Dietary Counseling Aimed at Reducing Sugar Intake Yields the Greatest Improvement in Management of Weight and Metabolic Dysfunction in Children with Obesity
Abstract
:1. Introduction
2. Methods
Statistical Analysis
3. Results
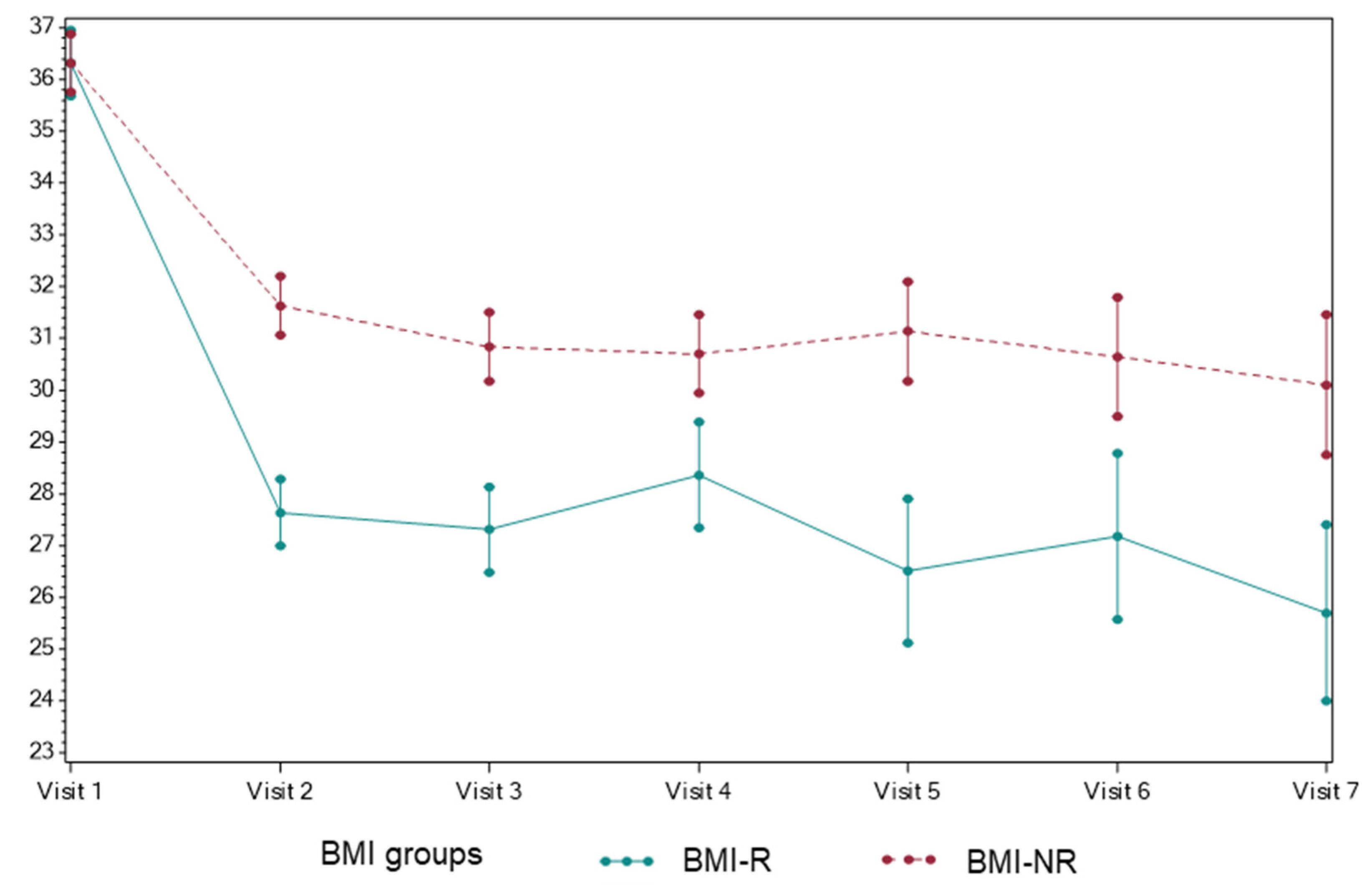
3.1. The Effect of Dietary Counseling on BMI
3.2. Dietary Changes Implemented by BMI Responders and Non-Responders
3.3. Metabolic Profile of BMI Responders versus Non-Responders
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fryar, C.D.; Carroll, M.D.; Afful, J. Prevalence of Overweight, Obesity and Severe Obesity among Children and Adolescents Aged 2–19 Years: United States, 1963–1965 through 2017–2018. NCHS Health E-Stats. 2020. Available online: https://www.cdc.gov/nchs/data/hestat/obesity-child-17-18/obesity-child.htm#Suggested%20citation (accessed on 11 March 2022).
