CRABPs Alter all-trans-Retinoic Acid Metabolism by CYP26A1 via Protein-Protein Interactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Expression and Purification of CRABP1 and CRABP2
2.3. Expression and Purification of FABP5
2.4. Preparation of CYP26A1 Microsomes from Sf9 Insect Cells
2.5. Determination of atRA-Binding Kinetics with CRABP1 and CRABP2 by Stopped-Flow
2.6. Effect of Binding Proteins on the 4-OH-atRA Formation by CYP3A4, CYP2C8, CYP26A1 and Human Liver Microsomes (HLMs)
2.7. Simulations of the Impact of CRABPs on the 4-OH-atRA Formation Assuming the Free Drug Hypothesis
2.8. 4-OH-atRA Formation Kinetics with CYP26A1 in the Presence and Absence of CRABPs
2.9. Impact of Increasing CRABP to atRA Ratio on the 4-OH-atRA Formation
2.10. Analysis of the Kinetics of Protein–Protein Interactions between CYP26A1, CYP26B1 and CRABPs
3. Results
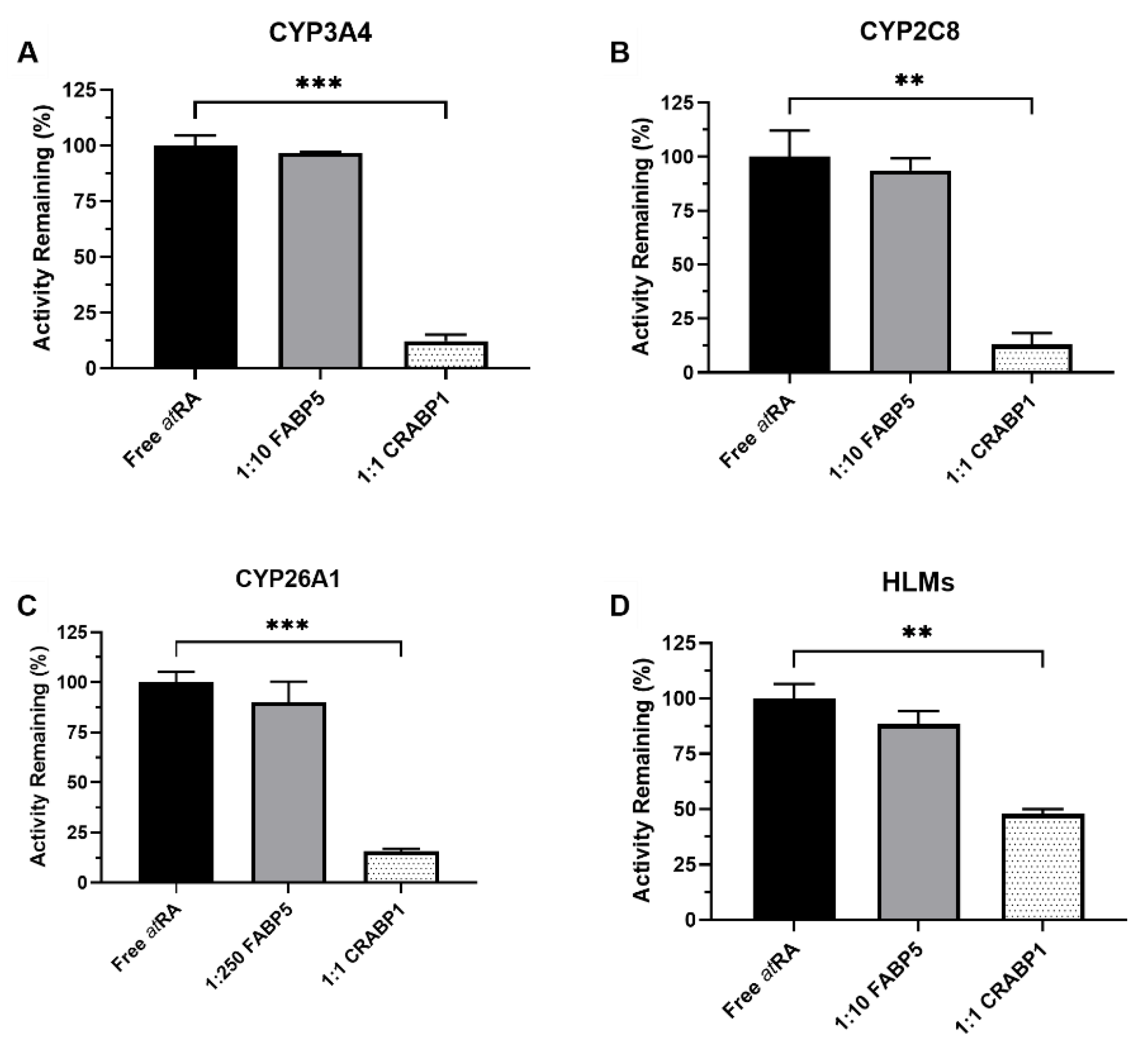
3.1. FABP5 Does Not Affect atRA Hydroxylation by CYPs
3.2. atRA Binds CRABPs with Nanomolar Affinity
3.3. CRABPs Sequester atRA from CYP3A4 and CYP2C8 as Predicted by the Free Drug Hypothesis
3.4. Determination of the Kinetics of the 4-OH-atRA Formation by CYP26A1 in the Presence of CRABPs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rhinn, M.; Dollé, P. Retinoic Acid Signalling during Development. Development 2012, 139, 843–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghiaur, G.; Yegnasubramanian, S.; Perkins, B.; Gucwa, J.L.; Gerber, J.M.; Jones, R.J. Regulation of Human Hematopoietic Stem Cell Self-Renewal by the Microenvironment’s Control of Retinoic Acid Signaling. Proc. Natl. Acad. Sci. USA 2013, 110, 16121–16126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.-H.; Gudas, L.J. Retinoids, Retinoic Acid Receptors, and Cancer. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ross, A.C. Retinoic Acid Regulates Cell Cycle Progression and Cell Differentiation in Human Monocytic THP-1 Cells. Exp. Cell Res. 2004, 297, 68–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, J.M.; Zhong, G.; Hogarth, C.; Huang, W.; Topping, T.; LaFrance, J.; Palau, L.; Czuba, L.C.; Griswold, M.; Ghiaur, G.; et al. Knockout of Cyp26a1 and Cyp26b1 during Postnatal Life Causes Reduced Lifespan, Dermatitis, Splenomegaly, and Systemic Inflammation in Mice. FASEB J. 2020, 34, 15788–15804. [Google Scholar] [CrossRef]
- Stevison, F.; Hogarth, C.; Tripathy, S.; Kent, T.; Isoherranen, N. Inhibition of the All-Trans Retinoic Acid (AtRA) Hydroxylases CYP26A1 and CYP26B1 Results in Dynamic, Tissue-Specific Changes in Endogenous AtRA Signaling. Drug Metab. Dispos. 2017, 45, 846–854. [Google Scholar] [CrossRef] [Green Version]
- Amory, J.K.; Muller, C.H.; Shimshoni, J.A.; Isoherranen, N.; Paik, J.; Moreb, J.S.; Amory, D.W.; Evanoff, R.; Goldstein, A.S.; Griswold, M.D. Suppression of Spermatogenesis by Bisdichloroacetyldiamines Is Mediated by Inhibition of Testicular Retinoic Acid Biosynthesis. J. Androl. 2011, 32, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Raverdeau, M.; Mills, K.H.G. Modulation of T Cell and Innate Immune Responses by Retinoic Acid. J. Immunol. 2014, 192, 2953–2958. [Google Scholar] [CrossRef]
