7.3. Intervention of the Gut Microbiome by Fecal Microbiota Transplantation
Alterations in the MG Microbiota of Mice after a Fecal Microbiota Transplant
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilhus, N.E.; Romi, F.; Hong, Y.; Skeie, G.O. Myasthenia gravis and infectious disease. J. Neurol. 2018, 265, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Jaretzki, A.; Barohn, R.J.; Ernstoff, R.M.; Kaminski, H.J.; Keesey, J.C.; Penn, A.S.; Sanders, D.B. Myasthenia gravis: Recommendations for clinical research standards. Neurology 2000, 55, 16–23. [Google Scholar] [PubMed]
- Jayam Trouth, A.; Dabi, A.; Solieman, N.; Kurukumbi, M.; Kalyanam, J. Myasthenia gravis: A review. Autoimmune Dis. 2012, 2012, 874680. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E.; Owe, J.F.; Hoff, J.M.; Romi, F.; Skeie, G.O.; Aarli, J.A. Myasthenia gravis: A review of available treatment approaches. Autoimmune Dis. 2011, 2011, 847393. [Google Scholar] [CrossRef]
- Gilhus, N.E. Myasthenia gravis. N. Engl. J. Med. 2016, 375, 2570–2581. [Google Scholar] [CrossRef]
- Gilhus, N.E.; Skeie, G.O.; Romi, F.; Lazaridis, K.; Zisimopoulou, P.; Tzartos, S. Myasthenia gravis—Autoantibody characteristics and their implications for therapy. Nat. Rev. Neurol. 2016, 12, 259–268. [Google Scholar] [CrossRef]
- Evoli, A. Myasthenia gravis: New developments in research and treatment. Curr. Opin. Neurol. 2017, 30, 464–470. [Google Scholar] [CrossRef]
- Bach, J.F. The etiology of autoimmune diseases: The case of myasthenia gravis. Ann. N. Y. Acad. Sci. 2012, 1274, 33–39. [Google Scholar] [CrossRef]
- Bach, J.-F. The hygiene hypothesis in autoimmunity: The role of pathogens and commensals. Nat. Rev. Immunol. 2018, 18, 105. [Google Scholar] [CrossRef]
- Grob, D.; Brunner, N.; Namba, T.; Pagala, M. Lifetime course of myasthenia gravis. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2008, 37, 141–149. [Google Scholar] [CrossRef]
- Andersen, J.; Heldal, A.; Engeland, A.; Gilhus, N. Myasthenia gravis epidemiology in a national cohort; combining multiple disease registries. Acta Neurol. Scand. 2014, 129, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.H. The epidemiology of myasthenia gravis. Ann. N. Y. Acad. Sci. 2003, 998, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.S.; Cardwell, C.R.; McCarron, P.O.; McConville, J. A systematic review of population based epidemiological studies in Myasthenia Gravis. BMC Neurol. 2010, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Alshekhlee, A.; Miles, J.; Katirji, B.; Preston, D.; Kaminski, H. Incidence and mortality rates of myasthenia gravis and myasthenic crisis in US hospitals. Neurology 2009, 72, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Bubuioc, A.-M.; Kudebayeva, A.; Turuspekova, S.; Lisnic, V.; Leone, M.A. The epidemiology of myasthenia gravis. J. Med. Life 2021, 14, 7. [Google Scholar] [CrossRef]
- Rinaldi, E.; Consonni, A.; Guidesi, E.; Elli, M.; Mantegazza, R.; Baggi, F. Gut microbiota and probiotics: Novel immune system modulators in myasthenia gravis? Ann. N. Y. Acad. Sci. 2018, 1413, 49–58. [Google Scholar] [CrossRef]
- Silvestri, N.J.; Wolfe, G.I. Myasthenia gravis. Semin. Neurol. 2012, 32, 215–226. [Google Scholar] [CrossRef]
- Diaz-Manera, J.; Rojas Garcia, R.; Illa, I. Treatment strategies for myasthenia gravis: An update. Expert Opin. Pharmacother. 2012, 13, 1873–1883. [Google Scholar] [CrossRef]
- Sanders, D.B.; Wolfe, G.I.; Benatar, M.; Evoli, A.; Gilhus, N.E.; Illa, I.; Kuntz, N.; Massey, J.M.; Melms, A.; Murai, H. International consensus guidance for management of myasthenia gravis: Executive summary. Neurology 2016, 87, 419–425. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, K.D.; Richman, D.P. Treatment of myasthenia gravis based on its immunopathogenesis. J. Clin. Neurol. 2011, 7, 173–183. [Google Scholar] [CrossRef]
- Nicolle, M.W. Myasthenia Gravis and Lambert-Eaton Myasthenic Syndrome. Continuum (Minneap. Minn) 2016, 22, 1978–2005. [Google Scholar] [CrossRef] [PubMed]
- Farmakidis, C.; Pasnoor, M.; Dimachkie, M.M.; Barohn, R.J. Treatment of myasthenia gravis. Neurol. Clin. 2018, 36, 311–337. [Google Scholar] [CrossRef] [PubMed]
- Bosch, E.; Subbiah, B.; Ross, M.A. Cholinergic crisis after conventional doses of anticholinesterase medications in chronic renal failure. Muscle Nerve 1991, 14, 1036–1037. [Google Scholar] [PubMed]
- Jowkar, A.A. Myasthenia Gravis. Medscape. Available online: https://emedicine.medscape.com/article/1171206-overview (accessed on 3 January 2022).