- Lange, S.J.; Kompaniyets, L.; Freedman, D.S.; Kraus, E.M.; Porter, R.; Blanck, H.M.; Goodman, A.B. Longitudinal Trends in Body Mass Index Before and During the COVID-19 Pandemic Among Persons Aged 2–19 Years—United States, 2018–2020. Morb. Mortal. Wkly. Rep. 2021, 70, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kelly, A.S. Review of Childhood Obesity: From Epidemiology, Etiology, and Comorbidities to Clinical Assessment and Treatment. Mayo Clin. Proc. 2017, 92, 251–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Styne, D.M.; Arslanian, S.A.; Connor, E.L.; Farooqi, I.S.; Murad, M.H.; Silverstein, J.H.; Yanovski, J.A. Pediatric Obesity-Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 709–757. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, A.; Dugan, A.J.; Killian, M.; Attia, S.L.; Mouzaki, M.; Fuchs, G.J.; Kohli, R.; Bada, H.; Kern, P.A.; Softic, S. Stratification by obesity class, rather than age, can identify a higher percent of children at risk for non-alcoholic fatty liver disease and metabolic dysfunction. Pediatr. Obes. 2021, 17, e12862. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Duster, M.; Roberts, T.; Devinsky, O. United States Dietary Trends Since 1800: Lack of Association Between Saturated Fatty Acid Consumption and Non-communicable Diseases. Front. Nutr. 2021, 8, 748847. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.L.; Softic, S.; Mouzaki, M. Evolving Role for Pharmacotherapy in NAFLD/NASH. Clin. Transl. Sci. 2021, 14, 11–19. [Google Scholar] [CrossRef]
- Softic, S.; Kohli, R. Pediatric NASH therapies: A speedbump on the road to success. Hepatology 2022, in press. [Google Scholar] [CrossRef]
- Barlow, S.E.; Expert, C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 2007, 120 (Suppl. 4), S164–S192. [Google Scholar] [CrossRef] [Green Version]
- Spear, B.A.; Barlow, S.E.; Ervin, C.; Ludwig, D.S.; Saelens, B.E.; Schetzina, K.E.; Taveras, E.M. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics 2007, 120 (Suppl. 4), S254–S288. [Google Scholar] [CrossRef] [Green Version]
- Rajjo, T.; Mohammed, K.; Alsawas, M.; Ahmed, A.T.; Farah, W.; Asi, N.; Almasri, J.; Prokop, L.J.; Murad, M.H. Treatment of Pediatric Obesity: An Umbrella Systematic Review. J. Clin. Endocrinol. Metab. 2017, 102, 763–775. [Google Scholar] [CrossRef] [Green Version]
- Kirk, S.; Scott, B.J.; Daniels, S.R. Pediatric obesity epidemic: Treatment options. J. Am. Diet Assoc. 2005, 105, S44–S51. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.H.; Squires, S. The Stoplight Diet for Children; Little, Brown & Company: Toronto, ON, Canada, 1988. [Google Scholar]
- Dietary Guidelines for Americans 2005, US Department of Health and Human Services, US Department of Agriculture. 2005. Available online: https://health.gov/sites/default/files/2020-01/DGA2005.pdf (accessed on 9 March 2022).
- Kavey, R.E.; Daniels, S.R.; Lauer, R.M.; Atkins, D.L.; Hayman, L.L.; Taubert, K. American Heart, A: American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. Circulation 2003, 107, 1562–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 2015, 8th ed. Available online: https://health.gov/our-work/food-nutrition/previous-dietary-guidelines/2015 (accessed on 11 March 2022).
