Blueberry Supplementation in Midlife for Dementia Risk Reduction
Abstract
1. Introduction
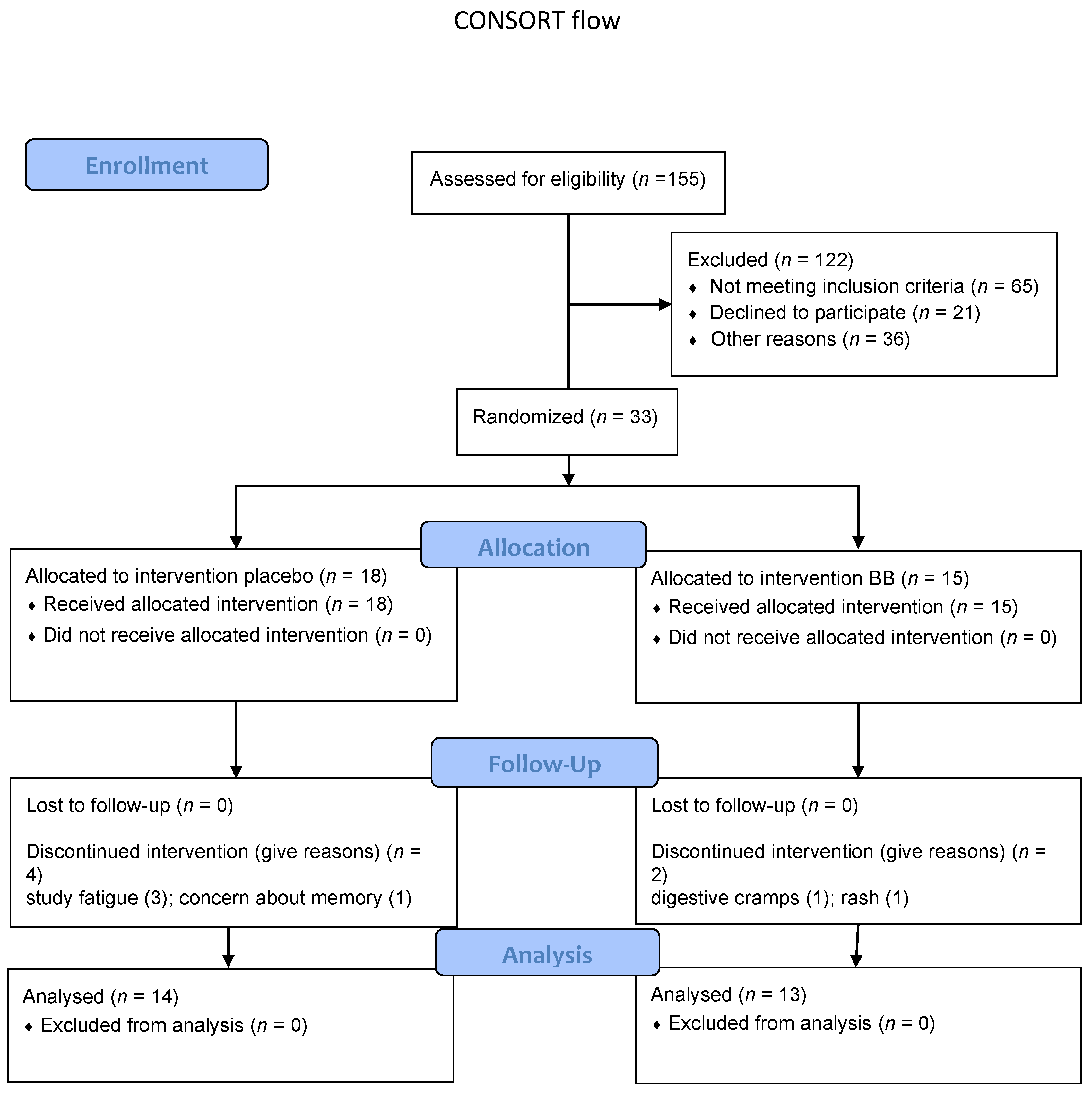
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Criteria
- (1)
- Men and women 50 to 65 years old;
- (2)
- Body mass index (BMI) = 25 or greater;
- (3)
- Subjective cognitive complaints reflecting awareness of decline in cognitive capability from a prior level;
- (4)
- Ability to comprehend and comply with the research protocol;
- (5)
- Provision of written informed consent.
2.3. Exclusion Criteria
- (1)
- Diagnosis of neurological condition or neurocognitive disorder, such as mild cognitive impairment, Alzheimer’s disease, or Parkinson’s disease;
- (2)
- Current or past psychiatric condition, such as psychosis or major mood disorder, causing a persisting change in level of occupational or social functioning;
- (3)
- Current or past substance use causing physiological dependence or change in functional capability;
- (4)
- Diabetes or kidney or liver disease;
- (5)
- Regular use of medication or dietary supplement that might affect outcome measures, such as benzodiazepines, psychostimulants, and berry fruit extracts.