- Morgutti, M.; Conti-Tronconi, B.M.; Sghirlanzoni, A.; Clementi, F. Cellular immune response to acetylcholine receptor in myasthenia gravis: II. Thymectomy and corticosteroids. Neurology 1979, 29, 734. [Google Scholar] [CrossRef]
- Conti-Fine, B.M.; Milani, M.; Kaminski, H.J. Myasthenia gravis: Past, present, and future. J. Clin. Investig. 2006, 116, 2843–2854. [Google Scholar] [CrossRef]
- Christadoss, P.; Goluszko, E. Treatment of experimental autoimmune myasthenia gravis with recombinant human tumor necrosis factor receptor Fc protein. J. Neuroimmunol. 2002, 122, 186–190. [Google Scholar] [CrossRef]
- Feferman, T.; Maiti, P.K.; Berrih-Aknin, S.; Bismuth, J.; Bidault, J.; Fuchs, S.; Souroujon, M.C. Overexpression of IFN-induced protein 10 and its receptor CXCR3 in myasthenia gravis. J. Immunol. 2005, 174, 5324–5331. [Google Scholar] [CrossRef]
- Shi, F.-D.; Wang, H.-B.; Li, H.; Hong, S.; Taniguchi, M.; Link, H.; Van Kaer, L.; Ljunggren, H.-G. Natural killer cells determine the outcome of B cell–mediated autoimmunity. Nat. Immunol. 2000, 1, 245–251. [Google Scholar] [CrossRef]
- Jander, S.; Stoll, G. Increased serum levels of the interferon-γ–inducing cytokine interleukin-18 in myasthenia gravis. Neurology 2002, 59, 287–289. [Google Scholar] [CrossRef]
- Blum, S.; Lee, D.; Gillis, D.; McEniery, D.F.; Reddel, S.; McCombe, P. Clinical features and impact of myasthenia gravis disease in Australian patients. J. Clin. Neurosci. 2015, 22, 1164–1169. [Google Scholar] [CrossRef]
- Gui, M.; Luo, X.; Lin, J.; Li, Y.; Zhang, M.; Zhang, X.; Yang, M.; Wang, W.; Bu, B. Long-term outcome of 424 childhood-onset myasthenia gravis patients. J. Neurol. 2015, 262, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Kalita, J.; Kohat, A.; Misra, U. Predictors of outcome of myasthenic crisis. Neurol. Sci. 2014, 35, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.; Wim, R.; Peter, V.E.; Tanja, R.; Jan, T.; Werner, V.S. Myasthenia gravis: Another autoimmune disease associated with hepatitis C virus infection. Dig. Dis. Sci. 1999, 44, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-L.; Lin, Y.-H.; Wang, P.-Y.; Chang, M.-H. HIV-associated myasthenia gravis and impacts of HAART: One case report and a brief review. Clin. Neurol. Neurosurg. (Dutch-Flemish Ed.) 2011, 113, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Levy, Y. Geoepidemiology of myasthenia gravis [corrected]. Autoimmun. Rev. 2010, 9, A383–A386. [Google Scholar] [CrossRef] [PubMed]
- Stübgen, J.P. Neuromuscular disorders associated with Hepatitis B vaccination. J. Neurol. Sci. 2010, 292, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Leis, A.A.; Szatmary, G.; Ross, M.A.; Stokic, D.S. West nile virus infection and myasthenia gravis. Muscle Nerve 2014, 49, 26–29. [Google Scholar] [CrossRef]
- Berrih-Aknin, S.; Le Panse, R. Myasthenia gravis: A comprehensive review of immune dysregulation and etiological mechanisms. J. Autoimmun. 2014, 52, 90–100. [Google Scholar] [CrossRef]
- Maniaol, A.H.; Elsais, A.; Lorentzen, Å.R.; Owe, J.F.; Viken, M.K.; Sæther, H.; Flåm, S.T.; Bråthen, G.; Kampman, M.T.; Midgard, R.; et al. Late onset myasthenia gravis is associated with HLA DRB1*15:01 in the Norwegian population. PLoS ONE 2012, 7, e36603. [Google Scholar] [CrossRef]
- Marx, A.; Pfister, F.; Schalke, B.; Saruhan-Direskeneli, G.; Melms, A.; Ströbel, P. The different roles of the thymus in the pathogenesis of the various myasthenia gravis subtypes. Autoimmun. Rev. 2013, 12, 875–884. [Google Scholar] [CrossRef]
- Romi, F.; Bø, L.; Skeie, G.O.; Myking, A.; Aarli, J.A.; Gilhus, N.E. Titin and ryanodine receptor epitopes are expressed in cortical thymoma along with costimulatory molecules. J. Neuroimmunol. 2002, 128, 82–89. [Google Scholar] [CrossRef]
- Köhling, H.L.; Plummer, S.F.; Marchesi, J.R.; Davidge, K.S.; Ludgate, M. The microbiota and autoimmunity: Their role in thyroid autoimmune diseases. Clin. Immunol. 2017, 183, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Münz, C.; Lünemann, J.D.; Getts, M.T.; Miller, S.D. Antiviral immune responses: Triggers of or triggered by autoimmunity? Nat. Rev. Immunol. 2009, 9, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Pestronk, A.; Drachman, D.B.; Self, S.G. Measurement of junctional acetylcholine receptors in myasthenia gravis: Clinical correlates. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 1985, 8, 245–251. [Google Scholar] [CrossRef]
- Grob, D.; Arsura, E.L.; Brunner, N.G.; Namba, T. The course of myasthenia gravis and therapies affecting outcome. Ann. N. Y. Acad. Sci. 1987, 505, 472–499. [Google Scholar] [CrossRef] [PubMed]
- Crisp, S.J.; Kullmann, D.M.; Vincent, A. Autoimmune synaptopathies. Nat. Rev. Neurosci. 2016, 17, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Sanyal, D. Jaw muscle weakness: A differential indicator of neuromuscular weakness—Preliminary observations. Muscle Nerve 2011, 43, 807–811. [Google Scholar] [CrossRef]
- Grob, D. Course and management of myasthenia gravis. J. Am. Med. Assoc. 1953, 153, 529–532. [Google Scholar] [CrossRef]
- Werner, P.; Kiechl, S.; Löscher, W.; Poewe, W.; Willeit, J. Distal myasthenia gravis–frequency and clinical course in a large prospective series. Acta Neurol. Scand. 2003, 108, 209–211. [Google Scholar] [CrossRef]
- Lederberg, J.; McCray, A.T. Ome SweetOmics—A genealogical treasury of words. Scientist 2001, 15, 8. [Google Scholar]
