Psychological Interventions and Bariatric Surgery among People with Clinically Severe Obesity—A Systematic Review with Bayesian Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.1.1. Population
2.1.2. Intervention
2.1.3. Outcomes
2.2. Search Strategy
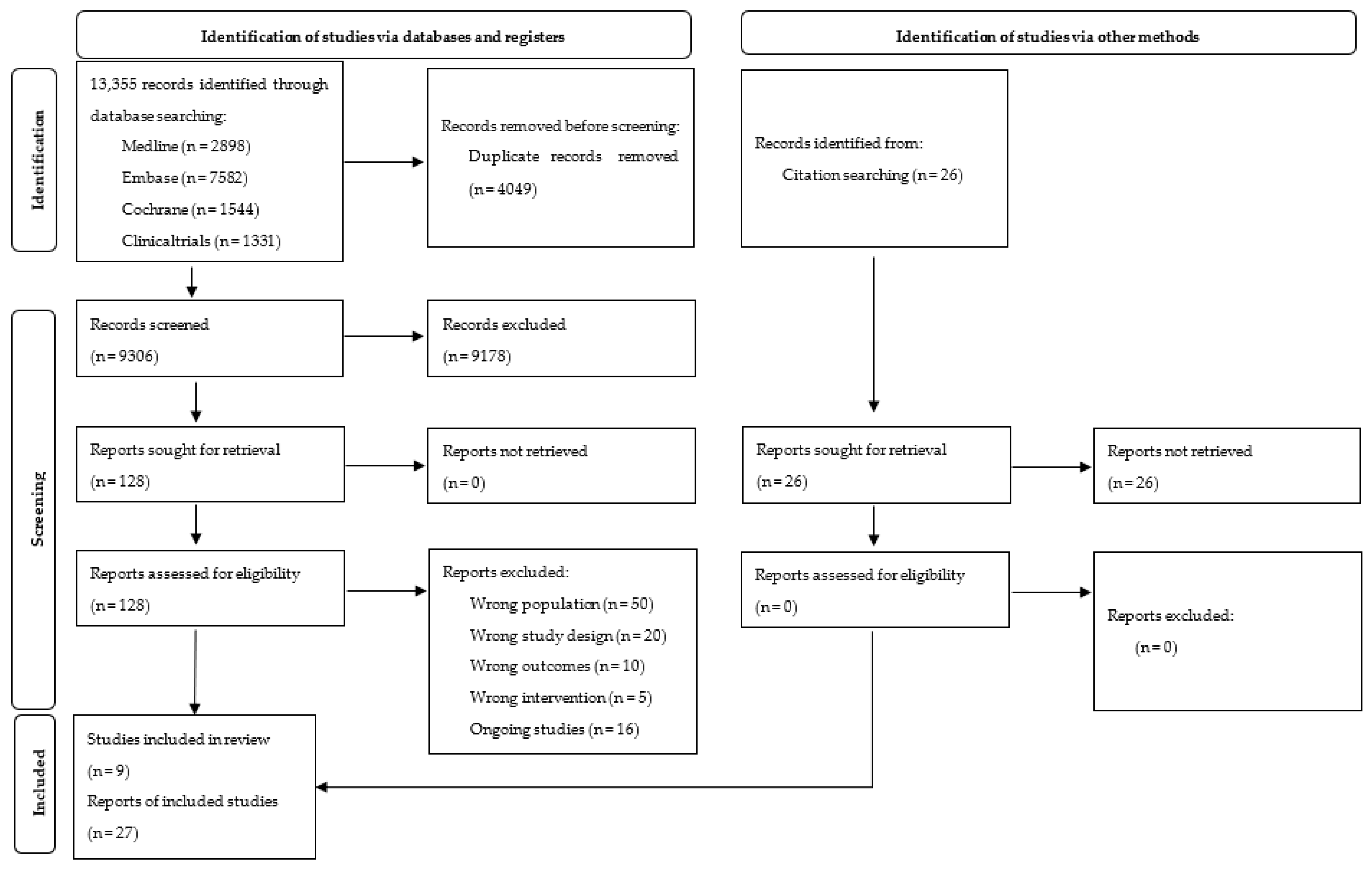
2.3. Study Selection
2.4. Data Extraction
2.5. Risk of Bias (ROB) Assessment
2.6. Data Analysis and Synthesis
2.7. Assessment of Reporting Bias
2.8. Certainty of Evidence
2.9. Sensitivity Analysis
3. Results
3.1. Included Studies
3.1.1. Participants
3.1.2. Intervention
3.1.3. Outcomes
3.2. Excluded Studies
3.3. ROB in Included Studies
3.4. Effects of Interventions
3.4.1. Primary Outcomes
Weight-Related Outcomes
3.4.2. Secondary Outcomes
3.4.3. Sensitivity Analysis
3.5. Certainty of the Evidence
4. Discussion
4.1. Summary of Main Results
4.2. Agreements and Disagreements with Other Studies or Reviews
5. Conclusions
6. Differences between the Protocol and Final Review
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDI | Beck Depression Inventory score |
| BF | Bayes factors |
| BMI | Body Mass Index |
| BS | Bariatric surgery |
| BT | Behavioural therapy |
| CAU | Care as usual |
| CBT | Cognitive-behavioural therapy |
| COI | Conflict of interest |
| CrI | Credible intervals |
| CSO | Clinically severe obesity |
| GB | Gastric Bypass |
| HADS | Hospital Anxiety and Depression Scale |
| IWQOL | Impact of Weight on Quality of Life |
| LAGB | Laparoscopic adjustable gastric banding |
| LRYGB | Laparoscopic Roux-En-Y Gastric Bypass |
| LSG | Laparoscopic sleeve gastrectomy |
| NR | Not reported |
| PHQ-9 | Patient Health Questionnaire |
| POMS | Profile of Mood States |
| PPI | Perioperative psychological interventions |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| QoL | Quality of life |
| RCT | Randomized controlled trials |
| RYGB | Roux-En-Y Gastric Bypass |
| SEIQoL | Schedule for the Evaluation of Individual Quality of Life |
| SG | Sleeve Gastrectomy |
| VGB | Vertical Banded Gastroplasty |
| WL | Weight loss |
References
- Nguyen, D.M.; El-Serag, H.B. The Epidemiology of Obesity. Gastrointest. Endosc. Clin. N. Am. 2010, 39, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Kit, B.K.; Orpana, H.; Graubard, B.I. Association of all-cause mortality with overweight and obesity using standard body mass index categories a systematic review and meta-analysis. JAMA 2013, 309, 71–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Obesity and Overweight. Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 19 May 2021).
- Wang, Y.; Lim, H. The global childhood obesity epidemic and the association between socio-economic status and childhood obesity. Int. Rev. Psychiatry 2012, 24, 176–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10), Fifth Version, 1st ed.; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- World Health Organization. The Challenge of Obesity in the WHO European Region. Fact Sheet EURO/13/05; World Health Organization: Copenhagen, Denmark; Bucharest, Romania, 2005; Volume 97. [Google Scholar]
- Griffiths, L.; Parsons, T.J.; Hill, A.J. Self-esteem and quality of life in obese children and adolescents: A systematic review. Int. J. Pediatr. Obes. 2010, 5, 282–304. [Google Scholar] [CrossRef] [PubMed]
- Olszanecka-Glinianowicz, M.; Ostrowska, L. Otyłość. In Choroby Wewnętrzne Kompendium Medycyny Praktycznej; Szczeklik, A., Gajewski, P., Eds.; Medycyna Praktyczna: Kraków, Poland, 2020; pp. 2694–2711. [Google Scholar]