2.4. Telephone Screening
2.5. Enrollment and Final Study Visits
2.6. Interim Visit
2.7. Whole, Freeze-Dried Blueberry and Placebo Powder and Supplementation Regimen
2.8. Neuropsychological Assessment
2.8.1. Controlled Oral Word Production
2.8.2. The California Verbal Learning Test, Second Edition
2.8.3. Verbal Paired Associate Learning
2.8.4. The Everyday Memory Questionnaire
2.8.5. The Beck Depression Inventory II
2.9. Metabolic Measures
2.10. Anthropometric Measures
2.11. Mitochondrial Oxygen-Consumption Rate
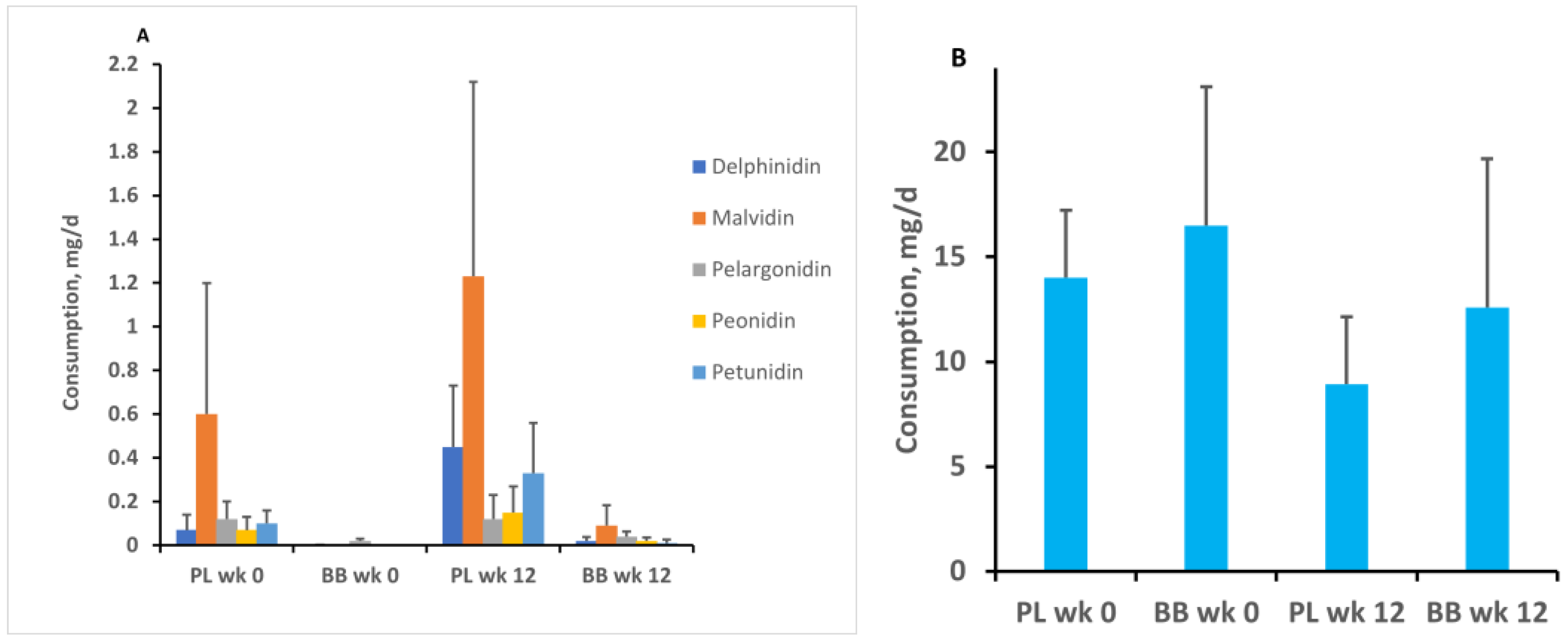
2.12. Diet Diary Records
2.13. Statistical Analyses and Power
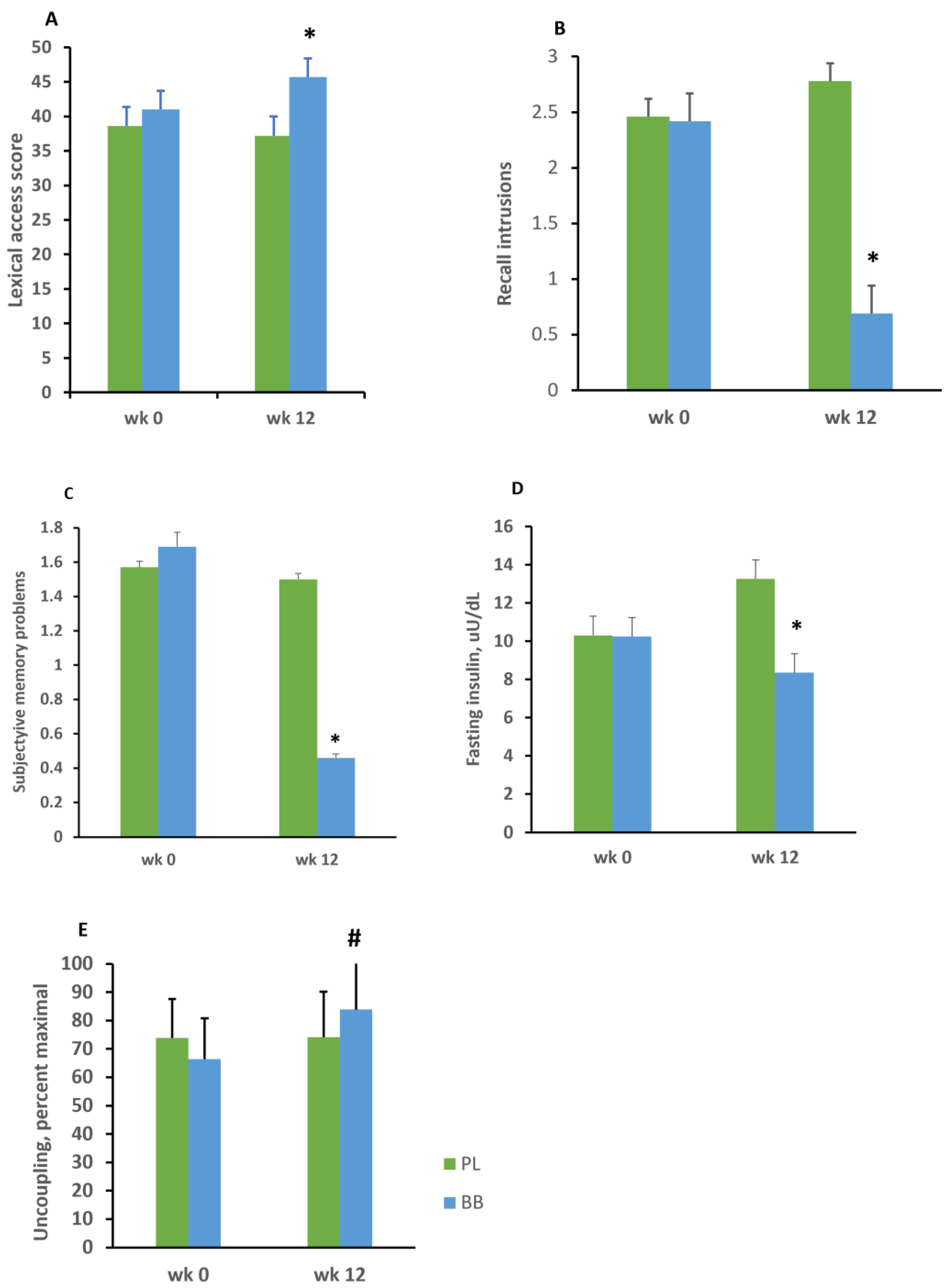
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Association. 2020 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, R.I.; Hristov, H.; Saif, N.; Hackett, K.; Hendrix, S.; Melendez, J.; Safdieh, J.; Fink, M.; Thambisetty, M.; Sadek, G.; et al. Individualized clinical management of patients at risk for Alzheimer’s dementia. Alzheimer’s Dement. 2019, 15, 1588–2602. [Google Scholar] [CrossRef] [PubMed]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomized controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- Alves, S.S.; Silva-Junior, R.M.P.D.; Servilha-Menezes, G.; Homolak, J.; Šalković-Petrišić, M.; Garcia-Cairasco, N. Insulin Resistance as a Common Link between Current Alzheimer’s Disease Hypotheses. J. Alzheimer’s Dis. 2021, 82, 71–105. [Google Scholar] [CrossRef]
- Hirode, G.; Wong, R.J. Trends in the prevalence of metabolic syndrome in the United States, 2011–2016. JAMA 2020, 323, 2526–2528. [Google Scholar] [CrossRef]
- Bikman, B. Why We Get Sick; BenBella Books, Inc.: Dallas, TX, USA, 2020. [Google Scholar]
- Kellar, D.; Craft, S. Brain insulin resistance in Alzheimer’s disease and related disorders: Mechanisms and therapeutic approaches. Lancet Neurol. 2020, 19, 758–766. [Google Scholar] [CrossRef]
- Kleinridders, A.; Ferris, H.A.; Cai, W.; Kahn, C.R. Insulin action in brain regulates systemic metabolism and brain function. Neuroscience 2014, 250, 140–150. [Google Scholar] [CrossRef]
- Schuh, A.F.; Reier, C.M.; Rizzi, L.; Chaves, M.; Roriz-Cruz, M. Mechanisms of brain aging regulation by insulin: Implications for neurodegeneration in late-onset Alzheimer’s disease. ISRN Neurol. 2011, 2011, 306905. [Google Scholar] [CrossRef][Green Version]
- Scheef, L.; Spottke, A.; Daerr, M.; Joe, A.; Striepens, N.; Kölsch, H.; Popp, J.; Daamen, M.; Gorris, D.; Heneka, M.T.; et al. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology 2012, 79, 1332–1339. [Google Scholar] [CrossRef]
- Jack, C.R.; Knopman, D.S.; Jagust, W.J.; Shaw, L.M.; Aisen, P.S.; Weiner, M.W.; Petersen, R.C.; Trojanowski, J.Q. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010, 9, 119–128. [Google Scholar] [CrossRef]
- Jessen, F.; Amariglio, R.E.; van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chételat, G.; Dubois, B.; Dufouil, C.; Ellis, K.A.; Van Der Flier, W.M.; et al. A conceptual framework for research on subjective cognitive decline. Alzheimer’s Dement. 2014, 10, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Saykin, A.J.; Wishart, H.A.; Rabin, L.A.; Santulli, R.B.; Flashman, L.A.; West, J.D.; McHugh, T.L.; Mamourian, A.C. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology 2006, 67, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Glodzik-Sobanska, L.; Reisberg, B.; De Santi, S.; Babb, J.S.; Pirraglia, E.; Rich, K.E.; Brys, M.; de Leon, M.J. Subjective memory complaints: Presence, severity and future outcome in normal older subjects. Dement. Geriatr. Cogn. Disord. 