- Collins, S.; Reid, G. Distant site effects of ingested prebiotics. Nutrients 2016, 8, 523. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J.; Michel, C. How to manipulate the microbiota: Prebiotics. In Microbiota of the Human Body; Springer: Berlin/Heidelberg, Germany, 2016; pp. 119–142. [Google Scholar]
- Sender, R.; Fuchs, S.; Milo, R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Sommer, F.; Bäckhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; de Agüero, M.G.; Ganal-Vonarburg, S.C. How nutrition and the maternal microbiota shape the neonatal immune system. Nat. Rev. Immunol. 2017, 17, 508. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Trinchieri, G. Microbiota: A key orchestrator of cancer therapy. Nat. Rev. Cancer 2017, 17, 271–285. [Google Scholar] [CrossRef]
- Zitvogel, L.; Ma, Y.; Raoult, D.; Kroemer, G.; Gajewski, T.F. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science 2018, 359, 1366–1370. [Google Scholar] [CrossRef]
- Pflughoeft, K.J.; Versalovic, J. Human microbiome in health and disease. Annu. Rev. Pathol. Mech. Dis. 2012, 7, 99–122. [Google Scholar] [CrossRef]
- Lederberg, J. Infectious history. Science 2000, 288, 287–293. [Google Scholar] [CrossRef]
- Gill, S.R.; Pop, M.; DeBoy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef]
- Kurokawa, K.; Itoh, T.; Kuwahara, T.; Oshima, K.; Toh, H.; Toyoda, A.; Takami, H.; Morita, H.; Sharma, V.K.; Srivastava, T.P. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res. 2007, 14, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; Zhu, W.; Sartor, R.B.; Li, E. Investigating the biological and clinical significance of human dysbioses. Trends Microbiol. 2011, 19, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Delzenne, N.M. Interplay between obesity and associated metabolic disorders: New insights into the gut microbiota. Curr. Opin. Pharmacol. 2009, 9, 737–743. [Google Scholar] [CrossRef]
- Jumpertz, R.; Le, D.S.; Turnbaugh, P.J.; Trinidad, C.; Bogardus, C.; Gordon, J.I.; Krakoff, J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 2011, 94, 58–65. [Google Scholar] [CrossRef]
- Claus, S.P.; Tsang, T.M.; Wang, Y.; Cloarec, O.; Skordi, E.; Martin, F.P.; Rezzi, S.; Ross, A.; Kochhar, S.; Holmes, E. Systemic multicompartmental effects of the gut microbiome on mouse metabolic phenotypes. Mol. Syst. Biol. 2008, 4, 219. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; Van Den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Saulnier, D.M.; Santos, F.; Roos, S.; Mistretta, T.-A.; Spinler, J.K.; Molenaar, D.; Teusink, B.; Versalovic, J. Exploring metabolic pathway reconstruction and genome-wide expression profiling in Lactobacillus reuteri to define functional probiotic features. PLoS ONE 2011, 6, e18783. [Google Scholar] [CrossRef]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Clarke, G.; O’Mahony, S.; Dinan, T.; Cryan, J. Priming for health: Gut microbiota acquired in early life regulates physiology, brain and behaviour. Acta Paediatr. 2014, 103, 812–819. [Google Scholar] [CrossRef]
- Neuman, H.; Debelius, J.W.; Knight, R.; Koren, O. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 2015, 39, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef]
- Wen, L.; Ley, R.E.; Volchkov, P.Y.; Stranges, P.B.; Avanesyan, L.; Stonebraker, A.C.; Hu, C.; Wong, F.S.; Szot, G.L.; Bluestone, J.A. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 2008, 455, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; San Yeoh, B.; Chassaing, B.; Xiao, X.; Saha, P.; Olvera, R.A.; Lapek, J.D., Jr.; Zhang, L.; Wang, W.-B.; Hao, S. Dysregulated microbial fermentation of soluble fiber induces cholestatic liver cancer. Cell 2018, 175, 679–694.e22. [Google Scholar] [CrossRef]
- Benakis, C.; Brea, D.; Caballero, S.; Faraco, G.; Moore, J.; Murphy, M.; Sita, G.; Racchumi, G.; Ling, L.; Pamer, E.G. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med. 2016, 22, 516–523. [Google Scholar] [CrossRef]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 2018, 173, 1728–1741.e13. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The central nervous system and the gut microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Liu, M.; Chen, J.; Pan, J.; Han, Y.; Liu, Y.; Cheng, K.; Zhou, C.; Wang, H. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci. Adv. 2019, 5, eaau8317. [Google Scholar] [CrossRef]
- Mayer, E.A. Gut feelings: The emerging biology of gut–brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef]
- Bienenstock, J.; Collins, S. 99th Dahlem Conference on Infection, Inflammation and Chronic Inflammatory Disorders: Psycho-neuroimmunology and the intestinal microbiota: Clinical observations and basic mechanisms. Clin. Exp. Immunol. 2010, 160, 85–91. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Schemann, M. Control of gastrointestinal motility by the “gut brain”-the enteric nervous system. J. Pediatric Gastroenterol. Nutr. 2005, 41, S4–S6. [Google Scholar] [CrossRef]
- Iyer, L.M.; Aravind, L.; Coon, S.L.; Klein, D.C.; Koonin, E.V. Evolution of cell–cell signaling in animals: Did late horizontal gene transfer from bacteria have a role? TRENDS Genet. 2004, 20, 292–299. [Google Scholar] [CrossRef]
