The Employment of Genera Vaccinium, Citrus, Olea, and Cynara Polyphenols for the Reduction of Selected Anti-Cancer Drug Side Effects
Abstract
1. Introduction
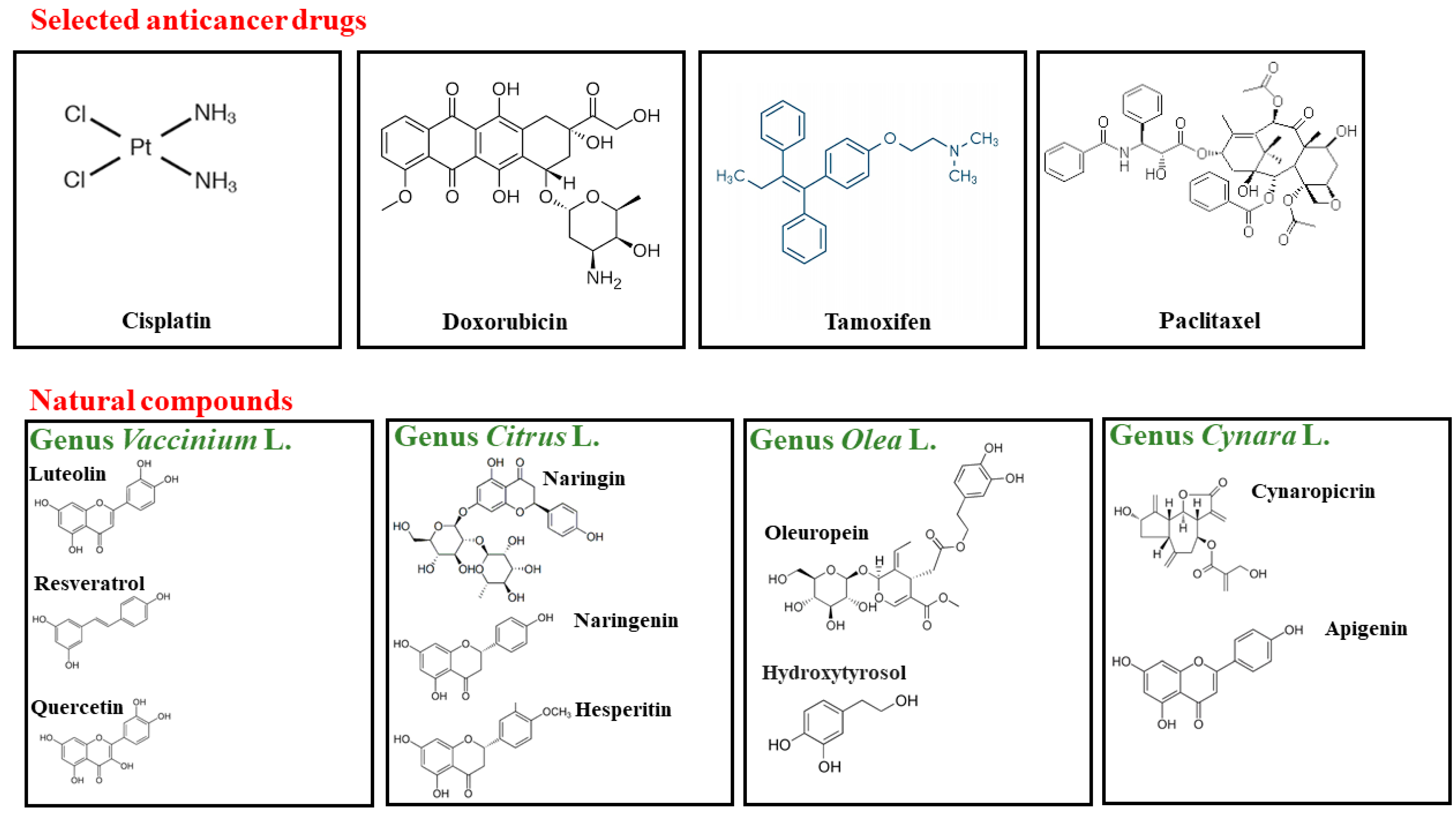
2. Cancer: Pharmacological Treatments and Their Side Effects
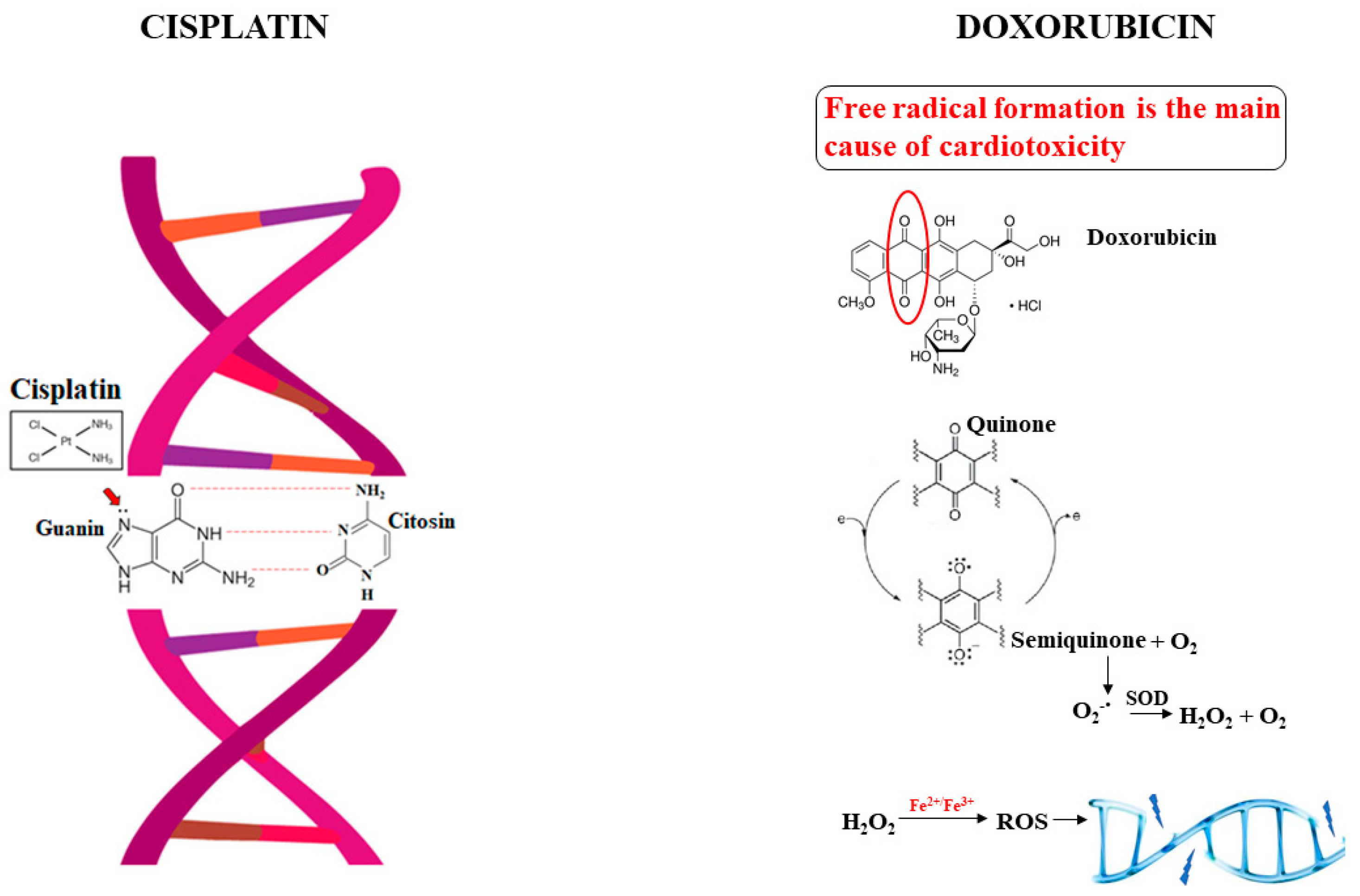
2.1. The Alkylating Agent Cisplatin
2.2. The Antimetabolite Doxorubicin
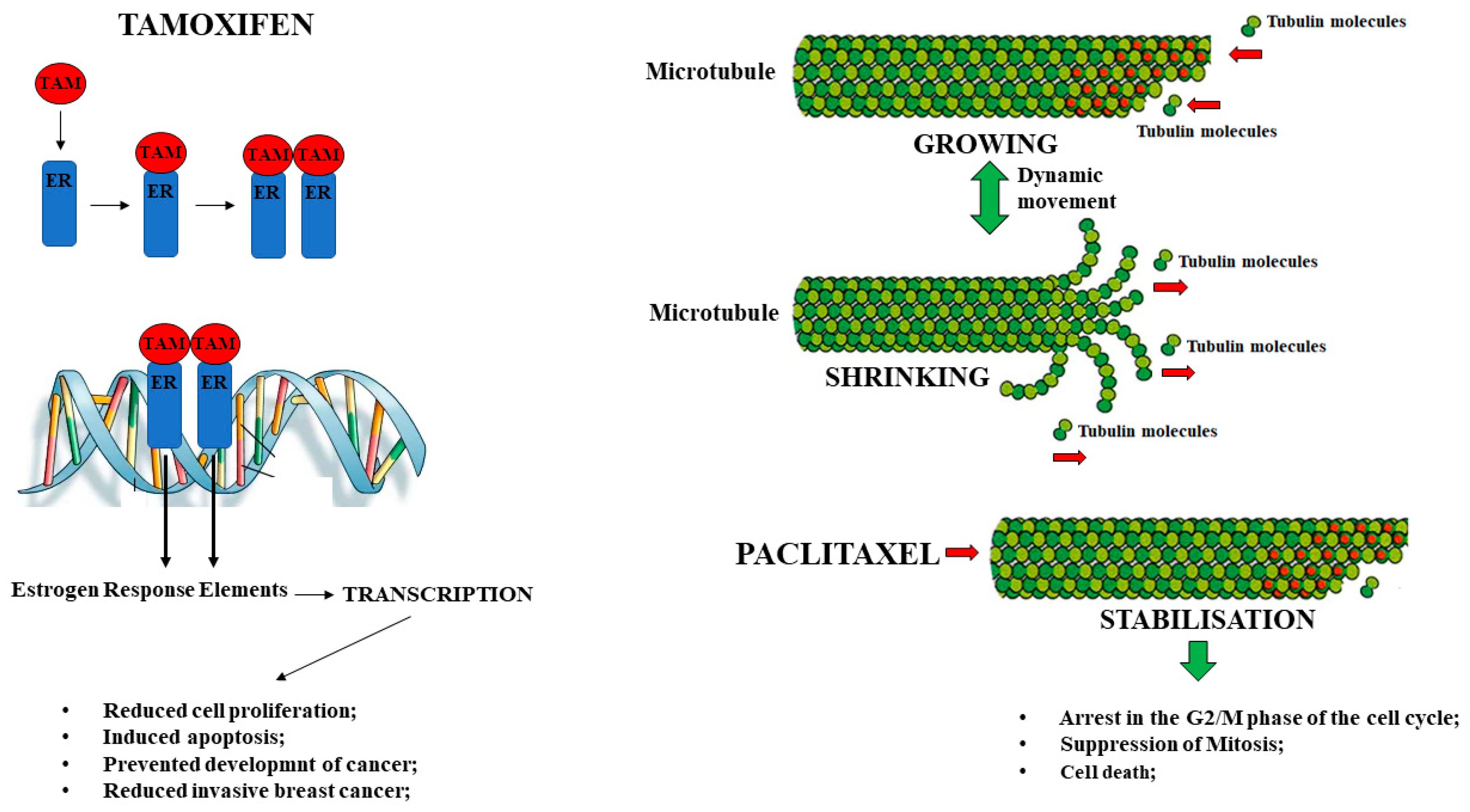
2.3. The Estrogen-Modulating Agent Tamoxifen
2.4. The Antimitotic Drug Paclitaxel
3. Genera Vaccinium L., Citrus L., Olea L., and Cynara L.: From Botany to Human Health
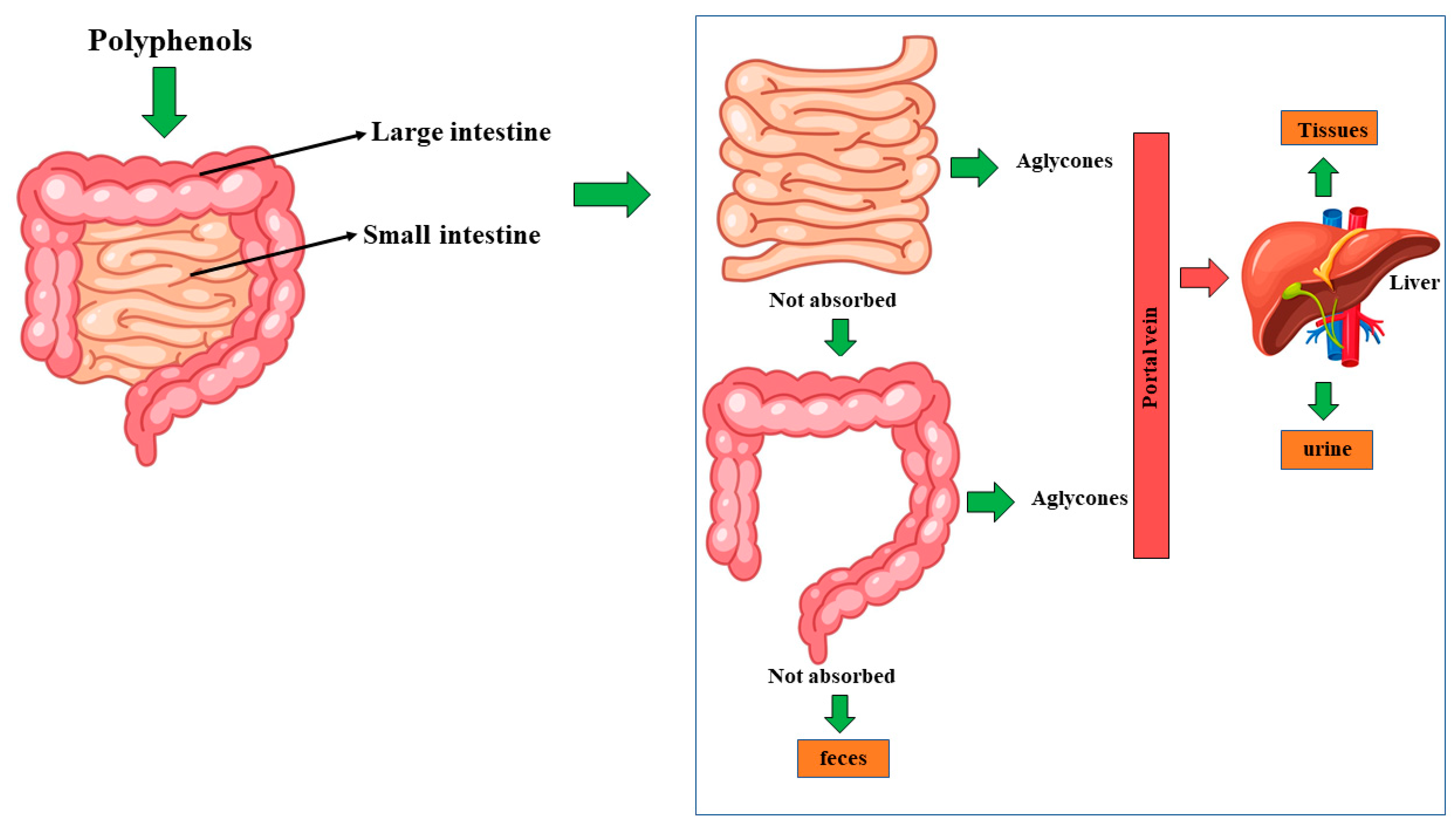
4. Effects of Polyphenols, Contained in Genera Vaccinium L., Citrus L., Olea L., and Cynara L, against the Side Effects Induced by Treatment with Selected Anti-Tumor Drugs
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Turati, F.; Carioli, G.; Bravi, F.; Ferraroni, M.; Serraino, D.; Montella, M.; Giacosa, A.; Toffolutti, F.; Negri, E.; Levi, F.; et al. Mediterranean Diet and Breast Cancer Risk. Nutrients 2018, 10, 326. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Selvaduray, K.R.; Radhakrishnan, A.K. Bioactive Compounds: Natural Defense Against Cancer? Biomolecules 2019, 9, 758. [Google Scholar] [CrossRef] [PubMed]
- Scaria, B.; Sood, S.; Raad, C.; Khanafer, J.; Jayachandiran, R.; Pupulin, A.; Grewal, S.; Okoko, M.; Arora, M.; Miles, L.; et al. Natural Health Products (NHP’s) and Natural Compounds as Therapeutic Agents for the Treatment of Cancer; Mechanisms of Anti-Cancer Activity of Natural Compounds and Overall Trends. Int. J. Mol. Sci. 2020, 21, 8480. [Google Scholar] [CrossRef]
- Drețcanu, G.; Iuhas, C.I.; Diaconeasa, Z. The Involvement of Natural Polyphenols in the Chemoprevention of Cervical Cancer. Int. J. Mol. Sci. 2021, 22, 8812. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.; Attri, S.; Mehta, V.; Udayabanu, M.; Goel, G. Microbe-bio-Chemical Insight: Reviewing Interactions between Dietary Polyphenols and Gut Microbiota. Mini-Rev. Med. Chem. 2018, 18, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Tatipamula, V.B.; Kukavica, B. Phenolic compounds as antidiabetic, anti-inflammatory, and anticancer agents and improvement of their bioavailability by liposomes. Cell Biochem. Funct. 2021, 39, 926–944. [Google Scholar] [CrossRef]
- Fraga, C.G.; Oteiza, P.I.; Galleano, M. Plant bioactives and redox signaling: (-)-Epicatechin as a paradigm. Mol. Aspects Med. 2018, 61, 31–40. [Google Scholar] [CrossRef]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Scarano, F.; Nucera, S.; Scicchitano, M.; Oppedisano, F.; Bosco, F.; Ruga, S.; et al. The Contribution of Gut Microbiota-Brain Axis in the Development of Brain Disorders. Front. Neurosci. 2021, 15, 616883. [Google Scholar] [CrossRef]
- Doroszkiewicz, J.; Groblewska, M.; Mroczko, B. The Role of Gut Microbiota and Gut-Brain Interplay in Selected Diseases of the Central Nervous System. Int. J. Mol. Sci. 2021, 22, 10028. [Google Scholar] [CrossRef]
- Wauquier, F.; Boutin-Wittrant, L.; Viret, A.; Guilhaudis, L.; Oulyadi, H.; Bourafai-Aziez, A.; Charpentier, G.; Rousselot, G.; Cassin, E.; Descamps, S.; et al. Metabolic and Anti-Inflammatory Protective Properties of Human Enriched Serum Following Artichoke Leaf Extract Absorption: Results from an Innovative Ex Vivo Clinical Trial. Nutrients 2021, 13, 2653. [Google Scholar] [CrossRef] [PubMed]
- Hausman, D.M. What Is Cancer? Perspect. Biol. Med. 2019, 62, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.S.; Saikia, B.J. Cancer and cure: A critical analysis. Indian J. Cancer 2016, 53, 441–442. [Google Scholar] [PubMed]
- Zahir, N.; Sun, R.; Gallahan, D.; Gatenby, R.A.; Curtis, C. Characterizing the ecological and evolutionary dynamics of cancer. Nat. Genet. 2020, 52, 759–767. [Google Scholar] [CrossRef]
- Arving, C.; Assmus, J.; Thormodsen, I.; Berntsen, S.; Nordin, K. Early rehabilitation of cancer patients. An individual randomized stepped-care stress-management intervention. Psychooncology 2019, 28, 301–308. [Google Scholar] [CrossRef]
- Ohdo, S.; Koyanagi, S.; Matsunaga, N. Chronopharmacological strategies focused on chrono-drug discovery. Pharmacol. Ther. 2019, 202, 72–90. [Google Scholar] [CrossRef]
- Lee, W.S.; Yang, H.; Chon, H.J.; Kim, C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp. Mol. Med. 2020, 52, 1475–1485. [Google Scholar] [CrossRef]
- Marques, V.A.; Ferreira-Junior, J.B.; Lemos, T.V.; Moraes, R.F.; Junior, J.R.d.S.; Alves, R.R.; Silva, M.S.; Freitas-Junior, R.; Vieira, C.A. Effects of Chemotherapy Treatment on Muscle Strength, Quality of Life, Fatigue, and Anxiety in Women with Breast Cancer. Int. J. Environ. Res. Public Health 2020, 17, 7289. [Google Scholar] [CrossRef]
- Haider, T.; Pandey, V.; Banjare, N.; Gupta, P.N.; Soni, V. Drug resistance in cancer: Mechanisms and tackling strategies. Pharmacol. Rep. 2020, 72, 1125–1151. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Wang, C.; Gu, Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Deliv. Rev. 2016, 98, 19–34. [Google Scholar] [CrossRef]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Sauter, B.; Gillingham, D. DNA Damaging Agents in Chemical Biology and Cancer. Chimia 2020, 74, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Long, D.; Yu, T.; Chen, X.; Liao, Y.; Wu, Y.; Lin, X. Vinblastine differs from Taxol as it inhibits the malignant phenotypes of NSCLC cells by increasing the phosphorylation of Op18/stathmin. Oncol. Rep. 2017, 37, 2481–2489. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Galanski, M.; Jakupec, M.A.; Keppler, B.K. Update of the Preclinical Situation of Anticancer Platinum Complexes: Novel Design Strategies and Innovative Analytical Approaches. Curr. Med. Chem. 2005, 12, 2075–2094. [Google Scholar] [CrossRef]
- Ho, G.Y.; Woodward, N.; Coward, J.I.G. Cisplatin versus carboplatin: Comparative review of therapeutic management in solid malignancies. Crit. Rev. Oncol./Hematol. 2016, 102, 37–46. [Google Scholar] [CrossRef]
- Ishida, S.; Lee, J.; Thiele, D.J.; Herskowitz, I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc. Natl. Acad. Sci. USA 2002, 99, 14298–14302. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 5, 364–378. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Dasari, S.; Noubissi, F.K.; Ray, P.; Kumar, S. Advances in Our Understanding of the Molecular Mechanisms of Action of Cisplatin in Cancer Therapy. J. Exp. Pharmacol. 2021, 13, 303–328. [Google Scholar] [CrossRef]
