Actions of Retinoic Acid in the Pathophysiology of HIV Infection
Abstract
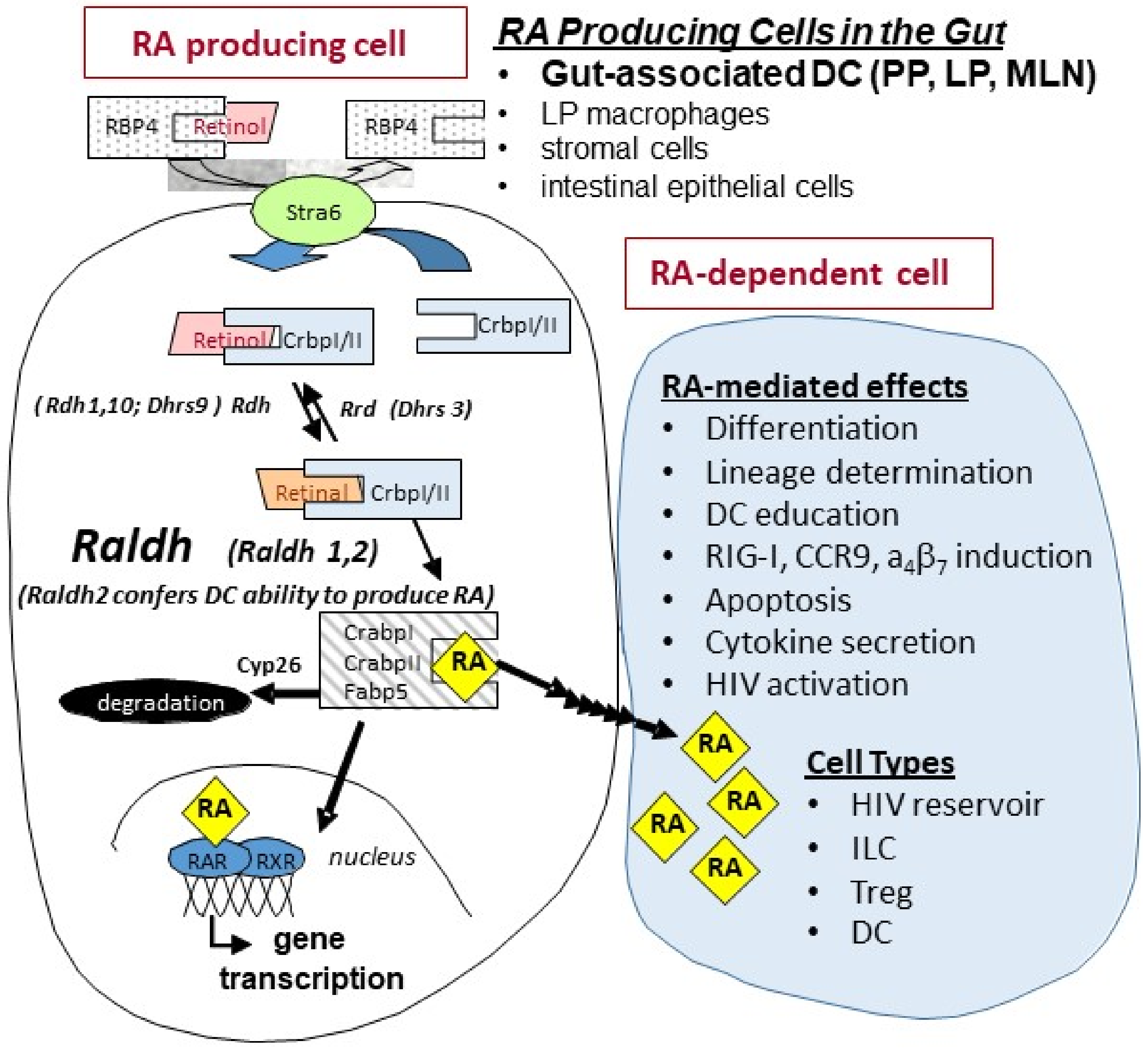
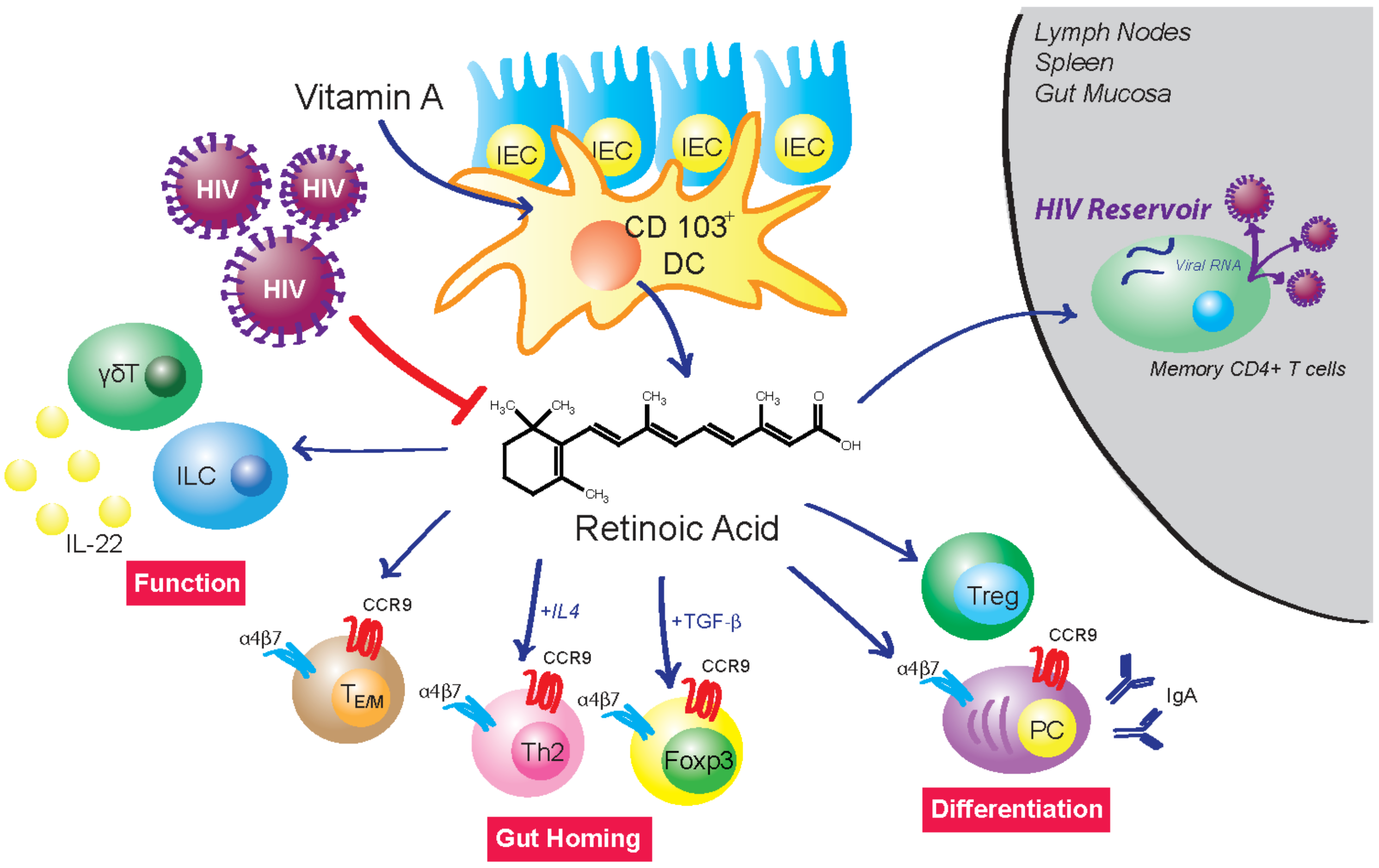
1. Retinoids in Gut Physiology
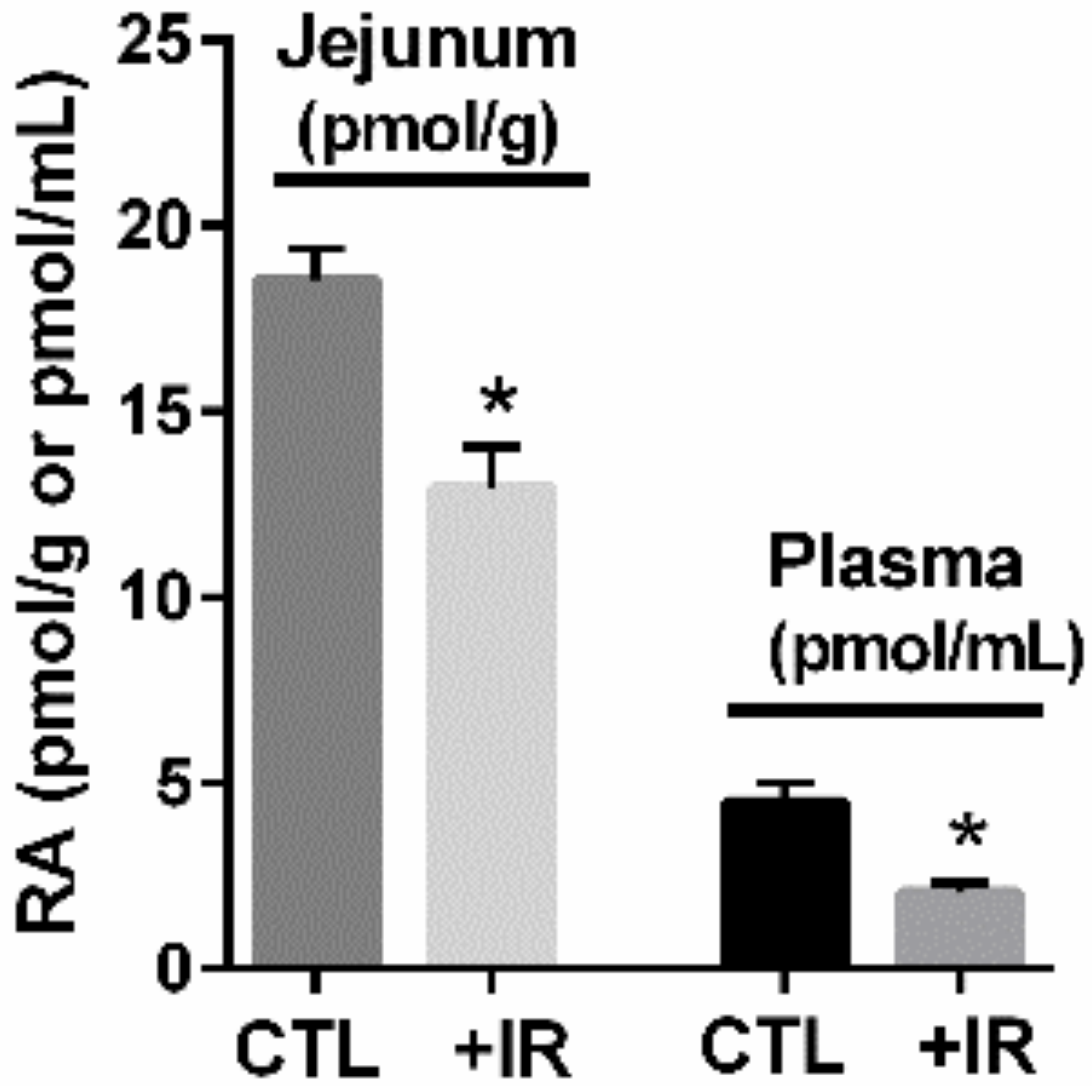
2. Mucosal Damage Results in RA Deficiency and Depletion of RA-Dependent Cell Populations
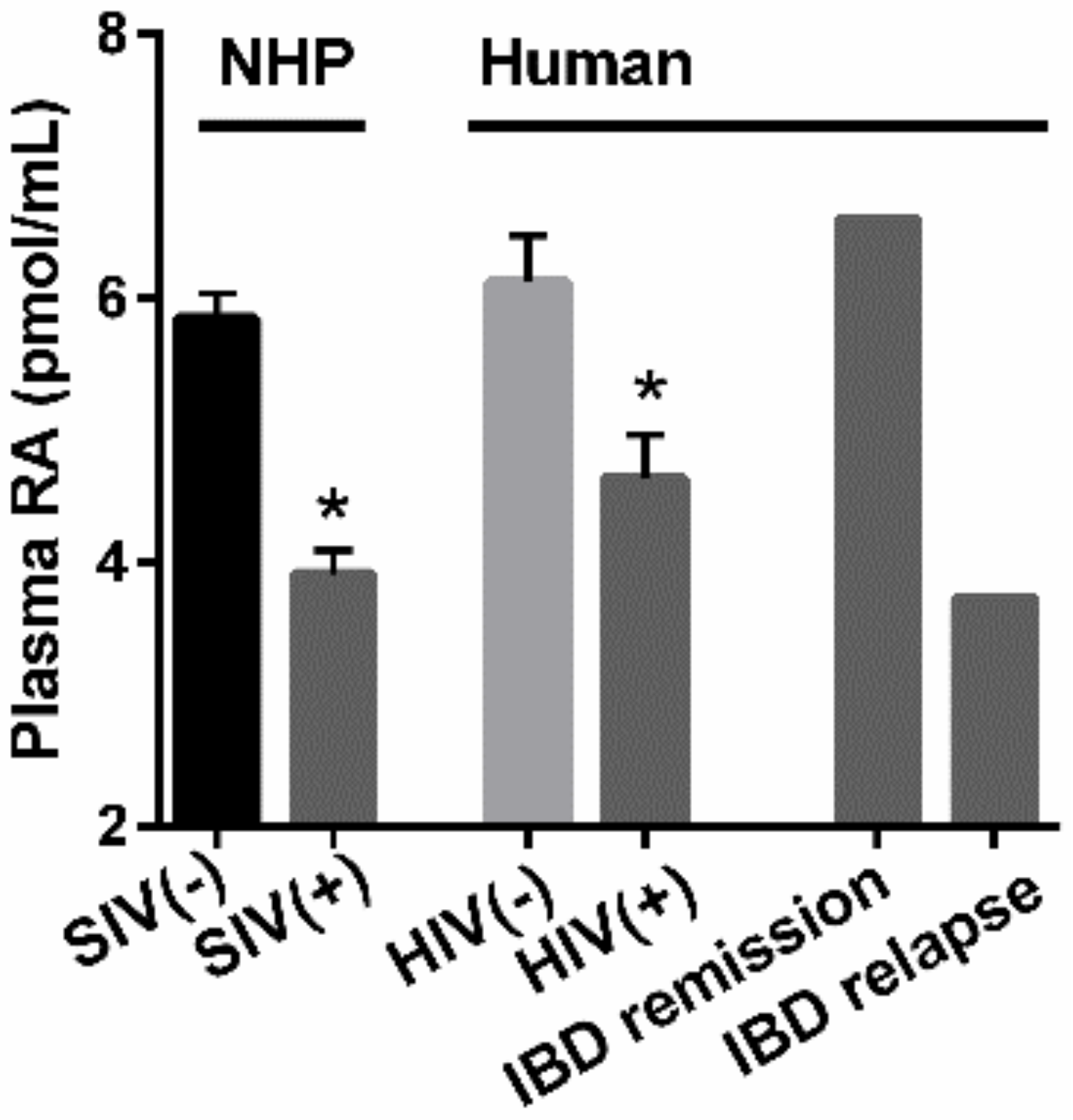
3. HIV and Gut Immunity
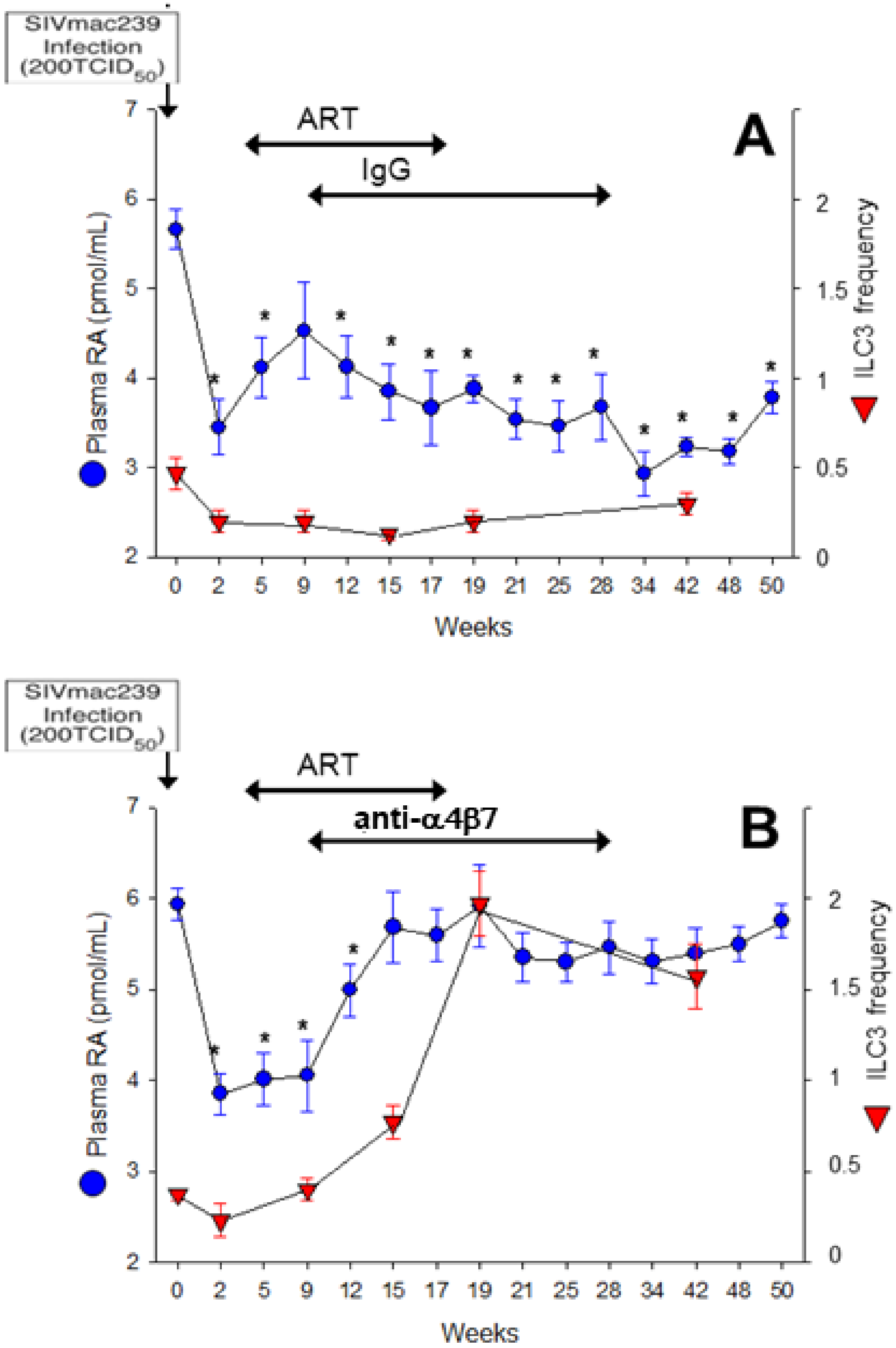
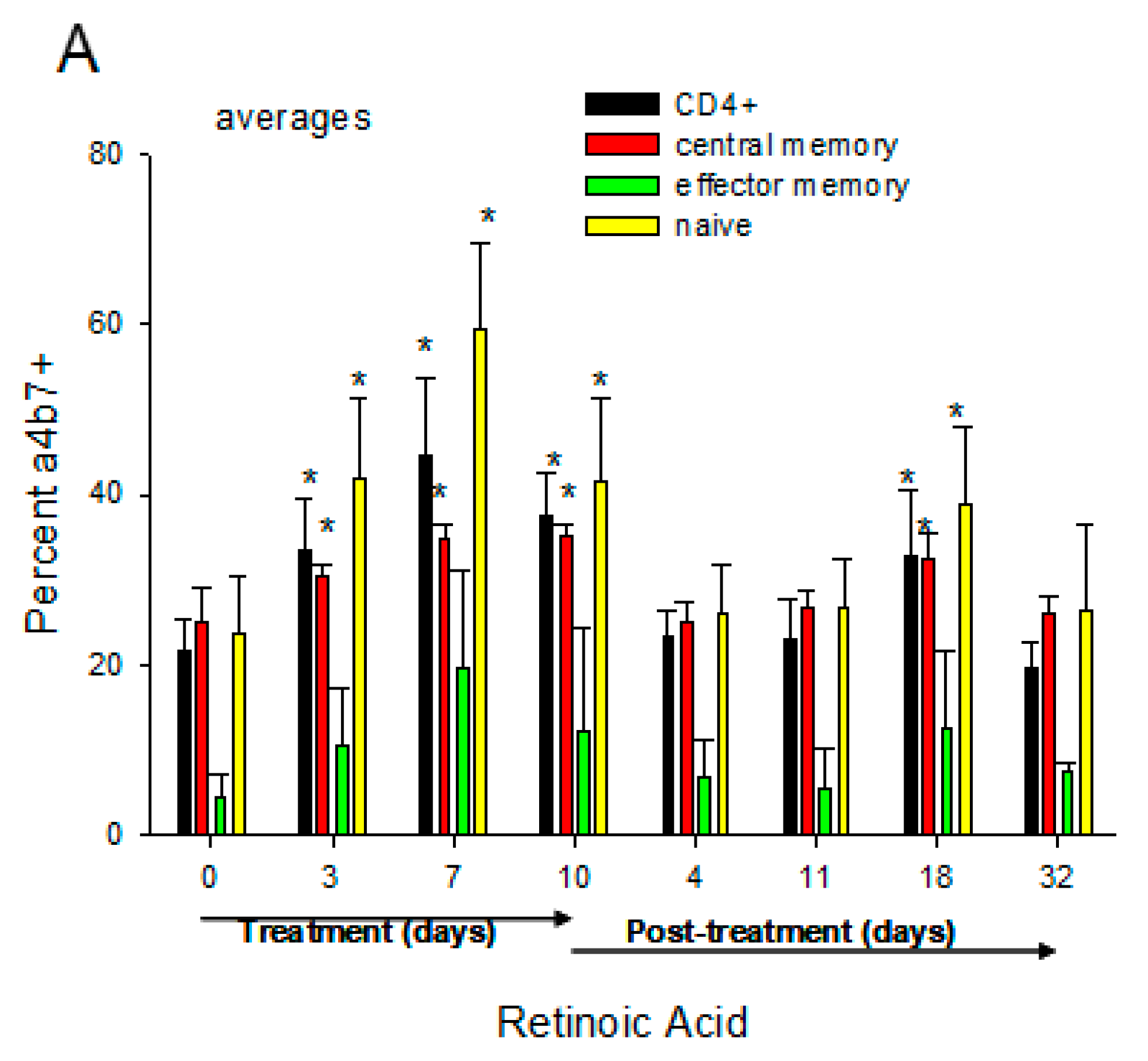
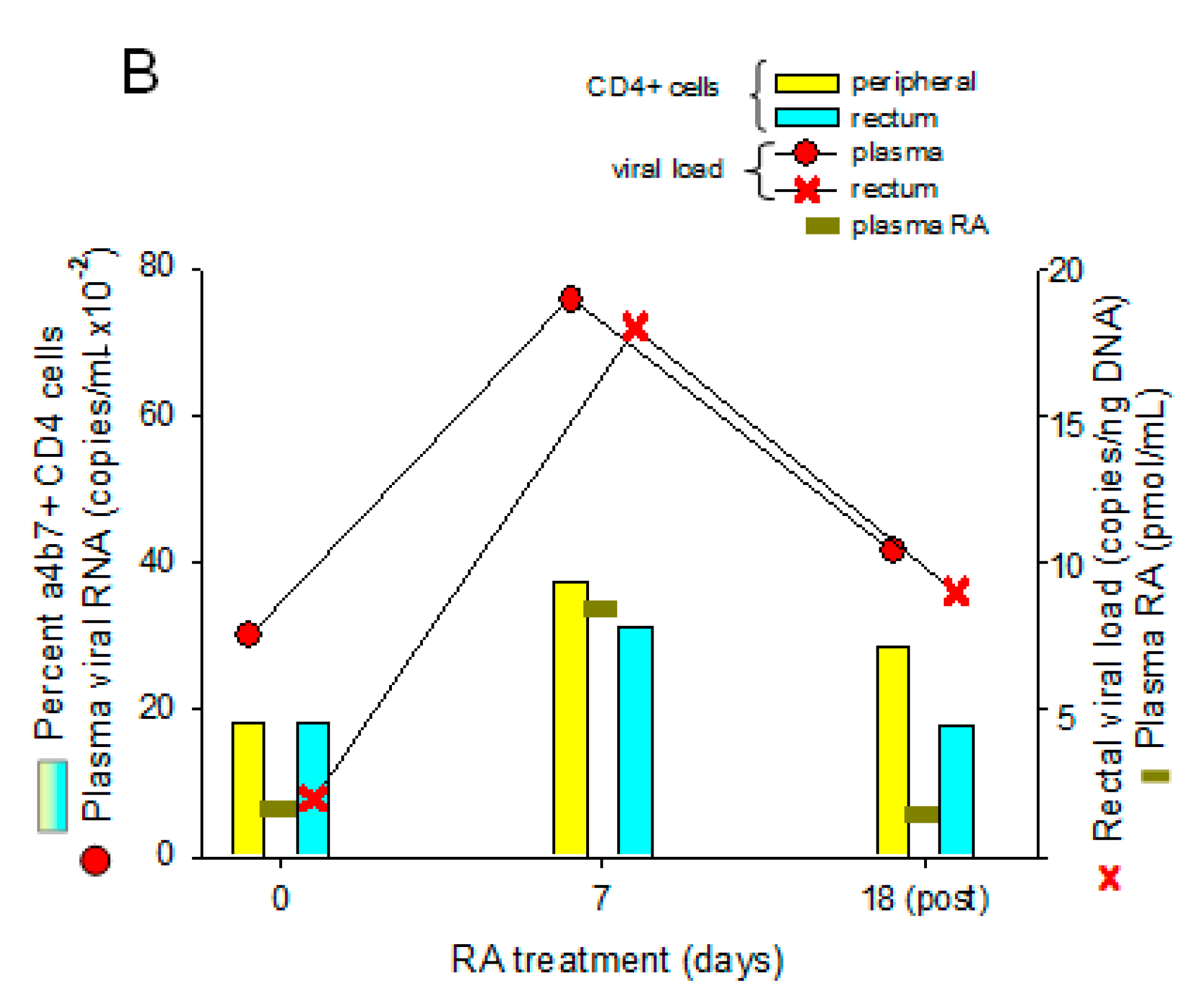
4. Therapeutic Potential of RA to Modify HIV Pathology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czarnewski, P.; Das, S.; Parigi, S.M.; Villablanca, E.J. Retinoic Acid and Its Role in Modulating Intestinal Innate Immunity. Nutrients 2017, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Cassani, B.; Villablanca, E.J.; De Calisto, J.; Wang, S.; Mora, J.R. Vitamin A and immune regulation: Role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance. Mol. Asp. Med. 2012, 33, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Larange, A.; Cheroutre, H. Retinoic Acid and Retinoic Acid Receptors as Pleiotropic Modulators of the Immune System. Annu. Rev. Immunol. 2016, 34, 369–394. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Hirakiyama, A.; Eshima, Y.; Kagechika, H.; Kato, C.; Song, S.-Y. Retinoic Acid Imprints Gut-Homing Specificity on T Cells. Immunity 2004, 21, 527–538. [Google Scholar] [CrossRef]
- Ross, A.C. Vitamin A and retinoic acid in T cell–related immunity. Am. J. Clin. Nutr. 2012, 96, 1166S–1172S. [Google Scholar] [CrossRef]
- Agace, W. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol. Lett. 2010, 128, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Sirisinha, S. The pleiotropic role of vitamin A in regulating mucosal immunity. Asian Pac. J. Allergy Immunol. 2015, 33, 71–89. [Google Scholar]
- Raverdeau, M.; Mills, K. Modulation of T Cell and Innate Immune Responses by Retinoic Acid. J. Immunol. 2014, 192, 2953–2958. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Brown, C.; Ortiz, C.; Noelle, R.J. Leukocyte Homing, Fate, and Function Are Controlled by Retinoic Acid. Physiol. Rev. 2015, 95, 125–148. [Google Scholar] [CrossRef]
- Hall, J.A.; Grainger, J.R.; Spencer, S.P.; Belkaid, Y. The Role of Retinoic Acid in Tolerance and Immunity. Immunity 2011, 35, 13–22. [Google Scholar] [CrossRef]
- Harrison, E.H. Mechanisms of Digestion and Absorption of Dietary Vitamin A. Annu. Rev. Nutr. 2005, 25, 87–103. [Google Scholar] [CrossRef] [PubMed]
- McDonald, K.G.; Leach, M.R.; Brooke, K.W.; Wang, C.; Wheeler, L.W.; Hanly, E.K.; Rowley, C.W.; Levin, M.S.; Wagner, M.; Li, E.; et al. Epithelial Expression of the Cytosolic Retinoid Chaperone Cellular Retinol Binding Protein II Is Essential for in Vivo Imprinting of Local Gut Dendritic Cells by Lumenal Retinoids. Am. J. Pathol. 2012, 180, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, B.; Patil, S.U.; Shreffler, W.G. Vitamins A and D have antagonistic effects on expression of effector cytokines and gut-homing integrin in human innate lymphoid cells. Clin. Exp. Allergy 2015, 45, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Semin. Immunol. 2009, 21, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.R.; Iwata, M.; Eksteen, B.; Song, S.Y.; Junt, T.; Senman, B.; Otipoby, K.L.; Yokota, A.; Takeuchi, H.; Ricciardi-Castagnoli, P.; et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 2006, 314, 1157–1160. [Google Scholar] [CrossRef]