2007, 24, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Perrotin, A.; Mormino, E.C.; Madison, C.M.; Hayenga, A.O.; Jagust, W.J. Subjective cognition and amyloid deposition imaging: A Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Arch. Neurol. 2012, 69, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, W.J.C.; Price, J.R.; Robinson, G.M.; Robinson, R. The distribution of anthocyanins in flowers, fruits and leaves. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1939, 230, 149–178. [Google Scholar]
- Timberlake, C.F.; Bridle, P. Distribution of Anthocyanins in Food Plants; Academic Press: New York, NY, USA, 1982. [Google Scholar]
- Mannino, G.; Gentile, C.; Ertani, A.; Serio, G.; Bertea, C.M. Anthocyanins: Biosynthesis, distribution, ecological role, and use of biostimulants to increase their content in plant foods—A review. Agriculture 2021, 11, 212. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, pharmacology and health benefits of anthocyanins. Phyther. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef]
- Mannino, G.; Chinigo, G.; Serio, G.; Genova, T.; Gentile, C.; Munaron, L.; Bertea, C.M. Proanthocyanidins and where to find them: A meta-analytic approach to investigate their chemistry, biosynthesis, distribution, and effect on human health. Antioxidants 2021, 10, 1229. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Patel, S.; Pan, X.; Naz, S.; Silva, A.S.; Saeed, F.; Suleria, H.A.R. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Francini-Pesenti, F.; Spinella, P.; Calò, L.A. Potential role of phytochemicals in metabolic syndrome prevention and therapy. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1987–2002. [Google Scholar] [CrossRef] [PubMed]
- Márquez Campos, E.; Jakobs, L.; Simon, M.C. Antidiabetic effects of flavan-3-ols and their microbial metabolites. Nutrients 2020, 12, 1592. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Rani, R.; Awana, M.; Pitale, D.; Kulshreshta, A.; Sharma, S.; Bollinedi, H.; Singh, A.; Singh, B.; Singh, A.K.; et al. Role of nutraceutical starch and proanthocyanidins of pigmented rice in regulating hyperglycemia: Enzyme inhibition, enhanced glucose uptake and hepatic glucose homeostasis using in vitro model. Food Chem. 2021, 335, 127505. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Herrera, I.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Cocoa flavonoids improve insulin signaling and modulate glucose production via AKT and AMPK in HepG2 cells. Mol. Nutr. Food Res. 2013, 57, 974–985. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Maykish, A. The gut microbiome and type 2 diabetes mellitus: Discussing a complex relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef]
- Kristo, A.; Klimis-Zacas, D.; Sikalidis, A. Protective Role of Dietary Berries in Cancer. Antioxidants 2016, 5, 37. [Google Scholar] [CrossRef]
- Joseph, J.A.; Shukitt-Hale, B.; Denisova, N.A.; Bielinski, D.; Martin, A.; McEwen, J.J.; Bickford, P.C. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J. Neurosci. 1999, 19, 8114–8121. [Google Scholar] [CrossRef]
- Shukitt-Hale, B.; Bielinski, D.F.; Lau, F.C.; Willis, L.M.; Carey, A.N.; Joseph, J.A. The beneficial effects of berries on cognition, motor behaviour and neuronal function in ageing. Br. J. Nutr. 2015, 114, 1542–1549. [Google Scholar] [CrossRef]