- Karkeni, E.; Bonnet, L.; Astier, J.; Couturier, C.; Dalifard, J.; Tourniaire, F.; Landrier, J.-F. All-Trans-Retinoic Acid Represses Chemokine Expression in Adipocytes and Adipose Tissue by Inhibiting NF-ΚB Signaling. J. Nutr. Biochem. 2017, 42, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, O.; Isoherranen, N.; Guo, M.H.; Lui, J.C.; Jee, Y.H.; Guttmann-Bauman, I.; Acerini, C.; Lee, W.; Allikmets, R.; Yanovski, J.A.; et al. Accelerated Skeletal Maturation in Disorders of Retinoic Acid Metabolism: A Case Report and Focused Review of the Literature. Horm. Metab. Res. 2016, 48, 737–744. [Google Scholar] [CrossRef] [Green Version]
- Blaner, W.S. Vitamin A Signaling and Homeostasis in Obesity, Diabetes, and Metabolic Disorders. Pharmacol. Ther. 2019, 197, 153–178. [Google Scholar] [CrossRef] [PubMed]
- Gudas, L.J.; Wagner, J.A. Retinoids Regulate Stem Cell Differentiation. J. Cell. Physiol. 2011, 226, 322–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chute, J.P.; Muramoto, G.G.; Whitesides, J.; Colvin, M.; Safi, R.; Chao, N.J.; McDonnell, D.P. Inhibition of Aldehyde Dehydrogenase and Retinoid Signaling Induces the Expansion of Human Hematopoietic Stem Cells. Proc. Natl. Acad. Sci. USA 2006, 103, 11707–11712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isoherranen, N.; Zhong, G. Biochemical and Physiological Importance of the CYP26 Retinoic Acid Hydroxylases. Pharmacol. Ther. 2019, 204, 107400. [Google Scholar] [CrossRef]
- Napoli, J.; Posch, K.; FioreIIa, P.; Boerman, M. Physiological Occurrence, Biosynthesis and Metabolism of Retinoic Acid: Evidence for Roles of Cellular Retinol-Binding Protein (CRBP) and Cellular Retinoic Acid-Binding Protein (CRABP) in the Pathway of Retinoic Acid Homeostasis. Biomed. Pharmacother. 1991, 45, 131–143. [Google Scholar] [CrossRef]
- Fiorella, P.D.; Napoli, J.L. Microsomal Retinoic Acid Metabolism. Effects of Cellular Retinoic Acid-Binding Protein (Type I) and C18-Hydroxylation as an Initial Step. J. Biol. Chem. 1994, 269, 10538–10544. [Google Scholar] [CrossRef]
- Lutz, J.D.; Dixit, V.; Yeung, C.K.; Dickmann, L.J.; Zelter, A.; Thatcher, J.E.; Nelson, W.L.; Isoherranen, N. Expression and Functional Characterization of Cytochrome P450 26A1, a Retinoic Acid Hydroxylase. Biochem. Pharmacol. 2009, 77, 258–268. [Google Scholar] [CrossRef] [Green Version]
- Topletz, A.R.; Thatcher, J.E.; Zelter, A.; Lutz, J.D.; Tay, S.; Nelson, W.L.; Isoherranen, N. Comparison of the Function and Expression of CYP26A1 and CYP26B1, the Two Retinoic Acid Hydroxylases. Biochem. Pharmacol. 2012, 83, 149–163. [Google Scholar] [CrossRef] [Green Version]