- Malik, V.S.; Hu, F.B. The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat. Rev. Endocrinol. 2022, 18, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Nielsen, S.J.; Popkin, B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 2004, 7s9, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Stanhope, K.L.; Boucher, J.; Divanovic, S.; Lanaspa, M.A.; Johnson, R.J.; Kahn, C.R. Fructose and hepatic insulin resistance. Crit. Rev. Clin. Lab. Sci. 2020, 57, 305–322. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.S.; Keim, N.L.; Stern, J.S.; Teff, K.; Havel, P.J. Fructose, weight gain, and the insulin resistance syndrome. Am. J. Clin. Nutr. 2002, 76, 911–922. [Google Scholar] [CrossRef]
- Le, K.A.; Ith, M.; Kreis, R.; Faeh, D.; Bortolotti, M.; Tran, C.; Boesch, C.; Tappy, L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am. J. Clin. Nutr. 2009, 89, 1760–1765. [Google Scholar] [CrossRef] [Green Version]
- Taskinen, M.R.; Soderlund, S.; Bogl, L.H.; Hakkarainen, A.; Matikainen, N.; Pietilainen, K.H.; Rasanen, S.; Lundbom, N.; Bjornson, E.; Eliasson, B.; et al. Adverse effects of fructose on cardiometabolic risk factors and hepatic lipid metabolism in subjects with abdominal obesity. J. Intern. Med. 2017, 282, 187–201. [Google Scholar] [CrossRef]
- Yilmaz, Y. Review article: Fructose in non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2012, 35, 1135–1144. [Google Scholar] [CrossRef]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.H.; et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef] [Green Version]
- Basciano, H.; Federico, L.; Adeli, K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr. Metab. 2005, 2, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helsley, R.N.; Moreau, F.; Gupta, M.K.; Radulescu, A.; DeBosch, B.; Softic, S. Tissue-Specific Fructose Metabolism in Obesity and Diabetes. Curr. Diabetes Rep. 2020, 20, 64. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Softic, S.; Gupta, M.K.; Wang, G.X.; Fujisaka, S.; O’Neill, B.T.; Rao, T.N.; Willoughby, J.; Harbison, C.; Fitzgerald, K.; Ilkayeva, O.; et al. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J. Clin. Investig. 2017, 127, 4059–4074. [Google Scholar] [CrossRef] [Green Version]
- Softic, S.; Kirby, M.; Berger, N.G.; Shroyer, N.F.; Woods, S.C.; Kohli, R. Insulin concentration modulates hepatic lipid accumulation in mice in part via transcriptional regulation of fatty acid transport proteins. PLoS ONE 2012, 7, e38952. [Google Scholar] [CrossRef] [Green Version]
- Softic, S.; Meyer, J.G.; Wang, G.X.; Gupta, M.K.; Batista, T.M.; Lauritzen, H.; Fujisaka, S.; Serra, D.; Herrero, L.; Willoughby, J.; et al. Dietary Sugars Alter Hepatic Fatty Acid Oxidation via Transcriptional and Post-translational Modifications of Mitochondrial Proteins. Cell Metab. 2019, 30, 735–753.e734. [Google Scholar] [CrossRef]
- Park, S.H.; Helsley, R.N.; Noetzli, L.; Tu, H.C.; Wallenius, K.; O’Mahony, G.; Boucher, J.; Liu, J.; Softic, S. A luminescence-based protocol for assessing fructose metabolism via quantification of ketohexokinase enzymatic activity in mouse or human hepatocytes. STAR Protoc. 2021, 2, 100731. [Google Scholar] [CrossRef]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef] [Green Version]
- Cohen, C.C.; Li, K.W.; Alazraki, A.L.; Beysen, C.; Carrier, C.A.; Cleeton, R.L.; Dandan, M.; Figueroa, J.; Knight-Scott, J.; Knott, C.J.; et al. Dietary sugar restriction reduces hepatic de novo lipogenesis in adolescent boys with fatty liver disease. J. Clin. Investig. 2021, 131, 131. [Google Scholar] [CrossRef]