- Sedletska, Y.; Giraud-Panis, M.J.; Malinge, J.M. Cisplatin is a DNA-damaging antitumour compound triggering multifactorial biochemical responses in cancer cells: Importance of apoptotic pathways. Curr. Med. Chem. Anticancer Agents 2005, 5, 251–265. [Google Scholar] [CrossRef]
- Jeon, J.; Lee, S.; Kim, H.; Kang, H.; Youn, H.; Jo, S.; Youn, B.; Kim, H.Y. Revisiting Platinum-Based Anticancer Drugs to Overcome Gliomas. Int. J. Mol. Sci. 2021, 22, 5111. [Google Scholar] [CrossRef]
- Wagner, J.M.; Karnitz, L.M. Cisplatin-induced DNA damage activates replication checkpoint signaling components that differentially affect tumor cell survival. Mol. Pharmacol. 2009, 76, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Mechanisms of cisplatin-induced ototoxicity and otoprotection. Front. Cell. Neurosci. 2017, 11, 338. [Google Scholar] [CrossRef]
- Xu, L.; Bai, Q.; Zhang, X.; Yang, H. Folate-mediated chemotherapy and diagnostics: An updated review and outlook. J. Control. Release 2017, 252, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Rancoule, C.; Guy, J.B.; Vallard, A.; Ben Mrad, M.; Rehailia, A.; Magné, N. 50th anniversary of cisplatin. Bull. Cancer 2017, 104, 167–176. [Google Scholar] [CrossRef]
- Makovec, T. Cisplatin and beyond: Molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol. Oncol. 2019, 53, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Kitano, H.; Masaoka, Y.; Mamiya, A.; Fujiwara, Y.; Miki, T.; Hidai, C. Combination Cancer Therapy of a Del1 Fragment and Cisplatin Enhanced Therapeutic Efficiency In Vivo. In Vivo 2021, 35, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Terada, M.; Hara, H.; Daiko, H.; Mizusawa, J.; Kadota, T.; Hori, K.; Ogawa, H.; Ogata, T.; Sakanaka, K.; Sakamoto, T.; et al. Phase III study of tri-modality combination therapy with induction docetaxel plus cisplatin and 5-fluorouracil versus definitive chemoradiotherapy for locally advanced unresectable squamous-cell carcinoma of the thoracic esophagus (JCOG1510: TRIANgLE). Jpn. J. Clin. Oncol. 2019, 49, 1055–1060. [Google Scholar] [CrossRef]
- Goyal, Y.; Koul, A.; Ranawat, P. Ellagic acid ameliorates cisplatin toxicity in chemically induced colon carcinogenesis. Mol. Cell. Biochem. 2019, 453, 205–215. [Google Scholar] [CrossRef]
- Kim, C.W.; Choi, K.C. Effects of anticancer drugs on the cardiac mitochondrial toxicity and their underlying mechanisms for novel cardiac protective strategies. Life Sci. 2021, 277, 119607. [Google Scholar] [CrossRef]
- Meredith, A.M.; Dass, C.R. Increasing role of the cancer chemotherapeutic doxorubicin in cellular metabolism. J. Pharm. Pharmacol. 2016, 68, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Menna, P.; Paz, O.G.; Chello, M.; Covino, E.; Salvatorelli, E.; Minotti, G. Anthracycline cardiotoxicity. Expert Opin. Drug Saf. 2012, 11 (Suppl. 1), S21–S36. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.-T.; Wu, C.-Y.; Chung, C.-Y.; Hwu, Y.; Cheng, S.H.; Mou, C.-Y.; Lo, L.-W. Probing the dynamics of doxorubicin-DNA intercalation during the initial activation of apoptosis by fluorescence lifetime imaging microscopy (FLIM). PLoS ONE 2012, 7, e44947. [Google Scholar]
- Lyon, P.C.; Suomi, V.; Jakeman, P.; Campo, L.; Coussios, C.; Carlisle, R. Quantifying cell death induced by doxorubicin, hyperthermia or HIFU ablation with flow cytometry. Sci. Rep. 2021, 11, 4404. [Google Scholar] [CrossRef] [PubMed]
- Ganapathi, R.N.; Ganapathi, M.K. Mechanisms regulating resistance to inhibitors of topoisomerase II. Front. Pharmacol. 2013, 4, 89. [Google Scholar] [CrossRef]
- Paulson, J.R.; Hudson, D.F.; Cisneros-Soberanis, F.; Earnshaw, W.C. Mitotic chromosomes. Semin. Cell Dev. Biol. 2021, 117, 7–29. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, H.; Li, X.; Yang, T.; Jiang, Q. Effects of PPARα/PGC-1α on the energy metabolism remodeling and apoptosis in the doxorubicin induced mice cardiomyocytes in vitro. Int. J. Clin. Exp. Pathol. 2015, 8, 12216–12224. [Google Scholar]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- Maiuolo, J.; Carresi, C.; Gliozzi, M.; Musolino, V.; Scarano, F.; Coppoletta, A.R.; Guarnieri, L.; Nucera, S.; Scicchitano, M.; Bosco, F.; et al. Effects of Bergamot Polyphenols on Mitochondrial Dysfunction and Sarcoplasmic Reticulum Stress in Diabetic Cardiomyopathy. Nutrients 2021, 13, 2476. [Google Scholar] [CrossRef]
- Maiuolo, J.; Maretta, A.; Gliozzi, M.; Musolino, V.; Carresi, C.; Bosco, F.; Mollace, R.; Scarano, F.; Palma, E.; Scicchitano, M.; et al. Ethanol-induced cardiomyocyte toxicity implicit autophagy and NFkB transcription factor. Pharmacol. Res. 2018, 133, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Demissei, B.G.; Fan, Y.; Qian, Y.; Cheng, H.G.; Smith, A.M.; Shimamoto, K.; Vedage, N.; Narayan, H.K.; Scherrer-Crosbie, M.; Davatzikos, C.; et al. Left ventricular segmental strain and the prediction of cancer therapy-related cardiac dysfunction. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Koleini, N.; Kardami, E. Autophagy and mitophagy in the context of doxorubicin-induced cardiotoxicity. Oncotarget 2017, 8, 46663–46680. [Google Scholar] [CrossRef] [PubMed]
- Al-Malky, H.S.; Al Harthi, S.E.; Osman, A.M. Major obstacles to doxorubicin therapy: Cardiotoxicity and drug resistance. J. Oncol. Pharm. Pract. 2020, 26, 434–444. [Google Scholar] [CrossRef]
- Fojtu, M.; Gumulec, J.; Stracina, T.; Raudenska, M.; Skotakova, A.; Vaculovicova, M.; Adam, V.; Babula, P.; Novakova, M.; Masarik, M. Reduction of Doxorubicin-Induced Cardiotoxicity Using Nanocarriers: A Review. Curr. Drug Metab. 2017, 18, 237–263. [Google Scholar] [CrossRef]
- Shabalala, S.; Muller, C.J.F.; Louwa, J.; Johnson, R. Polyphenols, autophagy and doxorubicin-induced cardiotoxicity. Life Sci. 2017, 180, 160–170. [Google Scholar] [CrossRef]
- Rondón-Lagos, M.; Villegas, V.E.; Rangel, N.; Sánchez, M.C.; Zaphiropoulos, P.G. Tamoxifen Resistance: Emerging Molecular Targets. Int. J. Mol. Sci. 2016, 17, 1357. [Google Scholar] [CrossRef]
- Moon, S.Y.; Lee, H.; Kim, S.; Hong, J.H.; Chun, S.H.; Lee, H.Y.; Kang, K.; Kim, H.S.; Won, H.S.; Ko, Y.H. Inhibition of STAT3 enhances sensitivity to tamoxifen in tamoxifen-resistant breast cancer cells. BMC Cancer. 2021, 21, 931. [Google Scholar] [CrossRef]
- Planey, S.L.; Kumar, R.; Arnott, J.A. Estrogen receptors (ER-alpha versus ER-beta): Friends or foes in human biology? J. Recept. Signal. Transduct. Res. 2014, 34, 1–5. [Google Scholar] [CrossRef]
- Freedman, O.C.; Fletcher, G.G.; Gandhi, S.; Mates, M.; Dent, S.F.; Trudeau, M.E.; Eisen, A. Adjuvant endocrine therapy for early breast cancer: A systematic review of the evidence for the 2014 cancer care ontario systemic therapy guideline. Curr. Oncol. 2015, 22, S95–S113. [Google Scholar] [CrossRef]