- Wild, B.; Herzog, W.; Lechner, S.; Niehoff, D.; Brenner, H.; Müller, H.; Rothenbacher, D.; Stegmaier, C.; Raum, E. Gender specific temporal and cross-sectional associations between BMI-class and symptoms of depression in the elderly. J. Psychosom. Res. 2012, 72, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.C.W.; Douketis, J.D.; Morrison, K.M.; Hramiak, I.M.; Sharma, A.M.; Ur, E. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children. CMAJ Can. Med. Assoc. J. 2007, 176, S1–S13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsen, G.S.; Småstuen, M.C.; Sandbu, R.; Nordstrand, N.; Hofsø, D.; Lindberg, M.; Hertel, J.K.; Hjelmesæth, J. Association of bariatric surgery vs medicalobesity treatment with long-term medical complications and obesity-related comorbidities. JAMA 2018, 319, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Colquitt, J.L.; Pickett, K.; Loveman, E.; Frampton, G.K. Surgery for weight loss in adults. Cochrane Database Syst. Rev. 2014, 8, CD003641. [Google Scholar] [CrossRef]
- Lauti, M.; Lemanu, D.; Zeng, I.S.L.; Su’a, B.; Hill, A.G.; MacCormick, A.D. Definition determines weight regain outcomes after sleeve gastrectomy. Surg. Obes. Relat. Dis. 2017, 13, 1123–1129. [Google Scholar] [CrossRef]
- Jirapinyo, P.; Dayyeh, B.K.A.; Thompson, C.C. Weight regain after Roux-en-Y gastric bypass has a large negative impact on the Bariatric Quality of Life Index. BMJ Open Gastroenterol. 2017, 4, e000153. [Google Scholar] [CrossRef]
- Maleckas, A.; Gudaitytė, R.; Petereit, R.; Venclauskas, L.; Veličkienė, D. Weight regain after gastric bypass: Etiology and treatment options. Gland Surg. 2016, 5, 617–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, A.H.; Adler, S.; Stevens, H.B.; Darcy, A.M.; Morton, J.M.; Safer, D.L. What variables are associated with successful weight loss outcomes for bariatric surgery after 1 year? Surg. Obes. Relat. Dis. 2014, 10, 697–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Garvey, W.T.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures—2019 update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endcrinology. Surg. Obes. Relat. Dis. 2019, 16, 175–247. [Google Scholar] [CrossRef]
- Elkins, G.; Whitfield, P.; Marcus, J.; Symmonds, R.; Rodriguez, J.; Cook, T. Noncompliance with behavioral recommendations following bariatric surgery. Obes. Surg. 2005, 15, 546–551. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Obesity: Identification, assessment and management. In Clinical Guideline 189; National Institute for Health and Care Excellence: Great Britain, UK, 2014. [Google Scholar]
- Stewart, F.; Avenell, A. Behavioural Interventions for Severe Obesity Before and/or After Bariatric Surgery: A Systematic Review and Meta-analysis. Obes. Surg. 2016, 26, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Kalarchian, M.A.; Marcus, M.D. Psychosocial Concerns Following Bariatric Surgery: Current Status. Curr. Obes. Rep. 2019, 8, 1–9. [Google Scholar] [CrossRef]
- Coulman, K.D.; MacKichan, F.; Blazeby, J.M.; Owen-Smith, A. Patient experiences of outcomes of bariatric surgery: A systematic review and qualitative synthesis. Obes. Rev. 2017, 18, 547–559. [Google Scholar] [CrossRef] [Green Version]
- Velapati, S.R.; Shah, M.; Kuchkuntla, A.R.; Abu-Dayyeh, B.; Grothe, K.; Hurt, R.T.; Mundi, M.S. Weight Regain after Bariatric Surgery: Prevalence, Etiology, and Treatment. Curr. Nutr. Rep. 2018, 7, 329–334. [Google Scholar] [CrossRef]
- Kalarchian, M.A.; Marcus, M.D. Management of the bariatric surgery patient: Is there a role for the cognitive behavior therapist? Cogn. Behav. Pract. 2003, 10, 112–119. [Google Scholar] [CrossRef]