- Damen, M.; Stankiewicz, T.E.; Park, S.H.; Helsley, R.N.; Chan, C.C.; Moreno-Fernandez, M.E.; Doll, J.R.; Szabo, S.; Herbert, D.R.; Softic, S.; et al. Non-hematopoietic IL-4Ralpha expression contributes to fructose-driven obesity and metabolic sequelae. Int. J. Obes. 2021, 45, 2377–2387. [Google Scholar] [CrossRef]
- Softic, S.; Boucher, J.; Solheim, M.H.; Fujisaka, S.; Haering, M.F.; Homan, E.P.; Winnay, J.; Perez-Atayde, A.R.; Kahn, C.R. Lipodystrophy Due to Adipose Tissue-Specific Insulin Receptor Knockout Results in Progressive NAFLD. Diabetes 2016, 65, 2187–2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.H.; Scapa, E.F.; Cohen, D.E.; Glimcher, L.H. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 2008, 320, 1492–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gugliucci, A. Formation of Fructose-Mediated Advanced Glycation End Products and Their Roles in Metabolic and Inflammatory Diseases. Adv. Nutr. 2017, 8, 54–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erkin-Cakmak, A.; Bains, Y.; Caccavello, R.; Noworolski, S.M.; Schwarz, J.M.; Mulligan, K.; Lustig, R.H.; Gugliucci, A. Isocaloric Fructose Restriction Reduces Serum d-Lactate Concentration in Children with Obesity and Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 3003–3011. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, J.M.; Noworolski, S.M.; Erkin-Cakmak, A.; Korn, N.J.; Wen, M.J.; Tai, V.W.; Jones, G.M.; Palii, S.P.; Velasco-Alin, M.; Pan, K.; et al. Effects of Dietary Fructose Restriction on Liver Fat, De Novo Lipogenesis, and Insulin Kinetics in Children with Obesity. Gastroenterology 2017, 153, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Gugliucci, A.; Lustig, R.H.; Caccavello, R.; Erkin-Cakmak, A.; Noworolski, S.M.; Tai, V.W.; Wen, M.J.; Mulligan, K.; Schwarz, J.M. Short-term isocaloric fructose restriction lowers apoC-III levels and yields less atherogenic lipoprotein profiles in children with obesity and metabolic syndrome. Atherosclerosis 2016, 253, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Lustig, R.H.; Mulligan, K.; Noworolski, S.M.; Tai, V.W.; Wen, M.J.; Erkin-Cakmak, A.; Gugliucci, A.; Schwarz, J.M. Isocaloric fructose restriction and metabolic improvement in children with obesity and metabolic syndrome. Obesity 2016, 24, 453–460. [Google Scholar] [CrossRef]
- Nishida, C.; Uauy, R.; Kumanyika, S.; Shetty, P. The joint WHO/FAO expert consultation on diet, nutrition and the prevention of chronic diseases: Process, product and policy implications. Public Health Nutr. 2004, 7, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.K.; Appel, L.J.; Brands, M.; Howard, B.V.; Lefevre, M.; Lustig, R.H.; Sacks, F.; Steffen, L.M.; Wylie-Rosett, J. American Heart Association Nutrition Committee of the Council on Nutrition PA, Metabolism, the Council on E, Prevention: Dietary sugars intake and cardiovascular health: A scientific statement from the American Heart Association. Circulation 2009, 120, 1011–1020. [Google Scholar] [CrossRef]
- Canadian Diabetes Association Clinical Practice Guidelines Expert, C.; Dworatzek, P.D.; Arcudi, K.; Gougeon, R.; Husein, N.; Sievenpiper, J.L.; Williams, S.L. Nutrition therapy. Can. J. Diabetes 2013, 37 (Suppl. 1), S45–S55. [Google Scholar] [CrossRef] [Green Version]