- Notas, G.; Pelekanou, V.; Kampa, M.; Alexakis, K.; Sfakianakis, S.; Laliotis, A.; Askoxilakis, J.; Tsentelierou, E.; Tzardi, M.; Tsapis, A.; et al. Tamoxifen induces a pluripotency signature in breast cancer cells and human tumors. Mol. Oncol. 2015, 9, 1744–1759. [Google Scholar] [CrossRef] [PubMed]
- Vaziri-Gohar, A.; Zheng, Y.; Houston, K.D. IGF-1 Receptor Modulates FoxO1 Mediated Tamoxifen Response in Breast Cancer Cells. Mol Cancer Res. 2017, 15, 489–497. [Google Scholar] [CrossRef]
- Hong, J.; Huang, J.; Shen, L.; Zhu, S.; Gao, W.; Wu, J.; Huang, O.; He, J.; Zhu, L.; Chen, W.; et al. A prospective, randomized study of Toremifene vs. tamoxifen for the treatment of premenopausal breast cancer: Safety and genital symptom analysis. BMC Cancer 2020, 20, 663. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.W.H.; Law, B.M.H.; Ng, M.S.N.; Wong, C.C.Y.; Wong, C.W.Y.; Quinley, M.; Orgusyan, J.M.; Chow, K.M.; Waye, M.M.Y. Association of single nucleotide polymorphisms of cytochrome P450 enzymes with experience of vasomotor, vaginal and musculoskeletal symptoms among breast cancer patients: A systematic review. BMC Cancer 2021, 21, 570. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.K.; Jang, M.J.; Lee, E.; Moon, H.G.; Noh, D.Y.; Han, W. Endocrine Treatment-Related Symptoms and Patient Outcomes in Breast Cancer: A Meta-Analysis. J. Breast Cancer 2018, 21, 37–44. [Google Scholar] [CrossRef] [PubMed]
- BeLow, M.; Osipo, C. Notch Signaling in Breast Cancer: A Role in Drug Resistance. Cells 2020, 9, 2204. [Google Scholar] [CrossRef]
- Yang, Y.H.; Mao, J.W.; Tan, X.L. Research progress on the source, production, and anti-cancer mechanisms of paclitaxel. Chin. J. Nat. Med. 2020, 18, 890–897. [Google Scholar] [CrossRef]
- Chimento, A.; De Luca, A.; Avena, P.; De Amicis, F.; Casaburi, I.; Sirianni, R.; Pezzi, V. Estrogen Receptors-Mediated Apoptosis in Hormone-Dependent Cancers. Int. J. Mol. Sci. 2022, 23, 1242. [Google Scholar] [CrossRef]
- Dimopoulou, I.; Bamias, A.; Lyberopoulos, P.; Dimopoulos, M.A. Pulmonary toxicity from novel antineoplastic agents. Ann. Oncol. 2006, 17, 372–379. [Google Scholar] [CrossRef]
- Shitara, K.; Baba, E.; Fujitani, K.; Oki, E.; Fujii, S.; Yamaguchi, K. Discovery and development of trastuzumab deruxtecan and safety management for patients with HER2-positive Gastric Cancer. Gastric Cancer 2021, 24, 780–789. [Google Scholar] [CrossRef]
- Rosa, A.C.; Fantozzi, R. The role of histamine in neurogenic inflammation. Br. J. Pharmacol. 2013, 170, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, I.; Tomassoni, D.; Roy, P.; Amenta, F.; Tayebati, S.K. Altered Brain Cholinergic and Synaptic Markers in Obese Zucker Rats. Cells 2021, 10, 2528. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zan, Y.; Wang, Z.J.; Hu, X.-Y.; Huang, F. Quercetin ameliorates paclitaxel-induced neuropathic pain by stabilizing mast cells, and subsequently blocking PKCε-dependent activation of TRPV1. Acta Pharmacologica Sinica 2016, 37, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Rasoulpoor, S.; Shaban, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791. [Google Scholar]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Hauner, K.; Maisch, P.; Retz, M. Side effects of chemotherapy. Urologe A 2017, 56, 472–479. [Google Scholar] [CrossRef]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. Biomed. Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and anticancer properties of berries. Crit. Rev. Food Sci. Nutr. 2018, 58, 2491–2507. [Google Scholar] [CrossRef]
- D’Urso, G.; Piacente, S.; Pizza, C.; Montoro, P. Metabolomics of Healthy Berry Fruits. Curr. Med. Chem. 2018, 25, 4888–4902. [Google Scholar] [CrossRef]
- Raudone, L.; Vilkickyte, G.; Pitkauskaite, L.; Raudonis, R.; Vainoriene, R.; Motiekaityte, V. Antioxidant Activities of Vaccinium vitis-idaea L. Leaves within Cultivars and Their Phenolic Compounds. Molecules 2019, 24, 844. [Google Scholar] [CrossRef] [PubMed]
- Mane, C.; Loonis, M.; Juhel, C.; Dufour, C.; Malien-Aubert, C. Food Grade Lingonberry Extract: Polyphenolic Composition and In Vivo Protective Effect against Oxidative Stress. J. Agric. Food Chem. 2011, 59, 3330–3339. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, A.S.; Siltari, A.; Ehlers, P.I.; Korpela, R.; Vapaatalo, H. Lingonberry juice negates the effects of a high salt diet on vascular function and low-grade inflammation. J. Funct. Foods 2014, 7, 238–245. [Google Scholar] [CrossRef]
- McDougall, G.J.; Ross, H.A.; Ikeji, M.; Stewart, D. Berry Extracts Exert Different Antiproliferative Effects against Cervical and Colon Cancer Cells Grown in Vitro. J. Agric. Food Chem. 2008, 56, 3016–3023. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Shea, E.; Daneshtalab, M.; Weber, J.T. Chemical Analysis of Extracts from Newfoundland Berries and Potential Neuroprotective Effects. Antioxidants 2016, 5, 36. [Google Scholar] [CrossRef]
- Reichert, K.P.; Schetinger, M.R.C.; Gutierres, J.M.; Pelinson, L.P.; Stefanello, N.; Dalenogare, D.P.; Baldissarelli, J.; Lopes, T.F.; Morsch, V.M. Lingonberry Extract Provides Neuroprotection by Regulating the Purinergic System and Reducing Oxidative Stress in Diabetic Rats. Mol. Nutr. Food Res. 2018, 62, e1800050. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen Radical Absorbing Capacity of Phenolics in Blueberries, Cranberries, Chokeberries, and Lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef]
- Wang, S.Y.; Feng, R.; Bowman, L.; Penhallegon, R.; Ding, M.; Lu, Y. Antioxidant Activity in Lingonberries (Vaccinium vitis-idaea L.) and Its Inhibitory Effect on Activator Protein-1, Nuclear Factor-_B, and Mitogen-Activated Protein Kinases Activation. J. Agric. Food Chem. 2005, 53, 3156–3166. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A.; Zieli´nska-Wasielica, J.; Olkowicz, M. Inhibitory effects of lingonberry (Vaccinium vitis-idaea L.) fruit extract on obesity-induced inflammation in 3T3-L1 adipocytes and RAW264.7 macrophages. J. Funct. Foods 2019, 54, 371–380. [Google Scholar] [CrossRef]
- Ogawa, K.; Kuse, Y.; Tsuruma, K.; Kobayashi, S.; Shimazawa, M.; Hara, H. Protective effects of bilberry and lingonberry extracts against blue light-emitting diode light-induced retinal photoreceptor cell damage in vitro. BMC Complement. Altern. Med. 2014, 14, 120. [Google Scholar] [CrossRef]