- Reis, B.S.; Rogoz, A.; Costa-Pinto, F.A.; Taniuchi, I.; Mucida, D. Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4(+) T cell immunity. Nat. Immunol. 2013, 14, 271–280. [Google Scholar] [CrossRef]
- Iwata, M.; Yokota, A. Retinoic Acid Production by Intestinal Dendritic Cells. Klotho 2011, 86, 127–152. [Google Scholar] [CrossRef]
- Bhattacharya, N.; Yuan, R.; Prestwood, T.R.; Penny, H.L.; DiMaio, M.A.; Reticker-Flynn, N.; Krois, C.R.; Kenkel, J.A.; Pham, T.D.; Carmi, Y.; et al. Normalizing Microbiota-Induced Retinoic Acid Deficiency Stimulates Protective CD8 + T Cell-Mediated Immunity in Colorectal Cancer. Immunity 2016, 45, 641–655. [Google Scholar] [CrossRef]
- Iliev, I.D.; Mileti, E.; Matteoli, G.; Chieppa, M.; Rescigno, M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2009, 2, 340–350. [Google Scholar] [CrossRef]
- Napoli, J.L.; Yoo, H.S. Retinoid metabolism and functions mediated by retinoid binding-proteins. Methods Enzymol. 2020, 637, 55–75. [Google Scholar] [CrossRef]
- Villablanca, E.J.; Wang, S.; de Calisto, J.; Gomes, D.C.; Kane, M.A.; Napoli, J.L.; Blaner, W.S.; Kagechika, H.; Blomhoff, R.; Rosemblatt, M.; et al. MyD88 and retinoic acid signaling pathways interact to modulate gastrointestinal activities of dendritic cells. Gastroenterology 2011, 141, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-M.; Wang, K.-P.; Ma, J.; Zheng, S.G. The role of all-trans retinoic acid in the biology of Foxp3+ regulatory T cells. Cell. Mol. Immunol. 2015, 12, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Villablanca, E.J. Retinoic acid-producing DCs and gut-tropic FOXP3+regulatory T cells in the induction of oral tolerance. OncoImmunology 2013, 2, e22987. [Google Scholar] [CrossRef] [PubMed]
- Bono, M.R.; Tejon, G.; Flores-Santibañez, F.; Fernandez, D.; Rosemblatt, M.; Sauma, D. Retinoic Acid as a Modulator of T Cell Immunity. Nutrients 2016, 8, 349. [Google Scholar] [CrossRef]
- Spencer, S.P.; Wilhelm, C.; Yang, Q.; Hall, J.A.; Bouladoux, N.; Boyd, A.; Nutman, T.B.; Urban, J.F.; Wang, J.; Ramalingam, T.R.; et al. Adaptation of Innate Lymphoid Cells to a Micronutrient Deficiency Promotes Type 2 Barrier Immunity. Science 2014, 343, 432–437. [Google Scholar] [CrossRef]
- Mielke, L.A.; Jones, S.A.; Raverdeau, M.; Higgs, R.; Stefanska, A.; Groom, J.R.; Misiak, A.; Dungan, L.S.; Sutton, C.E.; Streubel, G.; et al. Retinoic acid expression associates with enhanced IL-22 production by gammadelta T cells and innate lymphoid cells and attenuation of intestinal inflammation. J. Exp. Med. 2013, 210, 1117–1124. [Google Scholar] [CrossRef]
- Bernink, J.H.; Krabbendam, L.; Germar, K.; de Jong, E.; Gronke, K.; Kofoed-Nielsen, M.; Munneke, J.M.; Hazenberg, M.D.; Villaudy, J.; Buskens, C.J.; et al. Interleukin-12 and -23 Control Plasticity of CD127+ Group 1 and Group 3 Innate Lymphoid Cells in the Intestinal Lamina Propria. Immunity 2015, 43, 146–160. [Google Scholar] [CrossRef]
- Auci, D.L.; Egilmez, N.K. Synergy of Transforming Growth Factor Beta 1 and All Trans Retinoic Acid in the Treatment of Inflammatory Bowel Disease: Role of Regulatory T cells. J. Gastroenterol. Pancreatol. Liver Disord. 2016, 3. [Google Scholar] [CrossRef]
- Penny, H.L.; Prestwood, T.R.; Bhattacharya, N.; Sun, F.; Kenkel, J.A.; Davidson, M.G.; Shen, L.; Zuniga, L.A.; Seeley, E.S.; Pai, R.; et al. Restoring Retinoic Acid Attenuates Intestinal Inflammation and Tumorigenesis in APCMin/+ Mice. Cancer Immunol. Res. 2016, 4, 917–926. [Google Scholar] [CrossRef]