- European Association for the Study of The Liver; European Association for the Study of Diabetes. EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. Obes. Facts. 2016, 9, 65–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Horn, L.; Obarzanek, E.; Friedman, L.A.; Gernhofer, N.; Barton, B. Children’s adaptations to a fat-reduced diet: The Dietary Intervention Study in Children (DISC). Pediatrics 2005, 115, 1723–1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahreynian, M.; Qorbani, M.; Khaniabadi, B.M.; Motlagh, M.E.; Safari, O.; Asayesh, H.; Kelishadi, R. Association between Obesity and Parental Weight Status in Children and Adolescents. J. Clin. Res. Pediatr. Endocrinol. 2017, 9, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Fuemmeler, B.F.; Lovelady, C.A.; Zucker, N.L.; Ostbye, T. Parental obesity moderates the relationship between childhood appetitive traits and weight. Obesity 2013, 21, 815–823. [Google Scholar] [CrossRef] [Green Version]
- Whitaker, R.C.; Wright, J.A.; Pepe, M.S.; Seidel, K.D.; Dietz, W.H. Predicting obesity in young adulthood from childhood and parental obesity. N. Engl. J. Med. 1997, 337, 869–873. [Google Scholar] [CrossRef]
- Linabery, A.M.; Nahhas, R.W.; Johnson, W.; Choh, A.C.; Towne, B.; Odegaard, A.O.; Czerwinski, S.A.; Demerath, E.W. Stronger influence of maternal than paternal obesity on infant and early childhood body mass index: The Fels Longitudinal Study. Pediatr. Obes. 2013, 8, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Whitaker, K.L.; Jarvis, M.J.; Beeken, R.J.; Boniface, D.; Wardle, J. Comparing maternal and paternal intergenerational transmission of obesity risk in a large population-based sample. Am. J. Clin. Nutr. 2010, 91, 1560–1567. [Google Scholar] [CrossRef] [Green Version]
- Ruager-Martin, R.; Hyde, M.J.; Modi, N. Maternal obesity and infant outcomes. Early Hum. Dev. 2010, 86, 715–722. [Google Scholar] [CrossRef]
- Whitaker, R.C. Predicting preschooler obesity at birth: The role of maternal obesity in early pregnancy. Pediatrics 2004, 114, e29–e36. [Google Scholar] [CrossRef] [Green Version]
- Andela, S.; Burrows, T.L.; Baur, L.A.; Coyle, D.H.; Collins, C.E.; Gow, M.L. Efficacy of very low-energy diet programs for weight loss: A systematic review with meta-analysis of intervention studies in children and adolescents with obesity. Obes. Rev. 2019, 20, 871–882. [Google Scholar] [CrossRef]
- Maier, I.B.; Stricker, L.; Ozel, Y.; Wagnerberger, S.; Bischoff, S.C.; Bergheim, I. A low fructose diet in the treatment of pediatric obesity: A pilot study. Pediatr. Int. 2011, 53, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Kahn, C.R. Fatty liver disease: Is it nonalcoholic fatty liver disease or obesity-associated fatty liver disease? Eur. J. Gastroenterol. Hepatol. 2019, 31, 143. [Google Scholar] [CrossRef] [PubMed]
| Initial Visit | Last Visit | Interval Change | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | BMI-NR | BMI- R | p-Value | Total | BMI-NR | BMI- R | p-Value | p- Total | Mean ∆, BMI-NR | Mean∆, BMI-R | p-Value | |
| Age, y | 10.16 (3.54) | 10.05 (3.26) | 10.32 (3.88) | 0.62 | 10.97 (3.57) | 10.99 (3.19) | 10.94 (4.02) | 0.93 | 0.04 | 0.94 (4.56) | 0.62 (5.58) | 0.686 |
| Weight, kg | 71.78 (34.18) | 70.31 (34.58) | 73.67 (33.79) | 0.53 | 74.55 (31.82) | 74.82 (29.48) | 74.21 (34.82) | 0.90 | 0.11 | 4.50 (18.88) | 0.54 (12.38) | 0.107 |
| Height, cm | 145.02 (21.33) | 145.19 (19.10) | 144.80 (24.04) | 0.91 | 149.51 (19.16) | 150.55 (17.64) | 148.18 (21.02) | 0.43 | 0.10 | 5.35 (5.40) | 3.38 (10.73) | 0.157 |
| BMI | 31.49 (7.57) | 30.73 (6.88) | 32.48 (8.33) | 0.14 | 32.20 (11.67) | 33.13 (13.81) | 31.01 (8.04) | 0.22 | 0.51 | 2.40 (9.79) | –1.47 (1.31) | <0.001 |