- Popa-Wagner, A.; Dumitrascu, D.I.; Capitanescu, B.; Petcu, E.B.; Surugiu, R.; Fang, W.-H.; Dumbrava, D.A. Dietary habits, lifestyle factors and neurodegenerative diseases. Neural Regen. Res. 2020, 15, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Ciric, A.; Jelikic-Stankov, M.; Cvijovic, M.; Djurdjevic, P. Statistical optimization of an RP-HPLC method for the determination of selected flavonoids in berry juices and evaluation of their antioxidant activities. Biomed. Chromatogr. 2018, 32, e4150. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Nitecki, S.; Pereira-Caro, G.; McDougall, G.J.; Stewart, D.; Rowland, I.; Crozier, A.; Gill, C.I. Comparison of in vivo and in vitro digestion on polyphenol composition in lingonberries: Potential impact on colonic health. BioFactors 2014, 40, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Citrus limon (Lemon) Phenomenon-A Review of the Chemistry, Pharmacological Properties, Applications in the Modern Pharmaceutical, Food, and Cosmetics Industries, and Biotechnological Studies. Plants 2020, 9, 119. [Google Scholar] [CrossRef]
- Mabberley, D.J. Citrus (Rutaceae): A review of recent advances in etymology, systematics and medical applications. Blumea J. Plant Taxon. Plant Geogr. 2004, 49, 481–498. [Google Scholar] [CrossRef]
- Talon, M.; Gmitter, F.G. Citrus genomics. Int. J. Plant Genom. 2008, 2008, 1–17. [Google Scholar] [CrossRef]
- Ledesma-Escobar, C.A.; Priego-Capote, F.; Luque De Castro, M.D. Characterization of lemon (Citrus limon) polar extract by liquid chromatography-tandem mass spectrometry in high resolution mode. J. Mass Spectrom. 2015, 50, 1196–1205. [Google Scholar] [CrossRef]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant activity of Citrus fruits. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef]
- Akiyama, S.; Katsumata, S.; Suzuki, K.; Ishimi, Y.; Wu, J.; Uehara, M. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J. Clin. Biochem. Nutr. 2010, 46, 87–92. [Google Scholar] [CrossRef]
- Shen, W.; Xu, Y.; Lu, Y.H. Inhibitory effects of citrus flavonoids on starch digestion and antihyperglycemic effects in HepG2 cells. J. Agric. Food Chem. 2012, 60, 9609–9619. [Google Scholar] [CrossRef]
- Hwang, S.L.; Shih, P.H.; Yen, G.C. Neuroprotective effects of citrus flavonoids. J. Agric. Food Chem. 2012, 60, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Ilari, S.; Lauro, F.; Giancotti, L.A.; Malafoglia, V.; Dagostino, C.; Gliozzi, M.; Condemi, A.; Maiuolo, J.; Oppedisano, F.; Palma, E.; et al. The Protective Effect of Bergamot Polyphenolic Fraction (BPF) on Chemotherapy-Induced Neuropathic Pain. Pharmaceuticals 2021, 14, 975. [Google Scholar] [CrossRef]
- Ilari, S.; Giancotti, L.A.; Lauro, F.; Gliozzi, M.; Malafoglia, V.; Palma, E.; Tafani, M.; Russo, M.A.; Tomino, C.; Fini, M.; et al. Natural Antioxidant Control of Neuropathic Pain-Exploring the Role of Mitochondrial SIRT3 Pathway. Antioxidants 2020, 9, 1103. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, A.; Lee, H.J.; Saralamma, V.V.G.; Park, H.S.; Hong, G.E.; Yumnam, S.; Raha, S.; Charles, S.N.; Shin, S.C.; Kim, E.H.; et al. Flavonoids isolated from Citrus platymamma induced G2/M cell cycle arrest and apoptosis in A549 human lung cancer cells. Oncol. Lett. 2016, 12, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Degirmenci, H.; Erkurt, H. Relationship between volatile components, antimicrobial and antioxidant properties of the essential oil, hydrosol and extracts of Citrus aurantium L. flowers. J. Infect. Public Health 2019, 13, 58–67. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef]
- Emma, M.R.; Augello, G.; Di Stefano, V.; Azzolina, A.; Giannitrapani, L.; Montalto, G.; Cervello, M.; Cusimano, A. Potential Uses of Olive Oil Secoiridoids for the Prevention and Treatment of Cancer: A Narrative Review of Preclinical Studies. Int. J. Mol. Sci. 2021, 22, 1234. [Google Scholar] [CrossRef]
- Gorini, E.; Dangles, O.; Rakotomanomana, N.; Baracchinia, S.; Visioli, F. 3-O-Hydroxytyrosol glucuronide and 4-Ohydroxytyrosol glucuronide reduce endoplasmic reticulum stress in vitro. Food Funct. 2015, 6, 3275–3281. [Google Scholar]
- Reboredo-Rodríguez, P.; Varela-López, A.Y.; Forbes-Hernández, T.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Zhang, J.; Manna, P.P.; Bompadre, S.; Quiles, J.L.; et al. Phenolic compounds isolated from olive oil as nutraceutical tools for the prevention and management of cancer and cardiovascular diseases. Int. J. Mol. Sci. 2018, 19, 2305. [Google Scholar] [CrossRef]
- Zinnai, A.; Venturi, F.; Quartacci, M.F.; Sanmartin, C.; Favati, F.; Andrich, G. Solid carbon dioxide to promote the extraction of extra-virgin olive oil. Grasas Aceites 2016, 67, 121–129. [Google Scholar] [CrossRef]
- De Santis, S.; Galleggiante, V.; Scandiffio, L.; Liso, M.; Sommella, E.; Sobolewski, A.; Spilotro, V.; Pinto, A.; Campiglia, P.; Serino, G.; et al. Secretory leukoprotease inhibitor (Slpi) expression is required for educating murine dendritic cells inflammatory response following quercetin exposure. Nutrients 2017, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Nari, A.; Andrich, G.; Terzuoli, E.; Donnini, S.; Nicolella, C.; Zinnai, A. Development of phenol-enriched olive oil with phenolic compounds extracted from wastewater produced by physical refining. Nutrients 2017, 9, 916. [Google Scholar] [CrossRef] [PubMed]
- Bayram, B.; Ozcelik, B.; Grimm, S.; Roeder, T.; Schrader, C.; Ernst, I.M.; Wagner, A.E.; Grune, T.; Frank, J.; Rimbach, G. A diet rich in olive oil phenolics reduces oxidative stress in the heart of SAMP8 mice by induction of Nrf2-dependent gene expression. Rejuvenation Res. 2012, 15, 71–81. [Google Scholar] [CrossRef]
- Gill, C.I.R.; Boyd, A.; McDermott, E.; McCann, M.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G.; McGlynn, H.; et al. Potential anti-cancer effects of virgin olive oil phenols on colorectal carcinogenesis models in vitro. Int. J. Cancer 2005, 117, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.A.; Gualtieri, P.; Gratteri, S.; Ali, W.; Sergi, D.; Muscoli, S.; Cammarano, A.; Bernardini, S.; Di Renzo, L.; Romeo, F. Effects of postprandial hydroxytyrosol and derivates on oxidation of LDL, cardiometabolic state and gene expression: A nutrigenomic approach for cardiovascular prevention. J. Cardiovasc. Med. 2019, 20, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.A.B.; Santos, S.A.O.; Guerra, A.R.; Guerreiro, O.; Freire, C.S.R.; Rocha, S.M. Phenolic composition and antioxidant activity of different morphological parts of Cynara cardunculus L. var. altilis (DC). Ind. Crops Prod. 2014, 61, 460–471. [Google Scholar] [CrossRef]