- Keino, H.; Watanabe, T.; Sato, Y.; Okada, A.A. Anti-inflammatory effect of retinoic acid on experimental autoimmune uveoretinitis. Br. J. Ophthalmol. 2010, 94, 802–807. [Google Scholar] [CrossRef]
- Jijon, H.B.; Suarez-Lopez, L.; Diaz, O.E.; Das, S.; De Calisto, J.; Yaffe, M.B.; Pittet, M.J.; Mora, J.R.; Belkaid, Y.; Xavier, R.J.; et al. Intestinal epithelial cell-specific RARalpha depletion results in aberrant epithelial cell homeostasis and un-derdeveloped immune system. Mucosal Immunol. 2018, 11, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, C.A.; Spencer, S.P.; Torabi-Parizi, P.; Grainger, J.R.; Roychoudhuri, R.; Ji, Y.; Sukumar, M.; Muranski, P.; Scott, C.D.; Hall, J.A.; et al. Retinoic acid controls the homeostasis of pre-cDC-derived splenic and intestinal dendritic cells. J. Exp. Med. 2013, 210, 1961–1976. [Google Scholar] [CrossRef]
- Erkelens, M.N.; Mebius, R.E. Retinoic Acid and Immune Homeostasis: A Balancing Act. Trends Immunol. 2017, 38, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Klatt, N.R.; Estes, J.D.; Sun, X.; Ortiz, A.M.; Barber, J.S.; Harris, L.D.; Cervasi, B.; Yokomizo, L.K.; Pan, L.; Vinton, C.L.; et al. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol. 2012, 5, 646–657. [Google Scholar] [CrossRef]
- Huang, W.; Yu, J.; Liu, T.; Tudor, G.; Defnet, A.E.; Zalesak, S.; Kumar, P.; Booth, C.; Farese, A.M.; MacVittie, T.J.; et al. Proteomic Evaluation of the Natural History of the Acute Radiation Syndrome of the Gastrointestinal Tract in a Non-human Primate Model of Partial-body Irradiation with Minimal Bone Marrow Sparing Includes Dysregulation of the Retinoid Pathway. Health Phys. 2020, 119, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.; Brynjolfsson, S.; Dige, A.; Uronen-Hansson, H.; Börjesson, L.G.; Bengtsson, J.L.; Gudjonsson, S.; Ohman, L.; Agnholt, J.; Sjovall, H.; et al. Macrophage and dendritic cell subsets in IBD: ALDH+ cells are reduced in colon tissue of patients with ulcerative colitis regardless of inflammation. Mucosal Immunol. 2016, 9, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.B.; Aherne, C.M.; Kominsky, D.; McNamee, E.N.; Lebsack, M.D.; Eltzschig, H.; Jedlicka, P.; Rivera–Nieves, J. Retinoic Acid Attenuates Ileitis by Restoring the Balance Between T-Helper 17 and T Regulatory Cells. Gastroenterology 2011, 141, 1821–1831. [Google Scholar] [CrossRef]
- Coombes, J.L.; Siddiqui, K.R.; Arancibia-Carcamo, C.V.; Hall, J.; Sun, C.M.; Belkaid, Y.; Powrie, F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007, 204, 1757–1764. [Google Scholar] [CrossRef]
- Sun, C.-M.; Hall, J.A.; Blank, R.B.; Bouladoux, N.; Oukka, M.; Mora, J.R.; Belkaid, Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007, 204, 1775–1785. [Google Scholar] [CrossRef]
- Mucida, D.; Park, Y.; Kim, G.; Turovskaya, O.; Scott, I.; Kronenberg, M.; Cheroutre, H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 2007, 317, 256–260. [Google Scholar] [CrossRef]
- Xiao, S.; Jin, H.; Korn, T.; Liu, S.M.; Oukka, M.; Lim, B.; Kuchroo, V.K. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J. Immunol. 2008, 181, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Elias, K.M.; Laurence, A.; Davidson, T.S.; Stephens, G.; Kanno, Y.; Shevach, E.M.; O’Shea, J.J. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independ-ent signaling pathway. Blood 2008, 111, 1013–1020. [Google Scholar] [CrossRef]