- Beracochea, D.; Krazem, A.; Henkouss, N.; Haccard, G.; Roller, M.; Fromentin, E. Intake of wild blueberry powder improves episodic-like and working memory during normal aging in mice. Planta Med. 2016, 82, 1163–1168. [Google Scholar] [CrossRef]
- Shukitt-Hale, B.; Kalt, W.; Carey, A.N.; Vinqvist-Tymchuk, M.; McDonald, J.; Joseph, J.A. Plum juice, but not dried plum powder, is effective in mitigating cognitive deficits in aged rats. Nutrition 2009, 25, 567–573. [Google Scholar] [CrossRef]
- Papandreou, M.A.; Dimakopoulou, A.; Linardaki, Z.I.; Cordopatis, P.; Klimis-Zacas, D.; Margarity, M.; Lamari, F.N. Effect of a polyphenol-rich wild blueberry extract on cognitive performance of mice, brain antioxidant markers and acetylcholinesterase activity. Behav. Brain Res. 2009, 198, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Casadesus, G.; Shukitt-Hale, B.; Stellwagen, H.M.; Zhu, X.; Lee, H.-G.; Smith, M.A.; Joseph, J.A. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr. Neurosci. 2004, 7, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Rendeiro, C.; Vauzour, D.; Kean, R.J.; Butler, L.T.; Rattray, M.; Spencer, J.P.E.; Williams, C.M. Blueberry supplementation induces spatial memory improvements and region-specific regulation of hippocampal BDNF mRNA expression in young rats. Psychopharmacology 2012, 223, 319–330. [Google Scholar] [CrossRef]
- Tan, L.; Yang, H.; Pang, W.; Lu, H.; Hu, Y.; Ling, J.; Lu, S.; Zhang, W.; Jiang, Y. Cyanidin-3-O-galactoside and blueberry extracts supplementation improves spatial memory and regulates hippocampal ERK expression in senescence-accelerated mice. Biomed. Environ. Sci. 2014, 27, 186–196. [Google Scholar] [CrossRef]
- Williams, C.M.; El Mohsen, M.A.; Vauzour, D.; Rendeiro, C.; Butler, L.T.; Ellis, J.A.; Whiteman, M.; Spencer, J.P.E. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic. Biol. Med. 2008, 45, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Andres-Lacueva, C.; Shukitt-Hale, B.; Galli, R.L.; Jauregui, O.; Lamuela-Raventos, R.M.; Joseph, J.A. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr. Neurosci. 2005, 8, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Shukitt-Hale, B.; Carey, A.N.; Jenkins, D.; Rabin, B.M.; Joseph, J.A. Beneficial effects of fruit extracts on neuronal function and behavior in a rodent model of accelerated aging. Neurobiol. Aging 2007, 28, 1187–1194. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef]
- Miller, M.G.; Hamilton, D.A.; Joseph, J.A.; Shukitt-Hale, B. Dietary blueberry improves cognition among older adults in a randomized, double-blind, placebo-controlled trial. Eur. J. Nutr. 2018, 57, 1169–1180. [Google Scholar] [CrossRef]
- McNamara, R.K.; Kalt, W.; Shidler, M.D.; McDonald, J.; Summer, S.S.; Stein, A.L.; Stover, A.N.; Krikorian, R. Cognitive response to fish oil, blueberry, and combined supplementation in older adults with subjective cognitive impairment. Neurobiol. Aging 2018, 64, 147–156. [Google Scholar] [CrossRef]
- Bowtell, J.L.; Aboo-Bakkar, Z.; Conway, M.E.; Adlam, A.-L.R.; Fulford, J. Enhanced task-related brain activation and resting perfusion in healthy older adults after chronic blueberry supplementation. Appl. Physiol. Nutr. Metab. 2017, 42, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Boespflug, E.L.; Eliassen, J.C.; Dudley, J.A.; Shidler, M.D.; Kalt, W.; Summer, S.S.; Stein, A.L.; Stover, A.N.; Krikorian, R. Enhanced neural activation with blueberry supplementation in mild cognitive impairment. Nutr. Neurosci. 2018, 21, 297–305. [Google Scholar] [CrossRef] [PubMed]