- Scavo, A.; Rial, C.; Molinillo, J.M.G.; Varela, R.M.; Mauromicale, G.; Macías, F.A. Effect of shading on the sesquiterpene lactone content and phytotoxicity of cultivated cardoon leaf extracts. J. Agric. Food Chem. 2020, 68, 11946–11953. [Google Scholar] [CrossRef]
- Gominho, J.; Curt, M.D.; Lourenço, A.; Fernández, J.; Pereira, H. Cynara cardunculus L. as a biomass and multi-purpose crop: A review of 30 years of research. Biomass Bioenergy 2018, 109, 257–275. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Pereira, C.; Tzortzakis, N.; Barros, L.; Ferreira, I.C.F.R. Nutritional Value and Bioactive Compounds Characterization of Plant Parts from Cynara cardunculus L. (Asteraceae) Cultivated in Central Greece. Front. Plant Sci. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Sarmento, A.C.; Lopes, H.; Oliveira, C.S.; Vitorino, R.; Samyn, B.; Sergeant, K.; Debyser, G.; Van Beeumen, J.; Domingues, P.; Amado, F.; et al. Multiplicity of aspartic proteinases from Cynara cardunculus L. Planta 2009, 230, 429–439. [Google Scholar] [CrossRef]
- Ramos, P.A.B.; Ferro, A.M.; Oliveira, M.M.; Gonçalves, S.; Freire, C.S.R.; Silvestre, A.J.D. Biosynthesis and bioactivity of Cynara cardunculus L. guaianolides and hydroxycinnamic acids: A genomic, biochemical and health-promoting perspective. Phytochem. Rev. 2019, 4, 495–526. [Google Scholar] [CrossRef]
- Pandino, G.; Gattesco, F.; Bosisio, S.; Lombardo, S.; Russo, A.; Mauromicale, G. Cynaropicrin, total caffeoylquinic acids and flavonoids in leaves of Cynara cardunculus (cardoon) forms. Acta Hortic. 2019, 1284, 279–284. [Google Scholar] [CrossRef]
- Singh, N.; Yarla, N.S.; Siddiqi, N.J.; de Lourdes Pereira, M.; Sharma, B. Features, Pharmacological Chemistry, Molecular Mechanism and Health Benefits of Lemon. Med. Chem. 2021, 17, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Tsai, S.Y.; Lin, J.A.; Wu, C.H.; Yen, G.C. Cytoprotective effects of hesperetin and hesperidin against amyloid beta-induced impairment of glucose transport through downregulation of neuronal autophagy. Mol. Nutr. Food Res. 2012, 56, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Fogeiro, É.; Barracosa, P.; Oliveira, J.; Wessel, D. Influence of Cardoon Flower (Cynara cardunculus L.) and Flock Lactation Stage in PDO Serra da Estrela Cheese. Foods 2020, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Ben Amira, A.; Bauwens, J.; De Pauw, E.; Besbes, S.; Attia, H.; Francis, F.; Blecker, C. Identification of proteins from wild cardoon flowers (Cynara cardunculus L.) by a proteomic approach. J. Chem. Biol. 2016, 10, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.M.; Rosa, D.; Ferro, A.F.; Faustino, A.; Paulino, A.; Brás, T.; Machado, E.; Pinto, C.; Belo, A.D.F.; Nozes, P.; et al. Genetic diversity and population structure of Cynara cardunculus L. in southern Portugal. PLoS ONE 2021, 16, e0252792. [Google Scholar] [CrossRef]
- Barracosa, P.; Barracosa, M.; Pires, E. Cardoon as a Sustainable Crop for Biomass and Bioactive Compounds Production. Chem. Biodivers. 2019, 16, e1900498. [Google Scholar] [CrossRef]
- Ramos, P.A.B.; Guerra, A.R.; Guerreiro, O.; Freire, C.S.R.; Silva, A.M.S.; Duarte, M.F. Lipophilic extracts of Cynara cardunculus L. var. altilis (DC): A source of valuable bioactive terpenic compounds. J. Agric. Food Chem. 2013, 61, 8420–8429. [Google Scholar]
- Dias, M.I.; Barros, L.; Barreira, J.C.M.; Alves, M.J.; Barracosa, P.; Ferreira, I.C.F.R. Phenolic profile and bioactivity of cardoon (Cynara cardunculus L.) inflorescence parts: Selecting the best genotype for food applications. Food Chem. 2018, 268, 196–202. [Google Scholar] [CrossRef]
- Rial, C.; García, B.F.; Varela, R.M.; Torres, A.; Molinillo, J.M.; Macías, F.A. The Joint Action of Sesquiterpene Lactones from Leaves as an Explanation for the Activity of Cynara cardunculus. J. Agric. Food Chem. 2016, 64, 6416–6424. [Google Scholar] [CrossRef] [PubMed]
- Khaldi, S.; Naouari, M.; Jemaa, A. Cardoon (Cynara cardunculus L.) oil from cultivated and wild Tunisian populations and its antimicrobial activity. Ind. Crop. Prod. 2021, 171, 113852. [Google Scholar] [CrossRef]
- Mandim, F.; Petropoulos, S.A.; Giannoulis, K.D.; Dias, M.I.; Fernandes, Â.; Pinela, J.; Kostic, M.; Soković, M.; Barros, L.; Santos-Buelga, C.; et al. Seasonal variation of bioactive properties and phenolic composition of Cynara cardunculus var. altilis. Food Res. Int. 2020, 134, 109281. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.; Jacinto, T.A.; Coutinho, P. Bioactive Compounds from Cardoon as Health Promoters in Metabolic Disorders. Foods 2022, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.; Fernandes, Â.; Pereira, C.; Tzortzakis, N.; Vaz, J.; Soković, M.; Barros, L.; Ferreira, I.C.F.R. Bioactivities, chemical composition and nutritional value of Cynara cardunculus L. seeds. Food Chem. 2019, 289, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Villarini, M.; Acito, M.; di Vito, R.; Vannini, S.; Dominici, L.; Fatigoni, C.; Pagiotti, R.; Moretti, M. Pro-Apoptotic Activity of Artichoke Leaf Extracts in Human HT-29 and RKO Colon Cancer Cells. Int. J. Environ. Res. Public Health 2021, 18(8), 4166. [Google Scholar] [CrossRef]
- Wider, B.; Pittler, M.H.; Thompson-Coon, J.; Ernst, E. WITHDRAWN: Artichoke leaf extract for treating hypercholesterolaemia. Cochrane Database Syst. Rev. 2016, 5, CD003335. [Google Scholar]
- Zarkovic, N. Roles and Functions of ROS and RNS in Cellular Physiology and Pathology. Cells 2020, 9, 767. [Google Scholar] [CrossRef]
- Taira, J. Oxidative Stress Modulators and Functional Foods. Antioxidants 2021, 10, 191. [Google Scholar] [CrossRef]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef]
- García, N.; Zazueta, C.; Aguilera-Aguirre, L. Oxidative Stress and Inflammation in Cardiovascular Disease. Oxid. Med. Cell Longev. 2017, 2017, 5853238. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E. Oxidative Stress and Cancer. Curr. Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients. 2016, 8, 552. [Google Scholar] [CrossRef]
- Asano, T. Drug Resistance in Cancer Therapy and the Role of Epigenetics. J. Nippon Med. Sch. 2020, 87, 244–251. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, J.Q.; Assaraf, Y.G.; Ren, L.; Gupta, P.; Wei, L.; Ashby, C.R., Jr.; Yang, D.H.; Chen, Z.S. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist. Updat. 2018, 41, 1–25. [Google Scholar] [CrossRef]