- Strauch, U.G.; Grunwald, N.; Obermeier, F.; Gürster, S.; Rath, H.C. Loss of CD103+ intestinal dendritic cells during colonic inflammation. World J. Gastroenterol. 2010, 16, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Laffont, S.; Siddiqui, K.R.; Powrie, F. Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur. J. Immunol. 2010, 40, 1877–1883. [Google Scholar] [CrossRef]
- Brown, C.C.; Esterhazy, D.; Sarde, A.; London, M.; Pullabhatla, V.; Osma-Garcia, I.; Al-Bader, R.; Ortiz, C.; Elgueta, R.; Arno, M.; et al. Retinoic Acid Is Essential for Th1 Cell Lineage Stability and Prevents Transition to a Th17 Cell Program. Immunity 2015, 42, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Tejon, G.P.; Manríquez, V.; De Calisto, J.; Flores-Santibañez, F.; Hidalgo, Y.; Crisóstomo, N.; Fernandez, D.; Sauma, D.; Mora, J.R.; Bono, M.R.; et al. Vitamin A Impairs the Reprogramming of Tregs into IL-17-Producing Cells during Intestinal Inflammation. BioMed Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Yu, J.; Huang, W.; Liu, T.; Defnet, A.E.; Zalesak-Kravec, S.; Farese, A.M.; MacVittie, T.J.; Kane, M.A. Effect of Radiation on the Essential Nutrient Homeostasis and Signaling of Retinoids in a Non-human Primate Model with Minimal Bone Marrow Sparing. Health Phys. 2021, 121, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H. Control of Innate and Adaptive Lymphocytes by the RAR-Retinoic Acid Axis. Immune Netw. 2018, 18, e1. [Google Scholar] [CrossRef]
- Alter, G.; Teigen, N.; Davis, B.T.; Addo, M.; Suscovich, T.J.; Waring, M.T.; Streeck, H.; Johnston, M.N.; Staller, K.; Zaman, M.T.; et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood 2005, 106, 3366–3369. [Google Scholar] [CrossRef]
- Alter, G.; Heckerman, D.; Schneidewind, A.; Fadda, L.; Kadie, C.M.; Carlson, J.M.; Oniangue-Ndza, C.; Martin, M.; Li, B.; Khakoo, S.; et al. HIV-1 adaptation to NK-cell-mediated immune pressure. Nature 2011, 476, 96–100. [Google Scholar] [CrossRef]
- O’Connor, G.M.; Holmes, A.; Mulcahy, F.; Gardiner, C.M. Natural killer cells from long-term non-progressor HIV patients are characterized by altered pheno-type and function. Clin. Immunol. 2007, 124, 277–283. [Google Scholar] [CrossRef]
- Fehniger, T.; Herbein, G.; Yu, H.; Para, M.I.; Bernstein, Z.P.; O’Brien, W.A.; Caligiuri, M.A. Natural killer cells from HIV-1+ patients produce C-C chemokines and inhibit HIV-1 infection. J. Immunol. 1998, 161, 6433–6438. [Google Scholar] [PubMed]
- Shieh, T.M.; Carter, D.L.; Blosser, R.L.; Mankowski, J.L.; Zink, M.C.; Clements, J.E. Functional analyses of natural killer cells in macaques infected with neurovirulent simian immunodeficiency virus. J. Neurovirol. 2001, 7, 11–24. [Google Scholar] [PubMed]
- Vowels, B.R.; Gershwin, M.E.; Gardner, M.B.; McGRAW, T.P. Natural Killer Cell Activity of Rhesus Macaques Against Retrovirus-Pulsed CD4+ Target Cells. AIDS Res. Hum. Retrovir. 1990, 6, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Giavedoni, L.D.; Velasquillo, M.C.; Parodi, L.M.; Hubbard, G.B.; Hodara, V.L. Cytokine Expression, Natural Killer Cell Activation, and Phenotypic Changes in Lymphoid Cells from Rhesus Macaques during Acute Infection with Pathogenic Simian Immunodeficiency Virus. J. Virol. 2000, 74, 1648–1657. [Google Scholar] [CrossRef][Green Version]