- DeFuria, J.; Bennett, G.; Strissel, K.J.; Perfield, J.W.; 2nd Milbury, P.E.; Greenberg, A.S.; Obin, M.S. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J. Nutr. 2009, 139, 1510–1516. [Google Scholar] [CrossRef]
- Seymour, E.M.; Tanone, I.I.; Urcuyo-Llanes, D.E.; Lewis, S.K.; Kirakosyan, A.; Kondoleon, M.G.; Kaufman, P.B.; Bolling, S.F. Blueberry intake alters skeletal muscle and adipose tissue peroxisome proliferator-activated receptor activity and reduces insulin resistance in obese rats. J. Med. Food 2011, 14, 1511–1518. [Google Scholar] [CrossRef]
- Nair, A.R.; Elks, C.M.; Vila, J.; Del Piero, F.; Paulsen, D.B.; Francis, J. A blueberry-enriched diet improves renal function and reduces oxidative stress in metabolic syndrome animals: Potential mechanism of TLR4-MAPK signaling pathway. PLoS ONE 2014, 9, e111976. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Tang, Q.; Gao, Z.; Yu, Z.; Song, H.; Zheng, X.; Chen, W. Blueberry and mulberry juice prevent obesity development in C57BL/6 mice. PLoS ONE 2013, 8, e77585. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Kuhn, P.; Rojo, L.E.; Lila, M.A.; Raskin, I. Blueberry polyphenol-enriched soybean flour reduces hyperglycemia, body weight gain and serum cholesterol in mice. Pharmacol. Res. 2013, 68, 59–67. [Google Scholar] [CrossRef]
- Muraki, I.; Imamura, F.; Manson, J.E.; Hu, F.B.; Willett, W.C.; van Dam, R.M.; Sun, Q. Fruit consumption and risk of type 2 diabetes: Results from three prospective longitudinal cohort studies. BMJ 2013, 347, f5001. [Google Scholar] [CrossRef]
- Jennings, A.; Welch, A.A.; Spector, T.; Macgregor, A.; Cassidy, A. Intakes of anthocyanins and flavones are associated with biomarkers of insulin resistance and inflammation in women. J. Nutr. 2014, 144, 202–208. [Google Scholar] [CrossRef]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in blueberries improve insulin sensitivity in obese, insulin resistant men and women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef]
- Bell, L.; Lamport, D.J.; Butler Williams, C.M. A study of Glycaemic effects following acute anthocyanin-rich blueberry supplementation in healthy young adults. Food Funct. 2017, 8, 3104–3110. [Google Scholar] [CrossRef] [PubMed]
- Whyte, A.R.; Rahman, S.; Bell, L.; Edirisinghe, I.; Krikorian, R.; Williams, C.M.; Burton-Freeman, B. Improved metabolic function and cognitive performance in middle-aged adults following a single dose of wild blueberry. Eur. J. Nutr. 2021, 60, 1521–1536. [Google Scholar] [CrossRef] [PubMed]
- Craft, S. Insulin resistance syndrome and Alzheimer’s disease: Age- and obesity-related effect on memory, amyloid, and inflammation. Neurobiol. Aging 2005, 26, S65–S69. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, L.; Pupi, A.; De Leon, M.J. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2008, 1147, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Anselm, E.; Chataigneau, M.; Ndiaye, M.; Chataigneau, T.; Schini-Kerth, V.B. Grape juice causes endothelium-dependent relaxation via a redox-sensitive Src- and Akt-dependent activation of eNOS. Cardiovasc. Res. 2007, 73, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Borniquel, S.; Valle, I.; Cadenas, S.; Lamas, S.; Monsalve, M. Nitric oxide regulates mitochondrial oxidative stress protection via the transcriptional coactivator PGC-1 alpha. FASEB J. 2006, 11, 1889–1891. [Google Scholar] [CrossRef] [PubMed]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar] [CrossRef]