- Kim, E.K.; Jang, M.; Song, M.J.; Kim, D.; Kim, Y.; Jang, H.H. Redox-mediated mechanism of chemoresistance in cancer cells. Antioxidants 2019, 8, 471. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug Resistance (MDR): A Widespread Phenomenon in Pharmacological Therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Hashemi Sheikhshabani, S.; Amini-Farsani, Z.; Rahmati, S.; Jazaeri, A.; Mohammadi-Samani, M.; Asgharzade, S. Oleuropein reduces cisplatin resistance in ovarian cancer by targeting apoptotic pathway regulators. Life Sci. 2021, 278, 119525. [Google Scholar] [CrossRef] [PubMed]
- Geyikoğlu, F.; Çolak, S.; Türkez, H.; Bakır, M.; Koç, K.; Hosseinigouzdagani, M.K.; Çeriğ, S.; Sönmez, M. Oleuropein Ameliorates Cisplatin-induced Hematological Damages Via Restraining Oxidative Stress and DNA Injury. Indian J. Hematol. Blood Transfus. 2017, 33, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Bishr, A.; Sallam, N.; Nour El-Din, M.; Awad, A.S.; Kenawy, S.A. Ambroxol attenuates cisplatin-induced hepatotoxicity and nephrotoxicity via inhibition of p-JNK/p-ERK. Can. J. Physiol. Pharmacol. 2019, 97, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Crona, D.J.; Faso, A.; Nishijima, T.F.; McGraw, K.A.; Galsky, M.D.; Milowsky, M.I. A Systematic Review of Strategies to Prevent Cisplatin-Induced Nephrotoxicity. Oncologist 2017, 22, 609–619. [Google Scholar] [CrossRef]
- Chen, C.; Ai, Q.; Wei, Y. Hydroxytyrosol protects against cisplatin-induced nephrotoxicity via attenuating CKLF1 mediated inflammation, and inhibiting oxidative stress and apoptosis. Int. Immunopharmacol. 2021, 96, 107805. [Google Scholar] [CrossRef]
- Hazafa, A.; Rehman, K.U.; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef]
- Lopez-Lazaro, M. Distribution and biological activities of the flavonoid luteolin. Mini-Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Maatouk, M.; Abed, B.; Bouhlel, I.; Krifa, M.; Khlifi, R.; Ioannou, I.; Ghedira, K.; Ghedira, L.C. Heat treatment and protective potentials of luteolin-7-O-glucoside against cisplatin genotoxic and cytotoxic effects. Environ. Sci. Pollut. Res. Int. 2020, 27, 13417–13427. [Google Scholar] [CrossRef]
- Wang, H.; Luo, Y.; Qiao, T.; Wu, Z.; Huang, Z. Luteolin sensitizes the antitumor effect of cisplatin in drug-resistant ovarian cancer via induction of apoptosis and inhibition of cell migration and invasion. J. Ovarian Res. 2018, 11, 93. [Google Scholar] [CrossRef]
- Hamdy, A.A.; Basma, G. Cisplatin induced testicular damage through mitochondria mediated apoptosis, inflammation and oxidative stress in rats: Impact of resveratrol. Endocr. J. 2020, 67, 969–980. [Google Scholar]
- Bostan, M.; Mihaila, M.; Petrica-Matei, G.G.; Radu, N.; Hainarosie, R.; Stefanescu, C.D.; Roman, V.; Diaconu, C.C. Resveratrol Modulation of Apoptosis and Cell Cycle Response to Cisplatin in Head and Neck Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 6322. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zou, X.; Lin, S.; Hu, X.; Gao, J. Effects of naringin on reversing cisplatin resistance and the Wnt/beta-catenin pathway in human ovarian cancer SKOV3/CDDP cells. J. Int. Med. Res. 2020, 48, 300060519887869. [Google Scholar] [CrossRef] [PubMed]
- Ventura, G.J. Cardiotoxicity of epirubicin versus doxorubicin: Cost and clinical results. J. Clin. Oncol. 2005, 23, 2873. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Al Taee, H.; Goyal, S.; Mahajan, U.B.; Patil, C.R.; Arya, D.S.; Rajesh, M. Cardioprotective potentials of plant-derived small molecules against doxorubicin associated cardiotoxicity, Oxidative Med. Cell. Longev. 2016, 2016, 5724973. [Google Scholar]
- Tatlidede, E.; Sehirli, O.; Velioglu-Ogunc, A.; Cetinel, S.; Yegen, B.C.; Yarat, A.; Süleymanoğlu, S.; Şener, G. Resveratrol treatment protects against doxorubicin-induced cardiotoxicity by alleviating oxidative damage. Free Radic. Res. 2009, 43, 195–205. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 26. [Google Scholar] [CrossRef]
- Dutta, D.; Xu, J.; Dirain, M.L.; Leeuwenburgh, C. Calorie restriction combined with resveratrol induces autophagy and protects 26-month-old rat hearts from doxorubicin-induced toxicity. Free Radic. Biol. Med. 2014, 74, 252–262. [Google Scholar]
- Habauzit, V.; Morand, C. Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: An update for clinicians. Ther. Adv. Chronic Dis. 2012, 3, 87–106. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Sun, C.; Yang, J.; Wang, L.; Liu, J.; Gong, L.; Jing, Y. Apigenin attenuates experimental autoimmune myocarditis by modulating Th1/Th2 cytokine balance in mice. Inflammation 2016, 39, 678–686. [Google Scholar] [CrossRef]
- Zhang, T.; Yan, T.; Du, J.; Wang, S.; Yang, H. Apigenin attenuates heart injury in lipopolysaccharide-induced endotoxemic model by suppressing sphingosine kinase1/sphingosine 1-phosphate signaling pathway. Chem. Biol. Interact. 2015, 233, 46–55. [Google Scholar] [CrossRef]
- Gao, A.M.; Ke, Z.P.; Wang, J.N.; Yang, J.Y.; Chen, S.Y.; Chen, H. Apigenin sensitizes doxorubicin-resistant hepatocellular carcinoma BEL-7402/ADM cells to doxorubicin via inhibiting PI3K/Akt/Nrf2 pathway. Carcinogenesis 2013, 34, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Carresi, C.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Scarano, F.; Bosco, F.; Nucera, S.; Maiuolo, J.; Macrì, R.; Ruga, S.; et al. The Effect of Natural Antioxidants in the Development of Metabolic Syndrome: Focus on Bergamot Polyphenolic Fraction. Nutrients 2020, 12, 1504. [Google Scholar] [CrossRef]
- Carresi, C.; Musolino, V.; Gliozzi, M.; Maiuolo, J.; Mollace, R.; Nucera, S.; Maretta, A.; Sergi, D.; Muscoli, S.; Gratteri, S.; et al. Anti-oxidant effect of bergamot polyphenolic fraction counteracts doxorubicin-induced cardiomyopathy: Role of autophagy and c-kitposCD45negCD31neg cardiac stem cell activation. J. Mol. Cell Cardiol. 2018, 119, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.A. Cardioprotective Effect of Quercetin and Sitagliptin in Doxorubicin-Induced Cardiac Toxicity in Rats. Cancer Manag. Res. 2021, 13, 2349–2357. [Google Scholar] [CrossRef] [PubMed]
- Farinetti, A.; Zurlo, V.; Manenti, A.; Coppi, F.; Mattioli, A.V. Mediterranean diet and colorectal cancer: A systematic review. Nutrition 2017, 43–44, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Torić, J.; Marković, A.K.; Brala, C.J.; Barbarić, M. Anticancer effects of olive oil polyphenols and their combinations with anticancer drugs. Acta Pharm. 2019, 69, 461–482. [Google Scholar] [CrossRef]