- Pereira, L.; Johnson, R.; Ansari, A. Sooty mangabeys and rhesus macaques exhibit significant divergent natural killer cell responses during both acute and chronic phases of SIV infection. Cell. Immunol. 2008, 254, 10–19. [Google Scholar] [CrossRef]
- Bostik, P.; Kobkitjaroen, J.; Tang, W.; Villinger, F.; Pereira, L.E.; Little, D.M.; Stephenson, S.T.; Bouzyk, M.; Ansari, A.A. Decreased NK Cell Frequency and Function Is Associated with Increased Risk of KIR3DL Allele Polymorphism in Simian Immunodeficiency Virus-Infected Rhesus Macaques with High Viral Loads. J. Immunol. 2009, 182, 3638–3649. [Google Scholar] [CrossRef]
- Takahashi, Y.; Byrareddy, S.N.; Albrecht, C.; Brameier, M.; Walter, L.; Mayne, A.E.; Dunbar, R.; Russo, R.; Little, D.M.; Villinger, T.; et al. In Vivo Administration of a JAK3 Inhibitor during Acute SIV Infection Leads to Significant Increases in Viral Load during Chronic Infection. PLOS Pathog. 2014, 10, e1003929. [Google Scholar] [CrossRef]
- DeGottardi, M.Q.; Okoye, A.A.; Vaidya, M.; Talla, A.; Konfe, A.L.; Reyes, M.D.; Clock, J.A.; Duell, D.M.; Legasse, A.W.; Sabnis, A.; et al. Effect of Anti–IL-15 Administration on T Cell and NK Cell Homeostasis in Rhesus Macaques. J. Immunol. 2016, 197, 1183–1198. [Google Scholar] [CrossRef]
- Strauss-Albee, D.M.; Fukuyama, J.; Liang, E.C.; Yao, Y.; Jarrell, J.A.; Drake, A.L.; Kinuthia, J.; Montgomery, R.R.; John-Stewart, G.; Holmes, S.; et al. Human NK cell repertoire diversity reflects immune experience and correlates with viral suscepti-bility. Sci. Transl. Med. 2015, 7, 297ra115. [Google Scholar] [CrossRef]
- Byrareddy, S.N.; Arthos, J.; Cicala, C.; Villinger, F.; Ortiz, K.T.; Little, D.; Sidell, N.; Kane, M.A.; Yu, J.; Jones, J.W.; et al. Sustained virologic control in SIV+ macaques after antiretroviral and alpha4beta7 antibody therapy. Science 2016, 354, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Cella, M.; Fuchs, A.; Vermi, W.; Facchetti, F.; Otero, K.; Lennerz, J.K.M.; Doherty, J.M.; Mills, J.C.; Colonna, M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 2008, 457, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Takatori, H.; Kanno, Y.; Watford, W.T.; Tato, C.M.; Weiss, G.; Ivanov, I.I.; Littman, D.R.; O’Shea, J.J. Lymphoid tissue inducer–like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 2008, 206, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Spits, H.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate lymphoid cells—A proposal for uniform nomenclature. Nat. Rev. Immunol. 2013, 13, 145–149. [Google Scholar] [CrossRef]
- Hoyler, T.; Klose, C.S.; Souabni, A.; Turqueti-Neves, A.; Pfeifer, D.; Rawlins, E.L.; Voehringer, D.; Busslinger, M.; Diefenbach, A. The Transcription Factor GATA-3 Controls Cell Fate and Maintenance of Type 2 Innate Lymphoid Cells. Immunity 2012, 37, 634–648. [Google Scholar] [CrossRef]
- Mjösberg, J.; Bernink, J.; Golebski, K.; Karrich, J.J.; Peters, C.P.; Blom, B.; Velde, A.A.T.; Fokkens, W.J.; van Drunen, C.M.; Spits, H. The Transcription Factor GATA3 Is Essential for the Function of Human Type 2 Innate Lymphoid Cells. Immunity 2012, 37, 649–659. [Google Scholar] [CrossRef]
- Goverse, G.; Labao-Almeida, C.; Ferreira, M.; Molenaar, R.; Wahlen, S.; Konijn, T.; Koning, J.; Veiga-Fernandes, H.; Mebius, R.E. Vitamin A Controls the Presence of RORgamma+ Innate Lymphoid Cells and Lymphoid Tissue in the Small Intestine. J. Immunol. 2016, 196, 5148–5155. [Google Scholar] [CrossRef]