- Mitchell, J.E.; Christian, N.J.; Flum, D.R.; Pomp, A.; Pories, W.J.; Wolfe, B.M.; Courcoulas, A.P.; Belle, S.H. Postoperative Behavioral Variables and Weight Change 3 Years after Bariatric Surgery. JAMA Surg. 2016, 151, 752–757. [Google Scholar] [CrossRef]
- Jacob, A.; Moullec, G.; Lavoie, K.L.; Laurin, C.; Cowan, T.; Tisshaw, C.; Kazazian, C.; Raddatz, C.; Bacon, S.L. Impact of cognitive-behavioral interventions on weight loss and psychological outcomes: A meta-analysis. Health Psychol. 2018, 37, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; DeLany, J.P.; Otto, A.D.; Kuller, L.; Vockley, J.; South-Paul, J.E.; Thomas, S.B.; Brown, J.; McTigue, K.; Hames, K.C.; et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: A randomized trial. JAMA 2010, 304, 1795–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sogg, S.; Atwood, M.E.; Cassin, S.E.; Hawa, R.; Sockalingam, S. The Role of Psychosocial Interventions in Supporting Medical and Surgical Treatments for Severe Obesity. In Psychological Care in Severe Obesity; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- Kothandan, S.K. School based interventions versus family based interventions in the treatment of childhood obesity—A systematic review. Arch. Public Health 2014, 72, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadden, T.A.; Brownell, K.D.; Foster, G.D. Obesity: Responding to the global epidemic. J. Consult. Clin. Psychol. 2002, 70, 510. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.R.; Hill, J.O. Successful Weight Loss Maintenance. Annu. Rev. Nutr. 2001, 21, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Fabricatore, A.N. Behavior Therapy and Cognitive-Behavioral Therapy of Obesity: Is There a Difference? J. Am. Diet. Assoc. 2007, 107, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Öst, L.-G. Efficacy of the third wave of behavioral therapies: A systematic review and meta-analysis. Behav. Res. Ther. 2008, 46, 296–321. [Google Scholar] [CrossRef]
- Kulick, D.; Hark, L.; Deen, D. The Bariatric Surgery Patient: A Growing Role for Registered Dietitians. J. Am. Diet. Assoc. 2010, 110, 593–599. [Google Scholar] [CrossRef]
- Jumbe, S.; Hamlet, C.; Meyrick, J. Psychological Aspects of Bariatric Surgery as a Treatment for Obesity. Curr. Obes. Rep. 2017, 6, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Lindekilde, N.; Gladstone, B.P.; Lubeck, M.; Nielsen, J.; Clausen, L.; Vach, W.; Jones, A. The impact of bariatric surgery on quality of life: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 639–651. [Google Scholar] [CrossRef]
- Brennan, L.; Murphy, K.D.; de la Piedad Garcia, X.; Ellis, M.E.; Metzendorf, M.I.; Mckenzie, J.E. Psychological interventions for adults who are overweight or obese. Cochrane Database Syst. Rev. 2018, 3, CD012114. [Google Scholar] [CrossRef]
- Liu, R.H. Do Behavioral Interventions Delivered before Bariatric Surgery Impact Weight Loss in Adults? A Systematic Scoping Review. Bariatr. Surg. Pract. Patient Care 2016, 11, 39–48. [Google Scholar] [CrossRef]
- Groller, K.D. Systematic review of patient education practices in weight loss surgery. Surg. Obes. Relat. Dis. 2017, 13, 1072–1085. [Google Scholar] [CrossRef] [PubMed]
- van Zyl, N.; Andrews, L.; Williamson, H.; Meyrick, J. The effectiveness of psychosocial interventions to support psychological well-being in post-operative bariatric patients: A systematic review of evidence. Obes. Res. Clin. Pract. 2020, 14, 404–420. [Google Scholar] [CrossRef]
- Cheroutre, C.; Guerrien, A.; Rousseau, A. Contributing of Cognitive-Behavioral Therapy in the Context of Bariatric Surgery: A Review of the Literature. Obes. Surg. 2020, 30, 3154–3166. [Google Scholar] [CrossRef]