- Forsythe, P.; Sudo, N.; Dinan, T.; Taylor, V.H.; Bienenstock, J. Mood and gut feelings. Brain Behav. Immun. 2010, 24, 9–16. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Ochoa-Repáraz, J.; Mielcarz, D.W.; Begum-Haque, S.; Kasper, L.H. Gut, bugs, and brain: Role of commensal bacteria in the control of central nervous system disease. Ann. Neurol. 2011, 69, 240–247. [Google Scholar] [CrossRef]
- Berer, K.; Mues, M.; Koutrolos, M.; Al Rasbi, Z.; Boziki, M.; Johner, C.; Wekerle, H.; Krishnamoorthy, G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011, 479, 538–541. [Google Scholar] [CrossRef]
- Lavasani, S.; Dzhambazov, B.; Nouri, M.; Fåk, F.; Buske, S.; Molin, G.; Thorlacius, H.; Alenfall, J.; Jeppsson, B.; Weström, B. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS ONE 2010, 5, e9009. [Google Scholar] [CrossRef]
- Yokote, H.; Miyake, S.; Croxford, J.L.; Oki, S.; Mizusawa, H.; Yamamura, T. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am. J. Pathol. 2008, 173, 1714–1723. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Repáraz, J.; Mielcarz, D.W.; Ditrio, L.E.; Burroughs, A.R.; Foureau, D.M.; Haque-Begum, S.; Kasper, L.H. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2009, 183, 6041–6050. [Google Scholar] [CrossRef] [PubMed]
- Abegunde, A.T.; Muhammad, B.H.; Bhatti, O.; Ali, T. Environmental risk factors for inflammatory bowel diseases: Evidence based literature review. World J. Gastroenterol. 2016, 22, 6296. [Google Scholar] [CrossRef] [PubMed]
- Alverdy, J.C.; Luo, J.N. The influence of host stress on the mechanism of infection: Lost microbiomes, emergent pathobiomes, and the role of interkingdom signaling. Front. Microbiol. 2017, 8, 322. [Google Scholar] [CrossRef]
- Zheng, P.; Li, Y.; Wu, J.; Zhang, H.; Huang, Y.; Tan, X.; Pan, J.; Duan, J.; Liang, W.; Yin, B.; et al. Perturbed Microbial Ecology in Myasthenia Gravis: Evidence from the Gut Microbiome and Fecal Metabolome. Adv. Sci. 2019, 6, 1901441. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef]
- Hindson, J. A possible link between multiple sclerosis and gut microbiota. Nat. Rev. Neurol. 2017, 13, 705. [Google Scholar] [CrossRef]
- De Groot, P.F.; Belzer, C.; Aydin, Ö.; Levin, E.; Levels, J.H.; Aalvink, S.; Boot, F.; Holleman, F.; Van Raalte, D.H.; Scheithauer, T.P. Distinct fecal and oral microbiota composition in human type 1 diabetes, an observational study. PLoS ONE 2017, 12, e0188475. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Z.Z.; He, Y.; Yang, Y.; Liu, L.; Lin, Q.; Nie, Y.; Li, M.; Zhi, F.; Liu, S. Gut microbiota offers universal biomarkers across ethnicity in inflammatory bowel disease diagnosis and infliximab response prediction. MSystems 2018, 3, e00188-17. [Google Scholar] [CrossRef]
- Chen, J.; Wright, K.; Davis, J.M.; Jeraldo, P.; Marietta, E.V.; Murray, J.; Nelson, H.; Matteson, E.L.; Taneja, V. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Jangi, S.; Gandhi, R.; Cox, L.; Li, N.; Von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.; Liu, S.; Glanz, B. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef] [PubMed]
- Breban, M.; Tap, J.; Leboime, A.; Said-Nahal, R.; Langella, P.; Chiocchia, G.; Furet, J.-P.; Sokol, H. Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann. Rheum. Dis. 2017, 76, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Xia, Z.; Jiao, X.; Deng, J.; Zhang, L.; Li, J. Altered Gut Microbiota in Myasthenia Gravis. Front. Microbiol. 2018, 9, 2627. [Google Scholar] [CrossRef]
- Mu, L.; Sun, B.; Kong, Q.; Wang, J.; Wang, G.; Zhang, S.; Wang, D.; Liu, Y.; Liu, Y.; An, H. Disequilibrium of T helper type 1, 2 and 17 cells and regulatory T cells during the development of experimental autoimmune myasthenia gravis. Immunology 2009, 128, e826–e836. [Google Scholar] [CrossRef]
- Aricha, R.; Mizrachi, K.; Fuchs, S.; Souroujon, M.C. Blocking of IL-6 suppresses experimental autoimmune myasthenia gravis. J. Autoimmun. 2011, 36, 135–141. [Google Scholar] [CrossRef]
- Fattorossi, A.; Battaglia, A.; Buzzonetti, A.; Ciaraffa, F.; Scambia, G.; Evoli, A. Circulating and thymic CD4+ CD25+ T regulatory cells in myasthenia gravis: Effect of immunosuppressive treatment. Immunology 2005, 116, 134–141. [Google Scholar] [CrossRef]
- Li, X.; Xiao, B.-G.; Xi, J.-Y.; Lu, C.-Z.; Lu, J.-H. Decrease of CD4+ CD25highFoxp3+ regulatory T cells and elevation of CD19+ BAFF-R+ B cells and soluble ICAM-1 in myasthenia gravis. Clin. Immunol. 2008, 126, 180–188. [Google Scholar] [CrossRef]
- Masuda, M.; Matsumoto, M.; Tanaka, S.; Nakajima, K.; Yamada, N.; Ido, N.; Ohtsuka, T.; Nishida, M.; Hirano, T.; Utsumi, H. Clinical implication of peripheral CD4+ CD25+ regulatory T cells and Th17 cells in myasthenia gravis patients. J. Neuroimmunol. 2010, 225, 123–131. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef]
- Nagano, Y.; Itoh, K.; Honda, K. The induction of Treg cells by gut-indigenous Clostridium. Curr. Opin. Immunol. 2012, 24, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Feng, T.; Fujihashi, K.; Schoeb, T.R.; Elson, C.O. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc. Natl. Acad. Sci. USA 2009, 106, 19256–19261. [Google Scholar] [CrossRef] [PubMed]
- Lathrop, S.K.; Bloom, S.M.; Rao, S.M.; Nutsch, K.; Lio, C.-W.; Santacruz, N.; Peterson, D.A.; Stappenbeck, T.S.; Hsieh, C.-S. Peripheral education of the immune system by colonic commensal microbiota. Nature 2011, 478, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.S.; Jordan, A.; Basu, S.; Thomas, R.M.; Bandyopadhyay, S.; De Zoeten, E.F.; Wells, A.D.; Macian, F. Regulatory T cells suppress CD 4+ T cells through NFAT-dependent transcriptional mechanisms. EMBO Rep. 2014, 15, 991–999. [Google Scholar] [CrossRef]