- Van de Pavert, S.A.; Ferreira, M.; Domingues, R.G.; Ribeiro, H.; Molenaar, R.; Moreira-Santos, L.; Almeida, F.F.; Ibiza, S.; Barbosa, I.; Goverse, G.; et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature 2014, 508, 123–127. [Google Scholar] [CrossRef]
- Reeves, R.K.; Rajakumar, P.A.; Evans, T.I.; Connole, M.; Gillis, J.; Wong, F.E.; Kuzmichev, Y.V.; Carville, A.; Johnson, R.P. Gut inflammation and indoleamine deoxygenase inhibit IL-17 production and promote cytotoxic potential in NKp44+ mucosal NK cells during SIV infection. Blood 2011, 118, 3321–3330. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.; Liu, D.X.; Moroney-Rasmussen, T.; Lackner, A.A.; Veazey, R.S. IL-17-producing innate lymphoid cells are restricted to mucosal tissues and are depleted in SIV-infected macaques. Mucosal Immunol. 2012, 5, 658–669. [Google Scholar] [CrossRef]
- Khowawisetsut, L.; Pattanapanyasat, K.; Onlamoon, N.; Mayne, A.E.; Little, D.M.; Villinger, F.; Ansari, A.A. Relationships between IL-17+ Subsets, Tregs and pDCs That Distinguish among SIV Infected Elite Controllers, Low, Medium and High Viral Load Rhesus Macaques. PLoS ONE 2013, 8, e61264. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, N.P.; Gordon, S.N.; Doster, M.N.; Pegu, P.; Vaccari, M.; Shukur, N.; Schifanella, L.; Pise-Masison, C.A.; Lipinska, D.; Grubczak, K.; et al. Antiretroviral therapy partly reverses the systemic and mucosal distribution of NK cell subsets that is altered by SIVmac251 infection of macaques. Virology 2014, 450–451, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Richert-Spuhler, L.E.; Evans, T.I.; Gillis, J.; Connole, M.; Estes, J.D.; Keele, B.F.; Klatt, N.R.; Reeves, R.K. Hypercytotoxicity and Rapid Loss of NKp44+ Innate Lymphoid Cells during Acute SIV Infection. PLoS Pathog. 2014, 10, e1004551. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Reeves, R.K. Functional perturbation of classical natural killer and innate lymphoid cells in the oral mucosa during SIV infection. Front. Immunol. 2013, 3, 417. [Google Scholar] [CrossRef] [PubMed]
- Spits, H.; Di Santo, J.P. The expanding family of innate lymphoid cells: Regulators and effectors of immunity and tissue remodeling. Nat. Immunol. 2011, 12, 21–27. [Google Scholar] [CrossRef]
- Satoh-Takayama, N.; Vosshenrich, C.A.J.; Lesjean-Pottier, S.; Sawa, S.; Lochner, M.; Rattis, F.; Mention, J.-J.; Thiam, K.; Cerf-Bensussan, N.; Mandelboim, O.; et al. Microbial Flora Drives Interleukin 22 Production in Intestinal NKp46+ Cells that Provide Innate Mucosal Immune Defense. Immunity 2008, 29, 958–970. [Google Scholar] [CrossRef]
- Sonnenberg, G.F.; Monticelli, L.A.; Elloso, M.M.; Fouser, L.A.; Artis, D. CD4+ Lymphoid Tissue-Inducer Cells Promote Innate Immunity in the Gut. Immunity 2011, 34, 122–134. [Google Scholar] [CrossRef]
- Zheng, Y.; Valdez, P.A.; Danilenko, D.M.; Hu, Y.; Sa, S.M.; Gong, Q.; Abbas, A.R.; Modrusan, Z.; Ghilardi, N.; De Sauvage, F.J.; et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008, 14, 282–289. [Google Scholar] [CrossRef]
- Di Mascio, M.; Lifson, J.D.; Srinivasula, S.; Kim, I.; DeGrange, P.; Keele, B.F.; Belli, A.J.; Reimann, K.A.; Wang, Y.; Proschan, M.; et al. Evaluation of an antibody to alpha4beta7 in the control of SIVmac239-nef-stop infection. Science 2019, 365, 1025–1029. [Google Scholar] [CrossRef]
- Di Mascio, M.; Lifson, J.D.; Srinivasula, S.; Kim, I.; DeGrange, P.; Keele, B.F.; Belli, A.J.; Reimann, K.A.; Wang, Y.; Proschan, M.; et al. Lack of therapeutic efficacy of an antibody to alpha4beta7 in SIVmac251-infected rhesus macaques. Science 2019, 365, 1029–1033. [Google Scholar]