- Zhong, G.; Ortiz, D.; Zelter, A.; Nath, A.; Isoherranen, N. CYP26C1 Is a Hydroxylase of Multiple Active Retinoids and Interacts with Cellular Retinoic Acid Binding Proteins. Mol. Pharmacol. 2018, 93, 489–503. [Google Scholar] [CrossRef] [Green Version]
- Thatcher, J.E.; Zelter, A.; Isoherranen, N. The Relative Importance of CYP26A1 in Hepatic Clearance of All-Trans Retinoic Acid. Biochem. Pharmacol. 2010, 80, 903–912. [Google Scholar] [CrossRef] [Green Version]
- Zhong, G.; Hogarth, C.; Snyder, J.M.; Palau, L.; Topping, T.; Huang, W.; Czuba, L.C.; LaFrance, J.; Ghiaur, G.; Isoherranen, N. The Retinoic Acid Hydroxylase Cyp26a1 Has Minor Effects on Postnatal Vitamin A Homeostasis, but Is Required for Exogenous AtRA Clearance. J. Biol. Chem. 2019, 294, 11166–11179. [Google Scholar] [CrossRef] [PubMed]
- Napoli, J.L. Cellular Retinoid Binding-Proteins, CRBP, CRABP, FABP5: Effects on Retinoid Metabolism, Function and Related Diseases. Pharmacol. Ther. 2017, 173, 19–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorella, P.D.; Napoli, J.L. Expression of Cellular Retinoic Acid Binding Protein (CRABP) in Escherichia Coli. Characterization and Evidence That Holo-CRABP Is a Substrate in Retinoic Acid Metabolism. J. Biol. Chem. 1991, 266, 16572–16579. [Google Scholar] [CrossRef]
- Dong, D.; Ruuska, S.E.; Levinthal, D.J.; Noy, N. Distinct Roles for Cellular Retinoic Acid-Binding Proteins I and II in Regulating Signaling by Retinoic Acid. J. Biol. Chem. 1999, 274, 23695–23698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fogh, K.; Voorhees, J.J.; Aström, A. Expression, Purification, and Binding Properties of Human Cellular Retinoic Acid-Binding Protein Type I and Type II. Arch. Biochem. Biophys. 1993, 300, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Norris, A.W.; Cheng, L.; Giguère, V.; Rosenberger, M.; Li, E. Measurement of Subnanomolar Retinoic Acid Binding Affinities for Cellular Retinoic Acid Binding Proteins by Fluorometric Titration. Biochim. Biophys. Acta 1994, 1209, 10–18. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Yan, H. Structure-Function Relationships of Cellular Retinoic Acid-Binding Proteins: Quantitative analysis of the ligand binding properties of the wild-type proteins and site-directed mutants *. J. Biol. Chem. 1997, 272, 1541–1547. [Google Scholar] [CrossRef] [Green Version]
- Sanquer, S.; Gilchrest, B.A. Characterization of Human Cellular Retinoic Acid-Binding Protein-I and Protein-II: Ligand-Binding Affinities and Distribution in Skin. Arch. Biochem. Biophys. 1994, 311, 86–94. [Google Scholar] [CrossRef]
- Schug, T.T.; Berry, D.C.; Shaw, N.S.; Travis, S.N.; Noy, N. Opposing Effects of Retinoic Acid on Cell Growth Result from Alternate Activation of Two Different Nuclear Receptors. Cell 2007, 129, 723–733. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, A.; Petrescu, A.D.; Xiong, Y.; Noy, N. Nuclear Translocation of Cellular Retinoic Acid-Binding Protein II Is Regulated by Retinoic Acid-Controlled SUMOylation. J. Biol. Chem. 2011, 286, 42749–42757. [Google Scholar] [CrossRef] [Green Version]