- Yarza, P.; Yilmaz, P.; Pruesse, E.; Glöckner, F.O.; Ludwig, W.; Schleifer, K.-H.; Whitman, W.B.; Euzéby, J.; Amann, R.; Rosselló-Móra, R. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat. Rev. Microbiol. 2014, 12, 635–645. [Google Scholar] [CrossRef]
- Stewart, C.J.; Mansbach, J.M.; Wong, M.C.; Ajami, N.J.; Petrosino, J.F.; Camargo, C.A., Jr.; Hasegawa, K. Associations of nasopharyngeal metabolome and microbiome with severity among infants with bronchiolitis. A multiomic analysis. Am. J. Respir. Crit. Care Med. 2017, 196, 882–891. [Google Scholar] [CrossRef]
- Moris, G.; Arboleya, S.; Mancabelli, L.; Milani, C.; Ventura, M.; de Los Reyes-Gavilan, C.G.; Gueimonde, M. Fecal microbiota profile in a group of myasthenia gravis patients. Sci. Rep. 2018, 8, 14384. [Google Scholar] [CrossRef]
- Haro, C.; Montes-Borrego, M.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; Gómez-Delgado, F.; Pérez-Martínez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Tinahones, F.J.; Landa, B.B. Two healthy diets modulate gut microbial community improving insulin sensitivity in a human obese population. J. Clin. Endocrinol. 2016, 101, 233–242. [Google Scholar] [CrossRef]
- Walker, A.W.; Sanderson, J.D.; Churcher, C.; Parkes, G.C.; Hudspith, B.N.; Rayment, N.; Brostoff, J.; Parkhill, J.; Dougan, G.; Petrovska, L. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011, 11, 7. [Google Scholar] [CrossRef]
- Andoh, A.; Imaeda, H.; Aomatsu, T.; Inatomi, O.; Bamba, S.; Sasaki, M.; Saito, Y.; Tsujikawa, T.; Fujiyama, Y. Comparison of the fecal microbiota profiles between ulcerative colitis and Crohn’s disease using terminal restriction fragment length polymorphism analysis. J. Gastroenterol. 2011, 46, 479–486. [Google Scholar] [CrossRef]
- Surana, N.K.; Kasper, D.L. Moving beyond microbiome-wide associations to causal microbe identification. Nature 2017, 552, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.; Karpinets, T.; Prieto, P.; Vicente, D.; Hoffman, K.; Wei, S. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, K.A.; Stappenbeck, T.S. Peripheral education of the immune system by the colonic microbiota. Semin. Immunol. 2013, 25, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Narushima, S.; Sugiura, Y.; Oshima, K.; Atarashi, K.; Hattori, M.; Suematsu, M.; Honda, K. Characterization of the 17 strains of regulatory T cell-inducing human-derived Clostridia. Gut Microbes 2014, 5, 333–339. [Google Scholar] [CrossRef]
- Cummings, J.; Macfarlane, G. The control and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol. 1991, 70, 443–459. [Google Scholar] [CrossRef]
- Duncan, S.H.; Louis, P.; Flint, H.J. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microbiol. 2004, 70, 5810–5817. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K. T reg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Hase, K. Commensal microbiota regulates T cell fate decision in the gut. Semin. Immunol. 2015, 37, 17–25. [Google Scholar] [CrossRef]
- Maeda, T.; Towatari, M.; Kosugi, H.; Saito, H. Up-regulation of costimulatory/adhesion molecules by histone deacetylase inhibitors in acute myeloid leukemia cells. Blood J. Am. Soc. Hematol. 2000, 96, 3847–3856. [Google Scholar]
- Lührs, H.; Gerke, T.; Müller, J.; Melcher, R.; Schauber, J.; Boxberger, F.; Scheppach, W.; Menzel, T. Butyrate inhibits NF-κB activation in lamina propria macrophages of patients with ulcerative colitis. Scand. J. Gastroenterol. 2002, 37, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Miyake, S.; Kim, S.; Suda, W.; Oshima, K.; Nakamura, M.; Matsuoka, T.; Chihara, N.; Tomita, A.; Sato, W.; Kim, S.-W. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLoS ONE 2015, 10, e0137429. [Google Scholar] [CrossRef]
- Kaci, G.; Lakhdari, O.; Doré, J.; Ehrlich, S.D.; Renault, P.; Blottière, H.M.; Delorme, C. Inhibition of the NF-kappaB pathway in human intestinal epithelial cells by commensal Streptococcus salivarius. Appl. Environ. Microbiol. 2011, 77, 4681–4684. [Google Scholar] [CrossRef]
- Couvigny, B.; de Wouters, T.; Kaci, G.; Jacouton, E.; Delorme, C.; Dore, J.; Renault, P.; Blottière, H.M.; Guédon, E.; Lapaque, N. Commensal Streptococcus salivarius modulates PPARγ transcriptional activity in human intestinal epithelial cells. PLoS ONE 2015, 10, e0125371. [Google Scholar] [CrossRef] [PubMed]
- Cipolletta, D.; Feuerer, M.; Li, A.; Kamei, N.; Lee, J.; Shoelson, S.E.; Benoist, C.; Mathis, D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 2012, 486, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.P. Role of PPARgamma, transcriptional cofactors, and adiponectin in the regulation of nutrient metabolism, adipogenesis and insulin action: View from the chair. Int. J. Obes. 2005, 29 (Suppl. S1), S3–S4. [Google Scholar] [CrossRef]
- Choi, J.-M.; Bothwell, A.L.M. The nuclear receptor PPARs as important regulators of T-cell functions and autoimmune diseases. Mol. Cells 2012, 33, 217–222. [Google Scholar] [CrossRef]
- Kim, C.H.; Park, J.; Kim, M. Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw. 2014, 14, 277–288. [Google Scholar] [CrossRef]
- Giloteaux, L.; Goodrich, J.K.; Walters, W.A.; Levine, S.M.; Ley, R.E.; Hanson, M.R. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 2016, 4, 30. [Google Scholar] [CrossRef]