- Di Mascio, M.; Lifson, J.D.; Srinivasula, S.; Kim, I.; DeGrange, P.; Keele, B.F.; Belli, A.J.; Reimann, K.A.; Wang, Y.; Proschan, M.; et al. Blocking alpha4beta7 integrin binding to SIV does not improve virologic control. Science 2019, 365, 1033–1036. [Google Scholar]
- Frank, I.; Cigoli, M.; Arif, M.S.; Fahlberg, M.D.; Maldonado, S.; Calenda, G.; Pegu, A.; Yang, E.S.; Rawi, R.; Chuang, G.Y.; et al. Blocking alpha4beta7 integrin delays viral rebound in SHIVSF162P3-infected macaques treated with anti-HIV broadly neutralizing antibodies. Sci. Transl. Med. 2021, 13, eabf7201. [Google Scholar] [CrossRef] [PubMed]
- Zeissig, S.; Rosati, E.; Dowds, C.M.; Aden, K.; Bethge, J.; Schulte, B.; Pan, W.H.; Mishra, N.; Zuhayra, M.; Marx, M.; et al. Vedolizumab is associated with changes in innate rather than adaptive immunity in patients with inflammatory bowel disease. Gut 2019, 68, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Uzzan, M.; Tokuyama, M.; Rosenstein, A.K.; Tomescu, C.; SahBandar, I.N.; Ko, H.M.; Leyre, L.; Chokola, A.; Kaplan-Lewis, E.; Rodriguez, G.; et al. Anti-α4β7 therapy targets lymphoid aggregates in the gastrointestinal tract of HIV-1 infected individuals. Sci. Transl. Med. 2018, 10, eaau4711. [Google Scholar] [CrossRef]
- Paul, S.; Williet, N.; Di Bernado, T.; Berger, A.-E.; Boschetti, G.; Filippi, J.; Del Tedesco, E.; Nancey, S.; Flourie, B.; Roblin, X. Soluble Mucosal Addressin Cell Adhesion Molecule 1 and Retinoic Acid are Potential Tools for Therapeutic Drug Monitoring in Patients with Inflammatory Bowel Disease Treated with Vedolizumab: A Proof of Concept Study. J. Crohn’s Colitis 2018, 12, 1089–1096. [Google Scholar] [CrossRef]
- Da Mata, E.C.G.; Ombredane, A.; Joanitti, G.A.; Kanzaki, L.I.B.; Schwartz, E.F. Antiretroviral and cytotoxic activities of Tityus obscurus synthetic peptide. Arch. Pharm. (Weinheim) 2020, 353, e2000151. [Google Scholar] [CrossRef]
- Shannon, S.R.; Yu, J.; Defnet, A.E.; Bongfeldt, D.; Moise, A.R.; Kane, M.A.; Trainor, P.A. Identifying vitamin A signaling by visualizing gene and protein activity, and by quantification of vitamin A metabolites. Methods Enzymol. 2020, 637, 367–418. [Google Scholar] [CrossRef]
- Jones, J.W.; Pierzchalski, K.; Yu, J.; Kane, M.A. Use of fast HPLC multiple reaction monitoring cubed for endogenous retinoic acid quantification in com-plex matrices. Anal. Chem. 2015, 87, 3222–3230. [Google Scholar] [CrossRef]
- Arthos, J.; Cicala, C.; Martinelli, E.; Macleod, K.; Van Ryk, D.; Wei, D.; Xiao, Z.; Veenstra, T.D.; Conrad, T.P.; Lempicki, R.A.; et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol. 2008, 9, 301–309. [Google Scholar] [CrossRef]
- Nawaz, F.; Goes, L.R.; Ray, J.C.; Olowojesiku, R.; Sajani, A.; Ansari, A.A.; Perrone, I.; Hiatt, J.; Van Ryk, D.; Wei, D.; et al. MAdCAM costimulation through Integrin-alpha4beta7 promotes HIV replication. Mucosal Immunol. 2018, 11, 1342–1351. [Google Scholar] [CrossRef]
- Cicala, C.; Martinelli, E.; McNally, J.P.; Goode, D.J.; Gopaul, R.; Hiatt, J.; Jelicic, K.; Kottilil, S.; Macleod, K.; O’Shea, A.; et al. The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc. Natl. Acad. Sci. USA 2009, 106, 20877–20882. [Google Scholar] [CrossRef] [PubMed]