- Sealy, N.; Hankinson, S.E.; Houghton, S.C. Olive oil and risk of breast cancer: A systematic review and dose-response meta-analysis of observational studies. Br. J. Nutr. 2021, 125, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, N.M.; Bakar Siddique, A.; Ebrahim, H.Y.; Mohyeldin, M.M.; El Sayed, K.A. The olive oil phenolic (−)-oleocanthal modulates estrogen receptor expression in luminal breast cancer in vitro and in vivo and synergizes with tamoxifen treatment. Eur. J. Pharmacol. 2017, 810, 100–111. [Google Scholar] [CrossRef]
- Xu, J.; Guo, Z.; Yuan, S.; Li, H.; Xu, J. BCL2L1 is identified as a target of naringenin in regulating ovarian cancer progression. Mol. Cell. Biochem. 2022, 477, 1541–1553. [Google Scholar] [CrossRef]
- Meiyanto, E.; Hermawan, A.; Anindyajati, A. Natural products for cancer-targeted therapy: Citrus flavonoids as potent chemopreventive agents. Asian Pac. J. Cancer Prev. 2012, 13, 427–436. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsayed, A.R.; Barakat, M.; Abu-Taha, M.I.; Mahmod, A.I. Targeting Drug Chemo-Resistance in Cancer Using Natural Products. Biomedicines 2021, 9, 1353. [Google Scholar] [CrossRef] [PubMed]
- Kumarappan, C.; Vijayakumar, M.; Thilagam, E.; Balamurugan, M.; Thiagarajan, M.; Senthil, S.; Das, S.C.; Manda, S.C. Protective and curative effects of polyphenolicextracts from Ichnocarpus frutescense leaves on experimentalhepatotoxicity by carbon tretrachloride and tamoxifen. Annal. Hepatol. 2011, 10, 63–72. [Google Scholar] [CrossRef]
- Sinaga, E.; Fitrayadi, A.; Asrori, A.; Rahayu, S.E.; Suprihatin, S.; Prasasty, V.D. Hepatoprotective effect of Pandanus odoratissimus seed extracts on paracetamol-induced rats. Pharm. Biol. 2021, 59, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Musolino, V.; Gliozzi, M.; Bombardelli, E.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F.; et al. The synergistic effect of Citrus bergamia and Cynara cardunculus extracts on vascular inflammation and oxidative stress in non-alcoholic fatty liver disease. J. Tradit. Complement. Med. 2020, 10, 268–274. [Google Scholar] [CrossRef]
- Maiuolo, J.; Bava, I.; Carresi, C.; Gliozzi, M.; Musolino, V.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Ruga, S.; et al. The Effects of Bergamot Polyphenolic Fraction, Cynara cardunculus, and Olea europea L. Extract on Doxorubicin-Induced Cardiotoxicity. Nutrients 2021, 13, 2158. [Google Scholar] [CrossRef]
- Oppedisano, F.; Muscoli, C.; Musolino, V.; Carresi, C.; Macrì, R.; Giancotta, C.; Bosco, F.; Maiuolo, J.; Scarano, F.; Paone, S.; et al. The Protective Effect of Cynara Cardunculus Extract in Diet-Induced NAFLD: Involvement of OCTN1 and OCTN2 Transporter Subfamily. Nutrients 2020, 12, 1435. [Google Scholar] [CrossRef]
- De Faria, E.L.P.; Gomes, M.V.; Cláudio, A.F.M.; Freire, C.S.R.; Silvestre, A.J.D.; Freire, M.G. Extraction and recovery processes for cynaropicrin from Cynara cardunculus L. using aqueous solutions of surface-active ionic liquids. Biophys. Rev. 2018, 10, 915–925. [Google Scholar] [CrossRef]
- Elsebai, M.F.; Mocan, A.; Atanasov, A.G. Cynaropicrin: A Comprehensive Research Review and Therapeutic Potential as an Anti-Hepatitis C Virus Agent. Front. Pharmacol. 2016, 7, 472. [Google Scholar] [CrossRef]
- Beaver, C.C.; Magnan, M.A. Managing Chemotherapy Side Effects: Achieving Reliable and Equitable Outcomes. Clin. J. Oncol. Nurs. 2016, 20, 589–591. [Google Scholar] [CrossRef]
- Mansouri, R.; Takiguchi, Y. Monitoring the side effects of cancer chemotherapy. Nihon Rinsho. 2015, 73 (Suppl. 2), 102–106. [Google Scholar]
- Dallavalle, S.; Dobričić, V.; Lazzarato, L.; Gazzano, E.; Machuqueiro, M.; Pajeva, I.; Tsakovska, I.; Zidar, N.; Fruttero, R. Improvement of conventional anti-cancer drugs as new tools against multidrug resistant tumors. Drug Resist. Updat. 2020, 50, 100682. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussab, Y.E.; Wheate, N.J. The side effects of platinumbased chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Moghbeli, M. MicroRNAs as the critical regulators of Cisplatin resistance in ovarian cancer cells. J. Ovarian Res. 2021, 14, 127. [Google Scholar] [CrossRef]
- Gorini, S.; De Angelis, A.; Berrino, L.; Malara, N.; Rosano, G.; Ferraro, E. Chemotherapeutic Drugs and Mitochondrial Dysfunction: Focus on Doxorubicin, Trastuzumab, and Sunitinib. Oxid. Med. Cell Longev. 2018, 2018, 7582730. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Zhao, J.; Gutgesell, L.M.; Wang, Y.; Lee, S.; Karumudi, B.; Zhao, H.; Lu, Y.; Tonetti, D.A.; Thatcher, G.R. Novel Selective Estrogen Receptor Downregulators (SERDs) Developed against Treatment-Resistant Breast Cancer. J Med. Chem. 2017, 60, 1325–1342. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.; Chen, Z.; Muscoli, C.; Bryant, L.; Esposito, E.; Cuzzocrea, S.; Dagostino, C.; Ryerse, J.; Rausaria, S.; Kamadulski, A.; et al. Targeting the overproduction of peroxynitrite for the prevention and reversal of paclitaxel-induced neuropathic pain. J. Neurosci. 2012, 32, 6149–6160. [Google Scholar] [CrossRef] [PubMed]
- Rafehi, H.; Ververis, K.; Karagiannis, T.C. Mechanisms of action of phenolic compounds in olive. J. Diet. Suppl. 2012, 9, 96–109. [Google Scholar] [CrossRef]
- Malafoglia, V.; Tenti, M.; Ilari, S.; Balzani, E.; Fanelli, A.; Muscoli, C.; Raffaeli, W.; Bonci, A. Opportunities and challenges for nonaddictive interventions in chronic pain. Curr. Opin. Pharmacol. 2021, 57, 184–191. [Google Scholar] [CrossRef]
- Lauro, F.; Giancotti, L.A.; Ilari, S.; Dagostino, C.; Gliozzi, M.; Morabito, C.; Malafoglia, V.; Raffaeli, W.; Muraca, M.; Goffredo, B.M.; et al. Inhibition of Spinal Oxidative Stress by Bergamot Polyphenolic Fraction Attenuates the Development of Morphine Induced Tolerance and Hyperalgesia in Mice. PLoS ONE 2016, 11, e0156039. [Google Scholar] [CrossRef]
- Mileo, A.M.; Di Venere, D.; Mardente, S.; Miccadei, S. Artichoke Polyphenols Sensitize Human Breast Cancer Cells to Chemotherapeutic Drugs via a ROS-Mediated Downregulation of Flap Endonuclease 1. Oxid. Med. Cell Longev. 2020, 2020, 7965435. [Google Scholar] [CrossRef]
- Chen, L.; Alam, A.; Pac-Soo, A.; Chen, Q.; Shang, Y.; Zhao, H.; Yao, S.; Ma, D. Pretreatment with valproic acid alleviates pulmonary fibrosis through epithelial-mesenchymal transition inhibition in vitro and in vivo. Lab. Invest. 2021, 101, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, R. Trastuzumab-deruxtecan: An investigational agent for the treatment of HER2-positive breast cancer. Expert Opin. Investig. Drugs 2020, 29, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Carthy, E.; Ellender, T. Histamine, Neuroinflammation and Neurodevelopment: A Review. Front. Neurosci. 2021, 15, 680214. [Google Scholar] [CrossRef] [PubMed]