- Shukla, A.P.; He, D.; Saunders, K.H.; Andrew, C.; Aronne, L.J. Current concepts in management of weight regain following bariatric surgery. Expert Rev. Endocrinol. Metab. 2018, 13, 67–76. [Google Scholar] [CrossRef]
- Andromalos, L.; Crowley, N.; Brown, J.; Craggs-Dino, L.; Handu, D.; Isom, K.; Lynch, A.; DellaValle, D. Nutrition Care in Bariatric Surgery: An Academy Evidence Analysis Center Systematic Review. J. Acad. Nutr. Diet. 2019, 119, 678–686. [Google Scholar] [CrossRef]
- Cassin, S.E.; Atwood, M. Cognitive Behavioural Therapy for Severe Obesity. In Psychiatric Care in Severe Obesity; Springer International Publishing: New York City, NY, USA, 2017. [Google Scholar]
- David, L.A.; Sijercic, I.; Cassin, S.E. Preoperative and post-operative psychosocial interventions for bariatric surgery patients: A systematic review. Obes. Rev. 2020, 21, e12926. [Google Scholar] [CrossRef]
- Julien, C.A.; Lavoie, K.L.; Ribeiro, P.A.B.; Dragomir, A.I.; Mercier, L.A.; Garneau, P.Y.; Pescarus, R.; Bacon, S.L. Behavioral weight management interventions in metabolic and bariatric surgery: A systematic review and meta-analysis investigating optimal delivery timing. Obes. Rev. 2021, 22, e13168. [Google Scholar] [CrossRef]
- Swierz, M.J.; Storman, D.; Jasinska, K.W.; Storman, M.; Staskiewicz, W.; Gorecka, M.; Skuza, A.; Tobola, P.; Bala, M.M. Systematic review and meta-analysis of perioperative behavioral lifestyle and nutritional interventions in bariatric surgery: A call for better research and reporting. Surg. Obes. Relat. Dis. 2020, 16, 2088–2104. [Google Scholar] [CrossRef]
- Marshall, S.; Mackay, H.; Matthews, C.; Maimone, I.R.; Isenring, E. Does intensive multidisciplinary intervention for adults who elect bariatric surgery improve post-operative weight loss, co-morbidities, and quality of life? A systematic review and meta-analysis. Obes. Rev. 2020, 21, e13012. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.T.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 105906. [Google Scholar] [CrossRef]
- Cash, T.F.; Hrabosky, J.I. The Effects of Psychoeducation and Self-Monitoring in a Cognitive-Behavioral Program for Body-Image Improvement. Eat. Disord. 2003, 11, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Lv, N.; Azar, K.M.; Rosas, L.G.; Wulfovich, S.; Xiao, L.; Ma, J. Behavioral lifestyle interventions for moderate and severe obesity: A systematic review. Prev. Med. 2017, 100, 180–193. [Google Scholar] [CrossRef]
- Goldbeck, L.; Fidika, A.; Herle, M.; Quittner, A.L. Psychological interventions for individuals with cystic fibrosis and their families. Cochrane Database Syst. Rev. 2014, 2014, CD003148. [Google Scholar]
- Rusch, M.D.; Andris, D. Maladaptive eating patterns after weight-loss surgery. Nutr. Clin. Pr. 2007, 22, 41–49. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Wood, L.; Egger, M.; Gluud, L.L.; Schulz, K.F.; Jüni, P.; Altman, D.G.; Gluud, C.; Martin, R.; Wood, A.J.G.; Sterne, J.A.C. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: Meta-epidemiological study. BMJ 2008, 336, 601–605. [Google Scholar] [CrossRef] [Green Version]
- van Doorn, J.; van den Bergh, D.; Böhm, U.; Dablander, F.; Derks, K.; Draws, T.; Etz, A.; Evans, N.J.; Gronau, Q.F.; Haaf, J.M.; et al. The JASP guidelines for conducting and reporting a Bayesian analysis. Psychon. Bull. Rev. 2020, 28, 813–826. [Google Scholar] [CrossRef]
- Lee, M.D.; Wagenmakers, E.J. Bayesian cognitive modeling: A practical course. In Bayesian Cognitive Modeling: A Practical Course; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Spiegelhalter, D.J.; Abrams, K.R.; Myles, J.P. Bayesian Approaches to Clinical Trials and Health-Care Evaluation; Wiley: New York, NY, USA, 2004. [Google Scholar] [CrossRef]