- Krikorian, R.; Kalt, W.; McDonald, J.E.; Shidler, M.D.; Summer, S.S.; Stein, A.L. Cognitive performance in relation to urinary anthocyanins and their flavonoid-based products following blueberry supplementation in older adults at risk for dementia. J. Funct. Food 2020, 262, 103667. [Google Scholar] [CrossRef]
- Hubbard, W.B.; Banerjee, M.; Vekaria, H.; Prakhya, K.S.; Joshi, S.; Wang, Q.J.; Saatman, K.E.; Whiteheart, S.W.; Sullivan, P.G. Differential leukocyte and platelet profiles in distinct models of traumatic brain injury. Cells 2021, 10, 500. [Google Scholar] [CrossRef]
- Krikorian, R.; Zimmerman, M.E.; Fleck, D.E. Inhibitory control in obsessive compulsive disorder. Brain Cogn. 2004, 54, 257–259. [Google Scholar] [CrossRef]
- Royle, J.; Lincoln, N.B. The everyday memory questionnaire-revised: Development of a 13-item scale. Disabil. Rehabil. 2008, 30, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Delis, D.C.; Kramer, J.H.; Kaplan, E.; Ober, B.A. California Verbal Learning Test, 2nd ed.; Psychological Corporation: San Antonio, TX, USA, 2000. [Google Scholar]
- Krikorian, R. Independence of verbal and spatial paired associate learning. Brain Cogn. 1996, 32, 219–223. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Manual for the Beck Depression Inventory-II; Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Fisk, J.E.; Sharp, C.A. Age-related impairment in executive functioning: Updating, inhibition, shifting, and access. J. Clin. Exp. Neuropsychol. 2003, 26, 874–890. [Google Scholar] [CrossRef]
- Miceli, G.; Caltagirone, C.; Gainotti, G.; Masullo, C.; Silveri, M.C. Neuropsychological correlates of localized cerebral lesions in nonaphasic brain-damaged patients. J. Clin. Neuropsychol. 1981, 3, 53–63. [Google Scholar] [CrossRef]
- Sheeber, L.B.; Sorensen, E.D.; Howe, S.R. Data analytic techniques for treatment outcome studies with pretest/posttest measurements: An extensive primer. J. Psychiatr. Res. 1996, 30, 185–199. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Mahwak, NJ, USA, 1988. [Google Scholar]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Wahrenberg, H.; Hertel, K.; Leijonhufvud, B.; Persson, L.; Toft, E.; Arner, P. Use of waist circumference to predict insulin resistance: Retrospective study. Br. Med. J. 2005, 330, 1363–1364. [Google Scholar] [CrossRef]
- Shao, Z.; Janse, E.; Visser, K.; Meyer, A.S. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front. Psychol. 2014, 5, 772. [Google Scholar] [CrossRef]
- De Beni, R.; Palladino, P. Intrusion errors in working memory tasks. Are they related to reading comprehension ability? Learn. Individ. Differ. 2000, 12, 131–143. [Google Scholar] [CrossRef]
- Daum, I.; Graber, S.; Schugens, M.M.; Mayes, A.R. Memory dysfunction of the frontal type in normal ageing. Neuroreport 1996, 7, 15–17. [Google Scholar] [CrossRef]
- Daum, I.; Mayes, A.R. Memory and executive function impairment after frontal or posterior cortex lesions. Behav. Neurol. 2000, 12, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Kopelman, M.D.; Stanhope, N.; Kangley, D. Temporal and spatial context memory in patients with focal frontal, temporal lobe, and diencephalic lesions. Neuropsychologia 1996, 19, 32–41. [Google Scholar] [CrossRef]