| BMI % | 98.20 (1.98) | 98.13 (2.22) | 98.29 (1.63) | 0.59 | 97.62 (3.04) | 97.91 (2.67) | 97.25 (3.43) | 0.18 | 0.58 | –0.22 (2.11) | –1.04 (1.97) | 0.011 |
| Dietary Categories | BMI-NR | BMI-R | Treatment Effect (BMI = R-NR) | p-Value |
|---|---|---|---|---|
| Second helpings | –0.74 | –1.03 | –0.29 | 0.151 |
| Portion size | –0.52 | –0.75 | –0.23 | 0.086 |
| Sugar-sweetened beverages | –0.78 | –1.26 | –0.47 | 0.019 |
| Processed food | –0.38 | –0.87 | –0.49 | 0.002 |
| Sweets | –0.54 | –1.20 | –0.66 | <0.001 |
| Unhealthy snacks | –0.56 | –1.03 | –0.46 | 0.009 |
| Skipping meals | –0.04 | –0.14 | –0.09 | 0.346 |
| Meals eaten at school | –0.34 | –0.53 | –0.19 | 0.222 |
| Condiment use | –0.44 | –0.64 | –0.19 | 0.093 |
| Fruits and vegetables | –0.51 | –0.75 | –0.24 | 0.065 |
| Whole grains | –0.37 | –0.53 | –0.16 | 0.095 |
| Initial Visit | Last Visit | Interval Change | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | BMI- NR | BMI- R | p R-NR | Total | BMI-NR | BMI- R | p R-NR | p Total | Mean ∆, BMI-NR | Mean ∆, BMI-R | p R-NR | |
| HbA1c | 5.27 (0.38) | 5.25 (0.33) | 5.29 (0.44) | 0.51 | 5.25 (0.35) | 5.23 (0.32) | 5.28 (0.39) | 0.38 | 0.98 | –0.01 (0.19) | –0.01 (0.22) | 0.824 |
| Glucose, mg/dL | 87.10 (6.47) | 87.14 (6.46) | 87.04 (6.54) | 0.92 | 88.07 (6.22) | 88.91 (6.11) | 86.95 (46.26) | 0.07 | 0.33 | 1.76 (6.31) | –0.09 (2.39) | 0.021 |
| ALT, U/L | 30.76 (27.69) | 30.00 (30.91) | 31.75 (22.95) | 0.68 | 26.36 (18.09) | 26.73 (20.23) | 25.87 (14.93) | 0.76 | 0.14 | –3.27 (17.44) | –5.88 (16.39) | 0.338 |
| Triglycerides, mg/dL | 121.86 (65.90) | 120.50 (60.65) | 123.67 (72.74) | 0.76 | 116.52 (70.55) | 127.47 (82.77) | 101.91 (46.54) | 0.01 | 0.001 | 6.97 (57.14) | –21.75 (53.11) | 0.001 |
| Total Chol, mg/dL | 158.81 (29.03) | 154.27 (30.09) | 164.87 (26.57) | 0.02 | 154.91 (26.98) | 153.30 (28.22) | 157.04 (25.29) | 0.38 | 0.008 | –0.97 (15.52) | –7.83 (20.87) | 0.023 |
| LDL-c, mg/dL | 89.71 (24.88) | 86.38 (26.44) | 94.14 (22.05) | 0.05 | 86.99 (22.85) | 84.42 (23.67) | 90.41 (21.40) | 0.10 | 0.28 | –1.96 (15.65) | –3.74 (17.11) | 0.493 |
| HDL-c, mg/dL | 44.84 (8.56) | 43.51 (8.75) | 46.62 (8.03) | 0.02 | 44.53 (9.01) | 43.32 (9.46) | 46.16 (8.14) | 0.04 | 0.64 | –0.20 (5.09) | –0.46 (4.56) | 0.730 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radulescu, A.; Killian, M.; Kang, Q.; Yuan, Q.; Softic, S. Dietary Counseling Aimed at Reducing Sugar Intake Yields the Greatest Improvement in Management of Weight and Metabolic Dysfunction in Children with Obesity. Nutrients 2022, 14, 1500. https://doi.org/10.3390/nu14071500
Radulescu A, Killian M, Kang Q, Yuan Q, Softic S. Dietary Counseling Aimed at Reducing Sugar Intake Yields the Greatest Improvement in Management of Weight and Metabolic Dysfunction in Children with Obesity. Nutrients. 2022; 14(7):1500. https://doi.org/10.3390/nu14071500
Chicago/Turabian StyleRadulescu, Aurelia, Mary Killian, Qiwen Kang, Qingcong Yuan, and Samir Softic. 2022. "Dietary Counseling Aimed at Reducing Sugar Intake Yields the Greatest Improvement in Management of Weight and Metabolic Dysfunction in Children with Obesity" Nutrients 14, no. 7: 1500. https://doi.org/10.3390/nu14071500
APA StyleRadulescu, A., Killian, M., Kang, Q., Yuan, Q., & Softic, S. (2022). Dietary Counseling Aimed at Reducing Sugar Intake Yields the Greatest Improvement in Management of Weight and Metabolic Dysfunction in Children with Obesity. Nutrients, 14(7), 1500. https://doi.org/10.3390/nu14071500