- Sterne, J.A.; Gavaghan, D.; Egger, M. Publication and Related Bias in Meta-Analysis. J. Clin. Epidemiol. 2000, 53, 1119–1129. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0895435600002420 (accessed on 8 October 2021). [CrossRef]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalarchian, M.A.; Marcus, M.D.; Courcoulas, A.P.; Cheng, Y.; Levine, M.D. Preoperative lifestyle intervention in bariatric surgery: A randomized clinical trial. Surg. Obes. Relat. Dis. 2016, 12, 180–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hjelmesæth, J.; Rosenvinge, J.H.; Gade, H.; Friborg, O. Effects of Cognitive Behavioral Therapy on Eating Behaviors, Affective Symptoms, and Weight Loss After Bariatric Surgery: A Randomized Clinical Trial. Obes. Surg. 2019, 29, 61–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lier, H.Ø.; Biringer, E.; Stubhaug, B.; Tangen, T. The impact of preoperative counseling on postoperative treatment adherence in bariatric surgery patients: A randomized controlled trial. Patient Educ. Couns. 2012, 87, 336–342. [Google Scholar] [CrossRef]
- Hollywood, A.; Ogden, J.; Pring, C. The Impact of Psychological Support on Psychological Outcomes and Patients’ Experiences of the Bariatric Service 1 and 2 Years after Bariatric Surgery. J. Obes. Bariatr. 2015, 2, 1–7. [Google Scholar]
- Tucker, J.A.; Samo, J.A.; Rand, C.S.W.; Woodward, E.R. Behavioral interventions to promote adaptive eating behavior and lifestyle changes following surgery for obesity: Results of a two-year outcome evaluation. Int. J. Eat. Disord. 1991, 10, 689–698. [Google Scholar] [CrossRef]
- Wild, B.; Hünnemeyer, K.; Sauer, H.; Hain, B.; Mack, I.; Schellberg, D.; Müller-Stich, B.P.; Weiner, R.; Meile, T.; Rudofsky, G.; et al. A 1-year videoconferencing-based psychoeducational group intervention following bariatric surgery: Results of a randomized controlled study. Surg. Obes. Relat. Dis. 2015, 11, 1349–1360. [Google Scholar] [CrossRef]
- Kalarchian, M.A.; Marcus, M.D.; Courcoulas, A.P.; Cheng, Y.; Levine, M.D.; Josbeno, D. Optimizing long-term weight control after bariatric surgery: A pilot study. Surg. Obes. Relat. Dis. 2012, 8, 710–715. [Google Scholar] [CrossRef] [Green Version]
- Nijamkin, M.P.; Campa, A.; Nijamkin, S.S.; Sosa, J. Comprehensive Behavioral-Motivational Nutrition Education Improves Depressive Symptoms Following Bariatric Surgery: A Randomized, Controlled Trial of Obese Hispanic Americans. J. Nutr. Educ. Behav. 2013, 45, 620–626. [Google Scholar] [CrossRef]
- Paul, L.; van der Heiden, C.; van Hoeken, D.; Deen, M.; Vlijm, A.; Klaassen, R.A.; Biter, L.U.; Hoek, H.W. Cognitive Behavioral Therapy Versus Usual Care before Bariatric Surgery: One-Year Follow-Up Results of a Randomized Controlled Trial. Obes. Surg. 2021, 31, 970–979. Available online: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02204803/full (accessed on 8 October 2021). [CrossRef]
- Beck, N.N.; Johannsen, M.; Støving, R.K.; Mehlsen, M.; Zachariae, R. Do postoperative psychotherapeutic interventions and support groups influence weight loss following bariatric surgery? A systematic review and meta-analysis of randomized and nonrandomized trials. Obes. Surg. 2012, 22, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, A.; Hilbert, A. Post-operative behavioural management in bariatric surgery: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2013, 14, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.J.; Abrams, K.R. Bayesian methods in meta-analysis and evidence synthesis. Stat. Methods Med. Res. 2001, 10, 277–303. [Google Scholar] [CrossRef] [PubMed]