- Daoust, J.; Schaffer, J.; Zeighami, Y.; Dagher, A.; García-García, I.; Michaud, A. White matter integrity differences in obesity: A meta-analysis of diffusion tensor imaging studies. Neurosci. Biobehav. Rev. 2021, 129, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.F.; Caine, D.; Burns, A.; Herholz, K.; Ralph, M.A. Staging of the cognitive decline in Alzheimer’s disease: Insights from a detailed neuropsychological investigation of mild cognitive impairment and mild Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2012, 27, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, H.J.; Brunsdon, V.E.A.; Bradford, E.E.F. The developmental trajectories of executive function from adolescence to old age. Sci. Rep. 2021, 11, 1382. [Google Scholar] [CrossRef] [PubMed]
- Favieri, F.; Forte, G.; Casagrande, M. The executive functions in overweight and obesity: A systematic review of neuropsychological cross-sectional and longitudinal studies. Front. Psychol. 2019, 10, 2126. [Google Scholar] [CrossRef]
- Syan, S.K.; Owens, M.M.; Goodman, B.; Epstein, L.H.; Meyre, D.; Sweet, L.H.; MacKillop, J. Deficits in executive function and suppression of default mode network in obesity. NeuroImage Clin. 2019, 24, 102015. [Google Scholar] [CrossRef]
- Schmoller, A.; Hass, T.; Strugovshchikova, O.; Melchert, U.H.; Scholand-Engler, H.G.; Peters, A.; Schweiger, U.; Hohagen, F.; Oltmanns, K.M. Evidence for a relationship between body mass and energy metabolism in the human brain. J. Cereb. Blood Flow Metab. 2010, 30, 1403–1410. [Google Scholar] [CrossRef]
- Yang, Y.; Shields, G.S.; Guo, C.; Liu, Y. Executive function performance in obesity and overweight individuals: A meta-analysis and review. Neurosci. Biobehav. Rev. 2018, 84, 225–244. [Google Scholar] [CrossRef]
- Murman, D.L. The impact of age on cognition. Semin. Hear. 2015, 36, 111–121. [Google Scholar] [CrossRef]
- Buckner, R.L. Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron 2004, 44, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Belwal, T.; Nabavi, S.F.; Nabavi, S.M.; Habtemariam, S. Dietary anthocyanins and insulin resistance: When food becomes a medicine. Nutrients 2017, 9, 1111. [Google Scholar] [CrossRef]
- Jia, Y.; Wu, C.; Kim, Y.S.; Yang, S.O.; Kim, Y.; Kim, J.S.; Jeong, M.Y.; Lee, J.H.; Kim, B.; Lee, S.; et al. A dietary anthocyanin cyanidin-3-O-glucoside binds to PPARs to regulate glucose metabolism and insulin sensitivity in mice. Commun. Biol. 2020, 3, 514. [Google Scholar] [CrossRef] [PubMed]
- Scazzocchio, B.; Varì, R.; Filesi, C.; D’Archivio, M.; Santangelo, C.; Giovannini, C.; Iacovelli, A.; Silecchia, G.; Li Volti, G.; Galvano, F.; et al. Cyanidin-3-O-β-glucoside and protocatechuic acid exert insulin-like effects by upregulating PPARγ activity in human omental adipocytes. Diabetes 2011, 60, 2234–2244. [Google Scholar] [CrossRef] [PubMed]
- Akkarachiyasit, S.; Charoenlertkul, P.; Yibchok-Anun, S.; Adisakwattana, S. Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Int. J. Mol. Sci. 2010, 11, 3387–3396. [Google Scholar] [CrossRef]
- You, Q.; Chen, F.; Wang, X.; Luo, P.G.; Jiang, Y. Inhibitory effects of muscadine anthocyanins on α-glucosidase and pancreatic lipase activities. J. Agric. Food Chem. 2011, 59, 9506–9511. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, E.; Jensen, M.D. Quantification of adipose tissue insulin sensitivity. J. Investig. Med. 2016, 64, 989–991. [Google Scholar] [CrossRef]
- Ferré, P. The biology of peroxisome proliferator-activated receptors: Relationship with lipid metabolism and insulin sensitivity. Diabetes 2004, 53, S43–S50. [Google Scholar] [CrossRef]