- Forsythe, P.; Inman, M.D.; Bienenstock, J. Oral treatment with live Lactobacillus reuteri inhibits the allergic airway response in mice. Am. J. Respir. Crit. Care Med. 2007, 175, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Karimi, K.; Inman, M.D.; Bienenstock, J.; Forsythe, P. Lactobacillus reuteri–induced regulatory T cells protect against an allergic airway response in mice. Am. J. Respir. Crit. Care Med. 2009, 179, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Monnig, M.A.; Kahler, C.W.; Cioe, P.A.; Tucker, L.; Monti, P.M.; Mayer, K.H.; Ramratnam, B. Alcohol use predicts elevation in inflammatory marker soluble CD14 in men living with HIV. AIDS Care 2016, 28, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Eriguchi, Y.; Takashima, S.; Oka, H.; Shimoji, S.; Nakamura, K.; Uryu, H.; Shimoda, S.; Iwasaki, H.; Shimono, N.; Ayabe, T. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of α-defensins. Blood J. Am. Soc. Hematol. 2012, 120, 223–231. [Google Scholar] [CrossRef]
- Rani, R.A.; Ali, R.A.R.; Lee, Y.Y. Irritable bowel syndrome and inflammatory bowel disease overlap syndrome: Pieces of the puzzle are falling into place. Intest. Res. 2016, 14, 297. [Google Scholar] [CrossRef]
- Yim, H.C.; Li, J.C.; Lau, J.S.; Lau, A.S. HIV-1 Tat dysregulation of lipopolysaccharide-induced cytokine responses: Microbial interactions in HIV infection. Aids 2009, 23, 1473–1484. [Google Scholar] [CrossRef]
- Farhana, A.; Khan, Y.S. Biochemistry, lipopolysaccharide. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Allman, W.; Qi, H.; Saini, S.S.; Li, J.; Tuzun, E.; Christadoss, P. CD4 costimulation is not required in a novel LPS-enhanced model of myasthenia gravis. J. Neuroimmunol. 2012, 249, 1–7. [Google Scholar] [CrossRef]
- Rose, N.R. The adjuvant effect in infection and autoimmunity. Clin. Rev. Allergy Immunol. 2008, 34, 279–282. [Google Scholar] [CrossRef]
- Yoshino, S.; Sasatomi, E.; Ohsawa, M. Bacterial lipopolysaccharide acts as an adjuvant to induce autoimmune arthritisin mice. Immunology 2000, 99, 607–614. [Google Scholar] [CrossRef]
- Yang, D.; Su, Z.; Wu, S.; Bi, Y.; Li, X.; Li, J.; Lou, K.; Zhang, H.; Zhang, X. Low antioxidant status of serum bilirubin, uric acid, albumin and creatinine in patients with myasthenia gravis. Int. J. Neurosci. 2016, 126, 1120–1126. [Google Scholar] [CrossRef]
- Fuhua, P.; Xuhui, D.; Zhiyang, Z.; Ying, J.; Yu, Y.; Feng, T.; Jia, L.; Lijia, G.; Xueqiang, H. Antioxidant status of bilirubin and uric acid in patients with myasthenia gravis. Neuroimmunomodulation 2012, 19, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Roses, A.D.; Olanow, C.W.; McAdams, M.W.; Lane, R.J. No direct correlation between serum antiacetylcholine receptor antibody levels and clinical state of individual patients with myasthenia gravis. Neurology 1981, 31, 220. [Google Scholar] [CrossRef] [PubMed]
- Hotel, A.C.P.; Cordoba, A. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Prevention 2001, 5, 1–10. [Google Scholar]
- Metchnikoff, E. The Prolongation of Life: Optimistic Studies; Springer Publishing Company: New York, NY, USA, 2004. [Google Scholar]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. Int. Sch. Res. Not. 2013, 2013, 481651. [Google Scholar] [CrossRef]
- Stanton, C.; Gardiner, G.; Meehan, H.; Collins, K.; Fitzgerald, G.; Lynch, P.B.; Ross, R.P. Market potential for probiotics. Am. J. Clin. Nutr. 2001, 73, 476s–483s. [Google Scholar] [CrossRef]
- Food Processing. Modest growth for global probioitc market. Acesso Em 2009, 20, 4–10. Available online: http://www.foodprocessing.com/articles/2008/383.html. (accessed on 15 January 2022).
- Sheikh, S.; Pallagatti, S.; Kalucha, A.; Kaur, H. Probiotics. Going on the natural way. J. Clin. Exp. Dent. 2011, 3, e150–e154. [Google Scholar] [CrossRef]
- Ewe, J.-A.; Wan-Abdullah, W.-N.; Liong, M.-T. Viability and growth characteristics of Lactobacillus in soymilk supplemented with B-vitamins. Int. J. Food Sci. Nutr. 2010, 61, 87–107. [Google Scholar] [CrossRef]
- Sheehan, V.M.; Ross, P.; Fitzgerald, G.F. Assessing the acid tolerance and the technological robustness of probiotic cultures for fortification in fruit juices. Innov. Food Sci. Emerg. Technol. 2007, 8, 279–284. [Google Scholar] [CrossRef]
- Medina, L.; Jordano, R. Survival of constitutive microflora in commercially fermented milk containing bifidobacteria during refrigerated storage. J. Food Prot. 1994, 57, 731–733. [Google Scholar] [CrossRef]
- Gardiner, G.; Ross, R.; Collins, J.; Fitzgerald, G.; Stanton, C. Development of a probiotic Cheddar Cheese containing human-derived Lactobacillus paracaseiStrains. Appl. Environ. Microbiol. 1998, 64, 2192–2199. [Google Scholar] [CrossRef] [PubMed]
- Kehagias, C.; Koulouris, S.; Arkoudelos, J.; Samona, A. Viability and biochemical activity of bifidobacteria in association with yoghurt starter cultures in Bifidus milk and bio-yoghurt during storage at 4^ oc. Egypt. J. Dairy Sci. 2006, 34, 151. [Google Scholar]
- O’Toole, P.W.; Cooney, J.C. Probiotic bacteria influence the composition and function of the intestinal microbiota. Interdiscip. Perspect. Infect. Dis. 2008, 2008, 175285. [Google Scholar] [CrossRef] [PubMed]