- Harold, J. Theory of Probability. In Philosophy of Science; Cambridge University Press: Cambridge, UK, 1940. [Google Scholar]

| Study Name (Country) | Intervention | Control | Type of Surgery | Time Frame of PPI # | Follow-Up Post-Surgery (Months) | Outcomes | COI | Funding Reported | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description | Randomized n | Age Years Mean (SD) | Female n (%) | Description | Randomized n | Age Years Mean (SD) | Female n (%) | |||||||
| Kalarchian 2016 (USA) [62] | 6-month manualized behavioural lifestyle intervention: diet (1200–1400 calories/day) and PA goals + 12 individual, 1 h face-to-face counselling sessions pre-surgery + 12 telephone calls (15–20 min) pre-surgery + 3 monthly contacts + CAU | 121 | 43.9 (10.3) | 64 (90.1%) | Synopsis of information provided in intervention group; CAU: presurgical physician supervised diet + activity program | 119 | 45.9 (11.6) | 65 (90.3%) | RYGB, LAGB | 24 w before BS and 24 mo after BS | 6, 12, 24 | 1,2,5,6 | No | Yes |
| Hjelmesæth 2019 (Norway) [63] | 10 weekly individual sessions before BS aiming to improve dysfunctional eating behaviours | 50 | 44.1 (9.8) | 27 (64.3%) | 10 weeks of nutritional support and education pre-surgery | 52 | 41.2 (9.6) | 28 (73.7%) | RYGB, SG | 12 w before BS | 12, 48 | 1,2,5,6 | No | Yes |
| Lier 2012 (Norway) [64] | CBT (1 preoperative group session/week for 6 weeks + 3 postoperative group sessions (6 months, 1 year and 2 years post-surgery)—Semi-structured therapy manual | 49 | 43.5 (11.1) | 36 (74.0%) | CAU: 1 pre- and 1 post- surgery 4 h educational seminar on dietary strategies and behaviours | 50 | 42.4 (9.1) | 32 (67.0%) | GB | 6 w before BS and 24 mo after BS | 12 | 2 | No | Yes |
| Hollywood 2015 (UK) [65] | Bariatric rehabilitation service: 3 one-to-one 50 min sessions with psychologist 2 weeks pre-surgery, before discharge and at 3 months post-surgery + CAU | 82 | 45.6 (11.1) | 61 (74.4%) | CAU: Standard diet sheet postoperatively | 80 | 44.8 (10.6) | 61 (76.2%) | RYGB | 2 w before BS and 3 mo after BS | 12 | 1,2,4,5,6 | NR | Yes |
| Tucker 1991 (USA) [66] | Eating- and lifestyle-related materials every 2 weeks for 24 weeks post-surgery + 6 monthly consultations + CAU | 41 † | 40.18 † | 21 (65.6) † | CAU: Basic pre-surgery info on necessary eating-behaviour changes | 41 † | 40.18 † | 21 (65.6) † | GB, VBG | 6 m after BS | 6, 12, 24 | 1,2 | NR | NR |
| Wild 2015 (Germany) [67] | CAU + 1 year supervised video- conferencing-based psychoeducational group: eight 90 min face-to-face and six 50 min videoconferencing sessions + education in nutrition and exercise | 59 | 41.2 (9.0) | 35 (60.3%) | CAU: Conventional surgical visits at 1, 3, 6, and 12 months post-surgery | 58 | 41.9 (9.6) | 45 (80.4%) | LSG, RYGB, LAGB | 12 m after BS | 6, 12, 37.9 | 1,2,3,4,5,6 | No | Yes |
| Kalarchian 2012 (USA) [68] | 6-month behavioural intervention (6.6 year after surgery): instruction to intake 1200–1400 calorie/day and to follow postoperative guidelines + exercise program + 1 h face-to-face group meetings (12 weekly meetings) + 15–20 min telephone coaching (5 biweekly) | 18 | 51.0 (7.6) | 15 (83.3%) | Wait list control group | 18 | 53.9 (6.6) | 17 (94.4%) | GB, LAGB, VGB | 79 m after BS | 85, 91 | 2 | No | Yes |
| Nijamkin 2013 (USA) [69] | 6 nutrition and lifestyle education and behavioural–motivational group sessions every other week starting at 7 months post-surgery (use of Dietary Guidelines, nutrition education, exercises, motivational strategies) + e-mail reminders + telephone calls + CAU | 72 | 44.2 (12.6) | 62 (86.1%) | Brief printed guidelines; CAU: Optional counselling | 72 | 44.8 (14.4) | 58 (80.6%) | LRYGB | 6 m after BS | 12 | 1,2,6 | NR | Yes |