- Finley, J. Elimination of cancer stem cells and reactivation of latent HIV-1 via AMPK activation: Common mechanism of action linking inhibition of tumorigenesis and the potential eradication of HIV-1. Med. Hypotheses 2017, 104, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Olwenyi, O.A.; Acharya, A.; Routhu, N.K.; Pierzchalski, K.; Jones, J.W.; Kane, M.A.; Sidell, N.; Mohan, M.; Byrareddy, S.N. Retinoic Acid Improves the Recovery of Replication-Competent Virus from Latent SIV Infected Cells. Cells 2020, 9, 2076. [Google Scholar] [CrossRef] [PubMed]
- Bahraoui, E.; Yagello, M.; Billaud, J.N.; Sabatier, J.M.; Guy, B.; Muchmore, E.; Girard, M.; Gluckman, J.C. Immunogenicity of the human immunodeficiency virus (HIV) recombinant nef gene product. Mapping of T-cell and B-cell epitopes in immunized chimpanzees. AIDS Res. Hum. Retrovir. 1990, 6, 1087–1098. [Google Scholar] [CrossRef]
- Zhang, Y.; Planas, D.; Raymond Marchand, L.; Massanella, M.; Chen, H.; Wacleche, V.S.; Gosselin, A.; Goulet, J.P.; Filion, M.; Routy, J.P.; et al. Improving HIV Outgrowth by Optimizing Cell-Culture Conditions and Supplementing With all-trans Retinoic Acid. Front. Microbiol. 2020, 11, 902. [Google Scholar] [CrossRef]
- Li, P.; Kaiser, P.; Lampiris, H.W.; Kim, P.; Yukl, S.A.; Havlir, D.V.; Greene, W.C.; Wong, J.K. Stimulating the RIG-I pathway to kill cells in the latent HIV reservoir following viral reactivation. Nat. Med. 2016, 22, 807–811. [Google Scholar] [CrossRef]
- Garcia-Vidal, E.; Castellvi, M.; Pujantell, M.; Badia, R.; Jou, A.; Gomez, L.; Puig, T.; Clotet, B.; Ballana, E.; Riveira-Munoz, E.; et al. Evaluation of the Innate Immune Modulator Acitretin as a Strategy to Clear the HIV Reservoir. Antimicrob. Agents Chemother. 2017, 61, e01368-17. [Google Scholar] [CrossRef]
- Nelson, C.H.; Peng, C.C.; Lutz, J.D.; Yeung, C.K.; Zelter, A.; Isoherranen, N. Direct protein-protein interactions and substrate channeling between cellular retinoic acid binding proteins and CYP26B1. FEBS Lett. 2016, 590, 2527–2535. [Google Scholar] [CrossRef]
- Tian, K.; Norris, A.W.; Lin, C.L.; Li, E. The isolation and characterization of purified heterocomplexes of recombinant retinoic acid receptor and ret-inoid X receptor ligand binding domains. Biochemistry 1997, 36, 5669–5676. [Google Scholar] [CrossRef]
- Armstrong, J.L.; Ruiz, M.; Boddy, A.V.; Redfern, C.P.F.; Pearson, A.D.J.; Veal, G.J. Increasing the intracellular availability of all-trans retinoic acid in neuroblastoma cells. Br. J. Cancer 2005, 92, 696–704. [Google Scholar] [CrossRef]
- A5325, A Prospective Randomized, Controlled Study to Evaluate the Effect of Isotretinoin on Immune Activation among HIV-1 Infected Participants on Suppressive ART. ClinicalTrials.gov Identifier: NCT01969058. Available online: www.clinicaltrials.gov (accessed on 27 February 2022).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidell, N.; Kane, M.A. Actions of Retinoic Acid in the Pathophysiology of HIV Infection. Nutrients 2022, 14, 1611. https://doi.org/10.3390/nu14081611
Sidell N, Kane MA. Actions of Retinoic Acid in the Pathophysiology of HIV Infection. Nutrients. 2022; 14(8):1611. https://doi.org/10.3390/nu14081611
Chicago/Turabian StyleSidell, Neil, and Maureen A. Kane. 2022. "Actions of Retinoic Acid in the Pathophysiology of HIV Infection" Nutrients 14, no. 8: 1611. https://doi.org/10.3390/nu14081611
APA StyleSidell, N., & Kane, M. A. (2022). Actions of Retinoic Acid in the Pathophysiology of HIV Infection. Nutrients, 14(8), 1611. https://doi.org/10.3390/nu14081611