- Gomes, J.V.P.; Rigolon, T.C.R.; Souza, M.S.; Alvarez-Leite, J.I.A.; DellaLucia, C.M.; Martino, H.S.D.; Rosa, C. Antiobesity effects of anthocyanins on mitochondrial biogenesis, inflammation, and oxidative stress: A systematic review. Nutrition 2019, 66, 192–202. [Google Scholar] [CrossRef]
- Luchsinger, J.A.; Ming-Xiu, T.; Shea, S.; Mayeux, R. Hyperinsulinemia and risk of Alzheimer’s disease. Neurology 2004, 63, 1187–1192. [Google Scholar] [CrossRef]
- Mullins, R.J.; Diehl, T.C.; Chia, C.W.; Kapogiannis, D. Insulin resistance as a link between amyloid-beta and tau pathologies in Alzheimer’s disease. Front. Aging Neurosci. 2017, 91, 118. [Google Scholar] [CrossRef] [PubMed]
- Lagoa, R.; Graziani, I.; Lopez-Sanchez, C.; Garcia-Martinez, V.; Gutierrez-Merino, C. Complex I and cytochrome c are molecular targets of flavonoids that inhibit hydrogen peroxide production by mitochondria. Biochim. Biophys. Acta 2011, 1807, 1562–7152. [Google Scholar] [CrossRef] [PubMed]
- Trumbeckaitė, S.; Burdulis, D.; Raudonė, L.; Liobikas, J.; Toleikis, A.; Janulis, V. Direct effects of Vaccinium myrtillus L. fruit extracts on rat heart mitochondrial functions. Phytother. Res. 2013, 27, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Mookerjee, S.A.; Divakaruni, A.S.; Jastroch, M.; Brand, M.D. Mitochondrial uncoupling and lifespan. Mech. Ageing Dev. 2010, 131, 463–472. [Google Scholar] [CrossRef] [PubMed]
| Neurocognitive Measure | Cognitive Domain |
|---|---|
| Controlled Oral Word Association Test [64,65] | Executive ability |
| California Verbal Learning Test [64] | Learning/memory; Executive ability |
| Verbal Paired Associate Learning test [65] | Learning/memory |
| Everyday Memory Questionnaire [63] | Self-rated memory function |
| Beck Depression Inventory [66] | Mood |
| Factor | Placebo (n = 14) | Blueberry (n = 13) | t-Value | p |
|---|---|---|---|---|
| Age, years | 57.2 | 55.6 | 1.01 | 0.32 |
| Education, years | 14.8 | 16.3 | 1.70 | 0.10 |
| Body weight, kg | 94.2 | 93.0 | 0.15 | 0.87 |
| BMI | 33.2 | 31.7 | 0.62 | 0.53 |
| Waist circumference, cm | 107.3 | 106.7 | 0.10 | 0.91 |
| Fasting insulin, µU/mL | 10.3 | 10.2 | 0.01 | 0.98 |
| Fasting glucose, mg/dL | 109.5 | 99.3 | 0.64 | 0.52 |
| HbA1c, % | 6.16 | 5.67 | 0.89 | 0.38 |
| Total cholesterol, mg/dL | 197.6 | 200.8 | 0.66 | 0.51 |
| HDL, mg/dL | 53.3 | 61.0 | 1.34 | 0.18 |
| LDL, mg/dL | 116.1 | 128.6 | 1.00 | 0.32 |
| Triglycerides, mg/dL | 140.9 | 98.4 | 1.53 | 0.13 |
| BDI | 6.9 | 9.2 | 0.99 | 0.32 |
| EMQ | 20.7 | 15.4 | 1.53 | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krikorian, R.; Skelton, M.R.; Summer, S.S.; Shidler, M.D.; Sullivan, P.G. Blueberry Supplementation in Midlife for Dementia Risk Reduction. Nutrients 2022, 14, 1619. https://doi.org/10.3390/nu14081619
Krikorian R, Skelton MR, Summer SS, Shidler MD, Sullivan PG. Blueberry Supplementation in Midlife for Dementia Risk Reduction. Nutrients. 2022; 14(8):1619. https://doi.org/10.3390/nu14081619
Chicago/Turabian StyleKrikorian, Robert, Matthew R. Skelton, Suzanne S. Summer, Marcelle D. Shidler, and Patrick G. Sullivan. 2022. "Blueberry Supplementation in Midlife for Dementia Risk Reduction" Nutrients 14, no. 8: 1619. https://doi.org/10.3390/nu14081619
APA StyleKrikorian, R., Skelton, M. R., Summer, S. S., Shidler, M. D., & Sullivan, P. G. (2022). Blueberry Supplementation in Midlife for Dementia Risk Reduction. Nutrients, 14(8), 1619. https://doi.org/10.3390/nu14081619