| Paul 2021 (The Netherlands) [70] | cognitive behavioural therapy of 10 individual sessions of 45 min, conducted by a psychologist or cognitive behavioural therapeutic worker | 65 | 44.1 (8.2) | 46 (73%) | Conventional preparation procedure consisting of an information meeting by the surgeon or nurse practitioner and an information meeting by the dietitian. Patients also receive a detailed patient information booklet. | 65 | 39.3 (10.6) | 49 (75%) | GB | 10 w before BS | 12 | 1,2,4,5,6 | No | NR |
| Psychological interventions in patients with morbid obesity undergoing bariatric surgery | |||||
| Patient or population: patients with obesity undergoing bariatric surgery | |||||
| Settings: any | |||||
| Intervention: any psychological interventions (such as BT/CBT/related to those, combined psychological intervention, education) | |||||
| Comparison: any control (such as care as usual care or minimal intervention, diet with physical activity, nutrition counselling) | |||||
| Outcomes | Control | Psychological Intervention | No of Participants (Studies) | Quality of the Evidence (GRADE) | Comments |
| Changes in BMI [kg/m2] Follow-up: 6 to 12 months | The mean change in BMI ranged across control groups from: −10.59 kg/m2 to −13.03 kg/m2 | The mean change in BMI in the intervention groups was 0.29 kg/m2 lower (1.6 lower to 0.83 higher) | 176 (2) | ⊕ very low 1 | Lower units indicate greater WL |
| Changes in BMI [kg/m2] Follow-up: 1–2 years | The mean change in BMI ranged across control groups from: −16.65 kg/m2 to −13.03 kg/m2 | The mean change in BMI in the intervention groups was 0.59 kg/m2 lower (1.34 lower to 0.12 higher) | 742 (7) | ⊕ very low 2 | Lower units indicate greater WL |
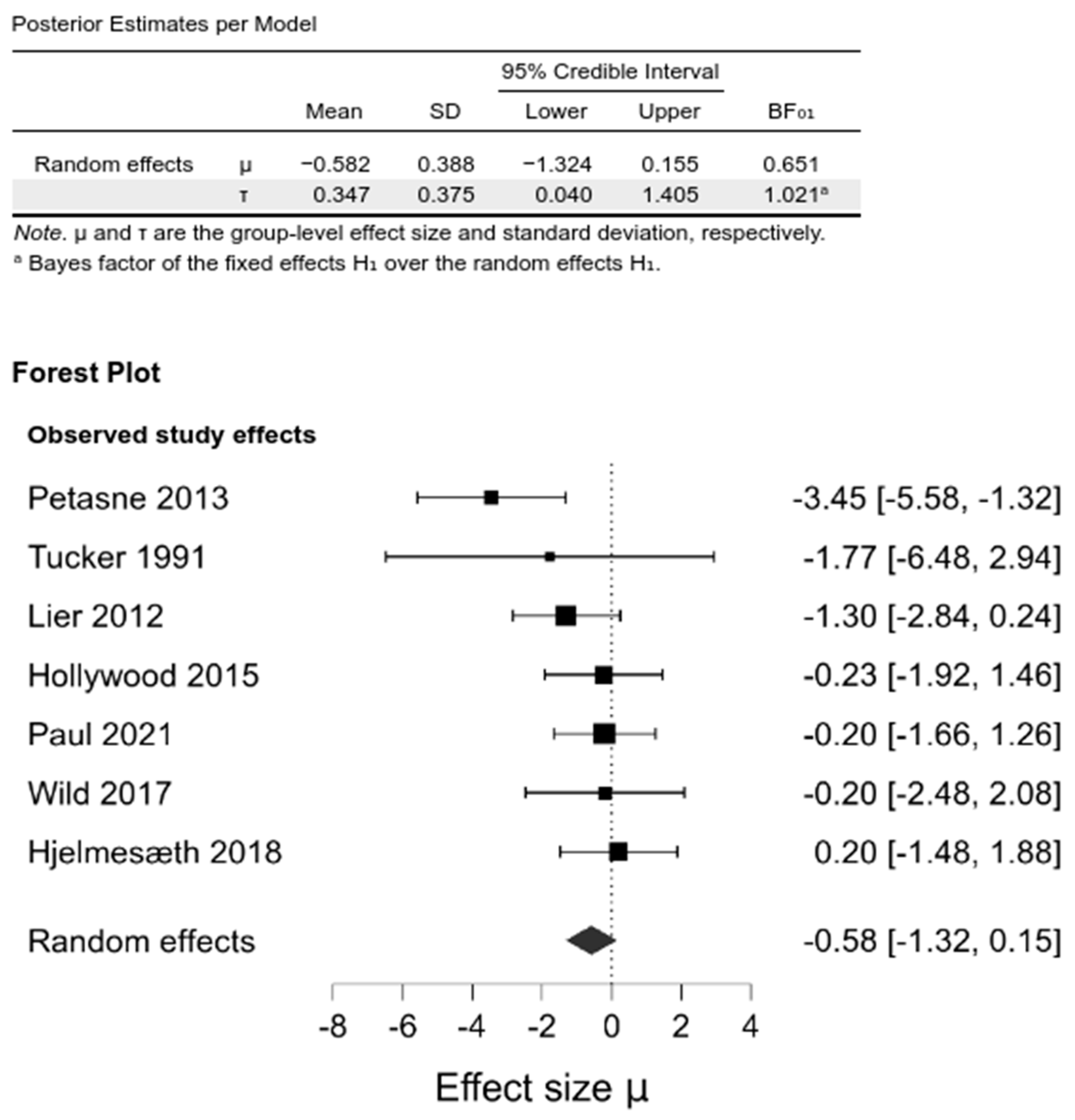
| Changes in BMI [kg/m2] Last follow-up | The mean change in BMI ranged across control groups from: −16.65 kg/m2 to −11.8 kg/m2 | The mean change in BMI in the intervention groups was 0.58 kg/m2 lower (1.32 lower to 0.15 higher) | 677 (7) | ⊕ very low 3 | Lower units indicate greater WL |
| WL [kg] Follow-up: 6 to 12 months | The mean WL [kg] ranged across control groups from: −37.90 kg to −29.75 kg | The mean WL [kg] in the intervention groups was 0.14 kg lower (1.43 lower to 1.97 higher) | 416 (4) | ⊕⊕ Low 4 | Lower units indicate greater WL |
| WL [kg] Follow-up: 1–2 years | The mean WL [kg] ranged across control groups from: −46.18 kg to −30.7 kg | The mean WL [kg] in the intervention groups was 0.56 kg higher (2.20 lower to 0.66 higher) | 842 (8) | ⊕ very low 5 | Lower units indicate greater WL |
| WL [kg] Last follow-up | The mean WL [kg] ranged across control groups from: −46.18 kg to −29.4 kg | The mean WL [kg] in the intervention groups was 0.50 kg higher (2.21 lower to 0.77 higher) | 731 (9) | ⊕ very low 6 | Lower units indicate greater WL |
| WL [%] Follow-up: 6 to 12 months | See comment | See comment | 143 (1) | ⊕ very low 7 | Higher units indicate greater WL Only one trial reported WL [%] within follow-up 6–12 months |
| WL [%] Follow-up: 1–2 years | The mean WL [%] ranged across control groups from: 29.4% to 30.1% | The mean WL [%] in the intervention groups was 0.54% lower (2.79 lower to 1.07 higher) | 223 (2) | ⊕⊕ Low 8 | Higher units indicate greater WL |
| WL [%] Last follow-up | The mean WL [%] ranged across control groups from: 27.9% to 29.5% | The mean WL [%] in the intervention groups was 1.06% lower (4.53 lower to 0.92 higher) | 204 (2) | ⊕⊕ Low 8 | Higher units indicate greater WL |