- van Baarlen, P.; Troost, F.; van der Meer, C.; Hooiveld, G.; Boekschoten, M.; Brummer, R.J.; Kleerebezem, M. Human mucosal in vivo transcriptome responses to three lactobacilli indicate how probiotics may modulate human cellular pathways. Proc. Natl. Acad. Sci. USA 2011, 108, 4562–4569. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Versalovic, J. Probiotics-host communication: Modulation of signaling pathways in the intestine. Gut Microbes 2010, 1, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Spinler, J.K.; Taweechotipatr, M.; Rognerud, C.L.; Ou, C.N.; Tumwasorn, S.; Versalovic, J. Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe 2008, 14, 166–171. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, E.F.; Cotter, P.D.; Stanton, C.; Ross, R.P.; Hill, C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: Bacteriocins and conjugated linoleic acid. Int. J. Food Microbiol. 2012, 152, 189–205. [Google Scholar] [CrossRef]
- Collado, M.; Meriluoto, J.; Salminen, S. Role of commercial probiotic strains against human pathogen adhesion to intestinal mucus. Lett. Appl. Microbiol. 2007, 45, 454–460. [Google Scholar] [CrossRef]
- Casas, I.; Dobrogosz, W. Validation of the probiotic concept: Lactobacillus reuteri confers broad-spectrum protection against disease in humans and animals. Microb. Ecol. Health Dis. 2000, 12, 247–285. [Google Scholar]
- Kabuki, T.; Saito, T.; Kawai, Y.; Uemura, J.; Itoh, T. Production, purification and characterization of reutericin 6, a bacteriocin with lytic activity produced by Lactobacillus reuteri LA6. Int. J. Food Microbiol. 1997, 34, 145–156. [Google Scholar] [CrossRef]
- Cox, M.J.; Huang, Y.J.; Fujimura, K.E.; Liu, J.T.; McKean, M.; Boushey, H.A.; Segal, M.R.; Brodie, E.L.; Cabana, M.D.; Lynch, S.V. Lactobacillus casei abundance is associated with profound shifts in the infant gut microbiome. PLoS ONE 2010, 5, e8745. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, N.; Scheu, S.; Jousset, A. Bacterial diversity stabilizes community productivity. PLoS ONE 2012, 7, e34517. [Google Scholar] [CrossRef] [PubMed]
- Bron, P.A.; Van Baarlen, P.; Kleerebezem, M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat. Rev. Microbiol. 2012, 10, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Bak, Y.-T. Irritable bowel syndrome, gut microbiota and probiotics. J. Neurogastroenterol. Motil. 2011, 17, 252. [Google Scholar] [CrossRef]
- Preidis, G.A.; Versalovic, J. Targeting the human microbiome with antibiotics, probiotics, and prebiotics: Gastroenterology enters the metagenomics era. Gastroenterology 2009, 136, 2015–2031. [Google Scholar] [CrossRef]
- Zhang, W.; Wen, K.; Azevedo, M.S.; Gonzalez, A.; Saif, L.J.; Li, G.; Yousef, A.E.; Yuan, L. Lactic acid bacterial colonization and human rotavirus infection influence distribution and frequencies of monocytes/macrophages and dendritic cells in neonatal gnotobiotic pigs. Vet. Immunol. Immunopathol. 2008, 121, 222–231. [Google Scholar] [CrossRef]
- Thomas, C.M.; Hong, T.; Van Pijkeren, J.P.; Hemarajata, P.; Trinh, D.V.; Hu, W.; Britton, R.A.; Kalkum, M.; Versalovic, J. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS ONE 2012, 7, e31951. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Prebiotics and probiotics: Are they functional foods? Am. J. Clin. Nutr. 2000, 71, 1682S–1687S. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef]
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Bouhnik, Y.; Raskine, L.; Simoneau, G.; Vicaut, E.; Neut, C.; Flourié, B.; Brouns, F.; Bornet, F.R. The capacity of nondigestible carbohydrates to stimulate fecal bifidobacteria in healthy humans: A double-blind, randomized, placebo-controlled, parallel-group, dose-response relation study. Am. J. Clin. Nutr. 2004, 80, 1658–1664. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.B.; Delzenne, N.M. Dietary fructans. Annu. Rev. Nutr. 1998, 18, 117–143. [Google Scholar] [CrossRef] [PubMed]
- Hoebregs, H. Fructans in foods and food products, ion-exchange chromatographic method: Collaborative study. J. AOAC Int. 1997, 80, 1029–1039. [Google Scholar] [CrossRef]
- Van Loo, J.; Coussement, P.; De Leenheer, L.; Hoebregs, H.; Smits, G. On the presence of inulin and oligofructose as natural ingredients in the western diet. Crit. Rev. Food Sci. Nutr. 1995, 35, 525–552. [Google Scholar] [CrossRef]
- Stinson, L.F.; Payne, M.S.; Keelan, J.A. Planting the seed: Origins, composition, and postnatal health significance of the fetal gastrointestinal microbiota. Crit. Rev. Microbiol. 2017, 43, 352–369. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Hernot, D.C.; Boileau, T.W.; Bauer, L.L.; Middelbos, I.S.; Murphy, M.R.; Swanson, K.S.; Fahey, G.C., Jr. In vitro fermentation profiles, gas production rates, and microbiota modulation as affected by certain fructans, galactooligosaccharides, and polydextrose. J. Agric. Food Chem. 2009, 57, 1354–1361. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Zheng, P.; Chen, X.; Yang, Y. Starch structure modulates metabolic activity and gut microbiota profile. Anaerobe 2013, 24, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.W.; Duncan, S.H.; Leitch, E.C.M.; Child, M.W.; Flint, H.J. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microbiol. 2005, 71, 3692–3700. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Louis, P.; Thomson, J.M.; Flint, H.J. The role of pH in determining the species composition of the human colonic microbiota. Environ. Microbiol. 2009, 11, 2112–2122. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.B.; Davis, K.M.; Lysenko, E.S.; Zhou, A.Y.; Yu, Y.; Weiser, J.N. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 2010, 16, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Li, H.; Zhang, M.; Li, X.-L.; Yue, L.-T.; Zhang, P.; Zhao, Y.; Wang, S.; Duan, R.-N.; Li, Y.-B. Caspase-1 inhibitor ameliorates experimental autoimmune myasthenia gravis by innate dendric cell IL-1-IL-17 pathway. J. Neuroinflammation 2015, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Li, H.; Zhang, M.; Xu, H.; Yue, L.-T.; Zhang, X.-X.; Wang, S.; Wang, C.-C.; Li, Y.-B.; Dou, Y.-C. Exosomes derived from atorvastatin-modified bone marrow dendritic cells ameliorate experimental autoimmune myasthenia gravis by up-regulated levels of IDO/Treg and partly dependent on FasL/Fas pathway. J. Neuroinflammation 2016, 13, 1–18. [Google Scholar] [CrossRef]