- Nelson, C.H.; Peng, C.-C.; Lutz, J.D.; Yeung, C.K.; Zelter, A.; Isoherranen, N. Direct Protein–Protein Interactions and Substrate Channeling between Cellular Retinoic Acid Binding Proteins and CYP26B1. FEBS Lett. 2016, 590, 2527–2535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glass, S.M.; Guengerich, F.P. Cellular Retinoid-Binding Proteins Transfer Retinoids to Human Cytochrome P450 27C1 for Desaturation. J. Biol. Chem. 2021, 297. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.L.; Porter, T.D.; Wilson, T.E.; Kasper, C.B. Structural Analysis of the FMN Binding Domain of NADPH-Cytochrome P-450 Oxidoreductase by Site-Directed Mutagenesis. J. Biol. Chem. 1989, 264, 7584–7589. [Google Scholar] [CrossRef]
- Berger, W.T.; Ralph, B.P.; Kaczocha, M.; Sun, J.; Balius, T.E.; Rizzo, R.C.; Haj-Dahmane, S.; Ojima, I.; Deutsch, D.G. Targeting Fatty Acid Binding Protein (FABP) Anandamide Transporters—A Novel Strategy for Development of Anti-Inflammatory and Anti-Nociceptive Drugs. PLoS ONE 2012, 7, e50968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omura, T.; Sato, R. The Carbon Monoxide-Binding Pigment of Liver Microsomes: I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J. Biol. Chem. 1964, 239, 2370–2378. [Google Scholar] [CrossRef]
- Hogarth, C.A.; Arnold, S.; Kent, T.; Mitchell, D.; Isoherranen, N.; Griswold, M.D. Processive Pulses of Retinoic Acid Propel Asynchronous and Continuous Murine Sperm Production. Biol. Reprod. 2015, 92, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Napoli, J.L. Physiological Insights into All-Trans-Retinoic Acid Biosynthesis. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2012, 1821, 152–167. [Google Scholar] [CrossRef] [Green Version]





| kon (M−1 min−1) | koff (min−1) | Kd (nM) | |
|---|---|---|---|
| CRABP1 | 1.07 × 109 ± 2.7 × 108 | 4.40 ± 2.4 | 4.7 ± 3.8 |
| CRABP2 | 0.96 × 109 ± 2.2 × 108 | 7.89 ± 6.0 | 7.6 ± 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yabut, K.C.B.; Isoherranen, N. CRABPs Alter all-trans-Retinoic Acid Metabolism by CYP26A1 via Protein-Protein Interactions. Nutrients 2022, 14, 1784. https://doi.org/10.3390/nu14091784
Yabut KCB, Isoherranen N. CRABPs Alter all-trans-Retinoic Acid Metabolism by CYP26A1 via Protein-Protein Interactions. Nutrients. 2022; 14(9):1784. https://doi.org/10.3390/nu14091784
Chicago/Turabian StyleYabut, King Clyde B., and Nina Isoherranen. 2022. "CRABPs Alter all-trans-Retinoic Acid Metabolism by CYP26A1 via Protein-Protein Interactions" Nutrients 14, no. 9: 1784. https://doi.org/10.3390/nu14091784
APA StyleYabut, K. C. B., & Isoherranen, N. (2022). CRABPs Alter all-trans-Retinoic Acid Metabolism by CYP26A1 via Protein-Protein Interactions. Nutrients, 14(9), 1784. https://doi.org/10.3390/nu14091784