| Self-efficacy Follow-up: 6 to 12 months | See comment | See comment | 97 (1) | ⊕ very low 9 | The direction of the effect was consistently in favour of intervention |
| Self-efficacy Follow-up: 1–2 years | See comment | See comment | 110 (1) | ⊕ very low 9 | The direction of the effect was consistently in favour of control |
| Self-efficacy Last follow-up | See comment | See comment | 74 (1) | ⊕ very low 9 | The direction of the effect was consistently in favour of intervention |
| Quality of life Follow-up: 6 to 12 months | See comment | See comment | 115 (1) | ⊕ very low 9 | The direction of the effect was inconsistent |
| Quality of life Follow-up: 1–2 years | See comment | See comment | 288 (3) | ⊕ very low 9 | The direction of the effect was inconsistent |
| Quality of life Last follow-up | See comment | See comment | 251 (3) | ⊕ very low 9 | The direction of the effect was inconsistent |
| Maladaptive eating behaviours Follow-up: 6 to 12 months | See comment | See comment | 205 (2) | ⊕ very low 9 | The direction of the effect was inconsistent |
| Maladaptive eating behaviours Follow-up: 1–2 years | See comment | See comment | 366 (3) | ⊕ very low 9 | The direction of the effect was inconsistent |
| Maladaptive eating behaviours Last follow-up | See comment | See comment | 498 (4) | ⊕⊕ Low 10 | The direction of the effect was inconsistent |
| Change in psychological symptoms Follow-up: 6 to 12 months | See comment | See comment | 428 (3) | ⊕⊕ Low 10 | The direction of the effect was inconsistent |
| Change in psychological symptoms Follow-up: 1–2 years | See comment | See comment | 498 (5) | ⊕⊕ Low 10 | The direction of the effect was consistently in favour of intervention |
| Change in psychological symptoms Last follow-up | See comment | See comment | 630 (6) | ⊕⊕ Low 10 | The direction of the effect was inconsistent |
| Change in problems with relationships | See comment | See comment | No RCTs reported this outcome | ||
| Change in cognitive function | See comment | See comment | No RCTs reported this outcome | ||
| Change in alcohol and other substances misuse Follow-up: 6 months | See comment | See comment | No RCTs reported this outcome | ||
| Change in suicidal behaviour | See comment | See comment | No RCTs reported this outcome | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Storman, D.; Świerz, M.J.; Storman, M.; Jasińska, K.W.; Jemioło, P.; Bała, M.M. Psychological Interventions and Bariatric Surgery among People with Clinically Severe Obesity—A Systematic Review with Bayesian Meta-Analysis. Nutrients 2022, 14, 1592. https://doi.org/10.3390/nu14081592
Storman D, Świerz MJ, Storman M, Jasińska KW, Jemioło P, Bała MM. Psychological Interventions and Bariatric Surgery among People with Clinically Severe Obesity—A Systematic Review with Bayesian Meta-Analysis. Nutrients. 2022; 14(8):1592. https://doi.org/10.3390/nu14081592
Chicago/Turabian StyleStorman, Dawid, Mateusz Jan Świerz, Monika Storman, Katarzyna Weronika Jasińska, Paweł Jemioło, and Małgorzata Maria Bała. 2022. "Psychological Interventions and Bariatric Surgery among People with Clinically Severe Obesity—A Systematic Review with Bayesian Meta-Analysis" Nutrients 14, no. 8: 1592. https://doi.org/10.3390/nu14081592
APA StyleStorman, D., Świerz, M. J., Storman, M., Jasińska, K. W., Jemioło, P., & Bała, M. M. (2022). Psychological Interventions and Bariatric Surgery among People with Clinically Severe Obesity—A Systematic Review with Bayesian Meta-Analysis. Nutrients, 14(8), 1592. https://doi.org/10.3390/nu14081592