- Yang, H.; Lei, P.; Xie, Y.; Han, Z.; Li, D.; Wang, S.; Sun, Z. Correlations of TNF-α gene promoter polymorphisms with the risk of thymoma-associated myasthenia gravis in a northern Chinese Han population. Cancer Gene Ther. 2017, 24, 259–266. [Google Scholar] [CrossRef][Green Version]
- Yi, J.; Guidon, A.; Sparks, S.; Osborne, R.; Juel, V.; Massey, J.; Sanders, D.; Weinhold, K.; Guptill, J. Characterization of CD4 and CD8 T cell responses in MuSK myasthenia gravis. J. Autoimmun. 2014, 52, 130–138. [Google Scholar] [CrossRef]
- Tüzün, E.; Huda, R.; Christadoss, P. Complement and cytokine based therapeutic strategies in myasthenia gravis. J. Autoimmun. 2011, 37, 136–143. [Google Scholar] [CrossRef]
- Maldonado, R.R.; Caram, A.L.A.; de Oliveira, D.S.; Kamimura, E.S.; Mazalli, M.R.; Aguiar-Oliveira, E. The Role of Probiotics and Prebiotics in the Composition of the Gut Microbiota and Their Influence on Inflammatory Bowel Disease, Obesity, and Diabetes. In Microbiome-Host Interactions; CRC Press: Boca Raton, FL, USA, 2021; pp. 113–128. [Google Scholar]
- Ser, H.-L.; Letchumanan, V.; Goh, B.-H.; Wong, S.H.; Lee, L.-H. The use of fecal microbiome transplant in treating human diseases: Too early for poop? Front. Microbiol. 2021, 12, 519836. [Google Scholar] [CrossRef]
| Studies | Changes in the Gut Microbiome |
|---|---|
| Zheng et al. [97] | 80 differential a OTUs were recognized and held accountable for distinguishing b MG subjects from c HCs. These 80 a OTUs mainly belonged to the phyla Firmicutes (59/80), Bacteroidetes (14/80), and Actinobacteria (3/80). In comparison with the c HCs, out of the 80 a OTUs that were recognized, 34 a OTUs belonging to the bacterial taxonomic families (Bacteroidaceae, Lachnospiraceae, Prevotellaceae, and Veillonellaceae) increased in abundance, while the remaining 46 a OTUs belonging to bacterial families (Lachnospiraceae, Ruminococcaceae, Erysipelotrichaceae, Clostridiaceae, and Peptostreptococcaceae) decreased in abundance in b MG subjects. |
| Firmicutes were the dominant fecal microbes of c HCs and b MG subjects. | |
| The level of Clostridium (under the phyla Firmicutes) was much greater in c HCs (p < 0.001). b MG subjects had a reduced abundance of bacteria belonging to Lachnospiraceae and Ruminococcaceae families from Clostridiales. | |
| The level of Actinobacteria was lower relative to c HCs. | |
| The level of Bacteroidetes was higher in b MG subjects. | |
| Qiu et al. [106] | In c HCs, the bacteria of genera Clostridium, Eubacterium, Faecalibacterium, Lactobacillus etc. were higher in abundance. Conversely, in b MG subjects, the bacteria of genera Streptococcus, Parasutterella, Escherichia, etc. were higher in abundance. The level of Clostridium (under the phyla Firmicutes) was the most depleted, with an absolute amount up to three-times less than in c HCs. |
| The level of Bacteroidetes was higher in b MG subjects. | |
| Moris et al. [119] | Firmicutes were the dominant fecal microbes of c HCs and b MG subjects. |
| The level of Actinobacteria was lower relative to c HCs. | |
| c HCs had high populations of Bifidobacterium longum subsp. longum followed by Bifidobacterium adolescentis, whereas b MG subjects had high relative proportions of Bifidobacterium animalis subsp. lactis, Bifidobacterium breve and Bifidobacterium dentium. | |
| The level of Bacteroidetes was higher in b MG subjects. | |
| Higher counts (p < 0.05) of total bacteria and the Desulfovibrio- and Bacteroides-groups based on a d qPCR analysis. |
| Findings | |
|---|---|
| Animal study on a FMT | Using an open field test, 4 weeks after a FMT [97]
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thye, A.Y.-K.; Law, J.W.-F.; Tan, L.T.-H.; Thurairajasingam, S.; Chan, K.-G.; Letchumanan, V.; Lee, L.-H. Exploring the Gut Microbiome in Myasthenia Gravis. Nutrients 2022, 14, 1647. https://doi.org/10.3390/nu14081647
Thye AY-K, Law JW-F, Tan LT-H, Thurairajasingam S, Chan K-G, Letchumanan V, Lee L-H. Exploring the Gut Microbiome in Myasthenia Gravis. Nutrients. 2022; 14(8):1647. https://doi.org/10.3390/nu14081647
Chicago/Turabian StyleThye, Angel Yun-Kuan, Jodi Woan-Fei Law, Loh Teng-Hern Tan, Sivakumar Thurairajasingam, Kok-Gan Chan, Vengadesh Letchumanan, and Learn-Han Lee. 2022. "Exploring the Gut Microbiome in Myasthenia Gravis" Nutrients 14, no. 8: 1647. https://doi.org/10.3390/nu14081647
APA StyleThye, A. Y.-K., Law, J. W.-F., Tan, L. T.-H., Thurairajasingam, S., Chan, K.-G., Letchumanan, V., & Lee, L.-H. (2022). Exploring the Gut Microbiome in Myasthenia Gravis. Nutrients, 14(8), 1647. https://doi.org/10.3390/nu14081647