- Tomassoni, D.; Martinelli, I.; Moruzzi, M.; Micioni Di Bonaventura, M.V.; Cifani, C.; Amenta, F.; Tayebati, S.K. Obesity and Age-Related Changes in the Brain of the Zucker Lepr fa/fa Rats. Nutrients 2020, 12, 1356. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Oka, Y.; Sashida, H.; Kanbe, T.; Abe, K.; Utsunomiya, I.; Taguchi, K. Vincristine-induced peripheral neuropathic pain and expression of transient receptor potential vanilloid 1 in rat. J. Pharmacol. Sci. 2017, 133, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Farhood, B.; Mortezaee, K.; Goradel, N.H.; Khanlarkhani, N.; Salehi, E.; Nashtaei, M.S.; Najafi, M.; Sahebkar, A. Curcumin as an anti-inflammatory agent: Implications to radiotherapy and chemotherapy. J. Cell. Physiol. 2019, 234, 5728–5740. [Google Scholar] [CrossRef]
- Feng, T.; Wei, Y.; Lee, R.J.; Zhao, L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017, 12, 6027–6044. [Google Scholar] [CrossRef]
- Shang, L.; Zhou, X.; Zhang, J.; Shi, Y.; Zhong, L. Metal Nanoparticles for Photodynamic Therapy: A Potential Treatment for Breast Cancer. Molecules 2021, 26, 6532. [Google Scholar] [CrossRef]
- Ma, J.; Zhong, M.; Xiong, Y.; Gao, Z.; Wu, Z.; Liu, Y.; Hong, X. Emerging roles of nucleotide metabolism in cancer development: Progress and prospect. Aging 2021, 13, 13349–13358. [Google Scholar] [CrossRef]
- Sato, J.; Kudo, K. Team medical care—Role of the pharmacist. Nihon Rinsho. 2015, 73 (Suppl. 2), 678–682. [Google Scholar]
- Rong, D.; Wang, C.; Zhang, X.; Wei, Y.; Zhang, M.; Liu, D.; Farhan, H.; Momen Ali, S.A.; Liu, Y.; Taouil, A.; et al. A novel taxane, difluorovinyl-ortataxel, effectively overcomes paclitaxel-resistance in breast cancer cells. Cancer Lett. 2020, 491, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Dilruba, S.; Kalayda, G.V. Platinum-based drugs: Past, present and future. Cancer Chemother. Pharmacol. 2016, 77, 1103–1124. [Google Scholar] [CrossRef] [PubMed]
- Samuel, P.; Pink, R.C.; Brooks, S.A.; Carter, D.R. miRNAs and ovarian cancer: A miRiad of mechanisms to induce cisplatin drug resistance. Expert Rev. Anticancer Ther. 2016, 16, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, V.W. The role of sirtuins in mitochondrial function and doxorubicin-induced cardiac dysfunction. Biol. Chem. 2017, 398, 955–974. [Google Scholar] [CrossRef]
- Patel, H.K.; Bihani, T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol. Ther. 2018, 186, 1–24. [Google Scholar] [CrossRef]
- Singh, M.; Zhou, X.; Chen, X.; Santos, G.S.; Peuget, S.; Cheng, Q.; Rihani, A.; Arnér, E.S.J.; Hartman, J.; Selivanova, G. Identification and targeting of selective vulnerability rendered by tamoxifen resistance. Breast Cancer Res. 2020, 22, 80. [Google Scholar] [CrossRef]
- Shitara, K.; Takashima, A.; Fujitani, K.; Koeda, K.; Hara, H.; Nakayama, N.; Hironaka, S.; Nishikawa, K.; Makari, Y.; Amagai, K.; et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): An open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol. Hepatol. 2017, 2, 277–287. [Google Scholar] [CrossRef]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S.; Ko, J.H. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Tan, B.L. Curcumin Combination Chemotherapy: The Implication and Efficacy in Cancer. Molecules 2019, 24, 2527. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, M.; Katnoria, J.K.; Nagpal, A.K.; Sharma, A. Polyphenols in Food: Cancer Prevention and Apoptosis Induction. Curr. Med. Chem. 2018, 25, 4740–4757. [Google Scholar] [CrossRef]
- Wu, H.; Chen, L.; Zhu, F.; Han, X.; Sun, L.; Chen, K.; Wu, H. The Cytotoxicity Effect of Resveratrol: Cell Cycle Arrest and Induced Apoptosis of Breast Cancer 4T1 Cells. Toxins 2019, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- Castro, N.; Novaes, G.M.; Pascoal, G.F.L.; Ong, T.P. Bioactive food compounds, epigenetics and chronic disease prevention: Focus on early-life interventions with polyphenols. Food Res. Int. 2019, 125, 108646. [Google Scholar]
- Bresciani, L.; Calani, L.; Bocchi, L.; Delucchi, F.; Savi, M.; Ray, S.; Brighenti, F.; Stilli, D.; Del Rio, D.; Bresciani, L. Bioaccumulation of resveratrol metabolites in myocardial tissue is dose-time dependent and related to cardiac hemodynamics in diabetic rats. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 408–415. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Zheng, Z.; Li, J.; Yan, Y.; Wu, W. Sulforaphane metabolites reduce resistance to paclitaxel via microtubule disruption. Cell Death Dis. 2018, 9, 1134. [Google Scholar] [CrossRef] [PubMed]
- Němcová-Fürstová, V.; Kopperová, D.; Balušíková, K.; Ehrlichová, M.; Brynychová, V.; Václavíková, R.; Daniel, P.; Souček, P.; Kovář, J. Characterization of acquired paclitaxel resistance of breast cancer cells and involvement of ABC transporters. Toxicol. Appl. Pharmacol. 2016, 310, 215–228. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B.; Singh, N. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Murata, M. Inflammation and cancer. Environ. Health Prev. Med. 2018, 23, 50. [Google Scholar] [CrossRef]
- Khandia, R.; Munjal, A. Interplay between inflammation and cancer. Adv. Protein Chem. Struct. Biol. 2020, 119, 199–245. [Google Scholar] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N.; Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; et al. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C.; Yahfoufi, N. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B.; Zhou, Y. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. Adipocyte and lipid metabolism in cancer drug resistance. Clin. Invest. 2019, 129, 3006–3017. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Shim, J.S.; Du, B. Targeting Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules 2016, 21, 965. [Google Scholar] [CrossRef] [PubMed]
- Namee, N.M.; O’Driscoll, L.; Namee, N.M. Extracellular vesicles and anti-cancer drug resistance. Biochim. Biophys. Acta Rev. Cancer 2018, 1870, 123–136. [Google Scholar] [CrossRef]
- Lin, Y.F.; Liu, J.J.; Chang, Y.J.; Yu, C.S.; Yi, W.; Lane, H.Y.; Lu, C.H. Predicting Anticancer Drug Resistance Mediated by Mutations. Pharmaceuticals 2022, 15, 136. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Singh, A.; Nazir, S.U.; Tulsyan, S.; Khan, A.; Kumar, R.; Bashir, N.; Tanwar, P.; Mehrotra, R.; Hussain, S. Cancer drug resistance: A fleet to conquer. J. Cell. Biochem. 2019, 120, 14213–14225. [Google Scholar] [CrossRef]
- Dana, P.M.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The role of polyphenols in overcoming cancer drug resistance: A comprehensive review. Cell. Mol. Biol. Lett. 2022, 27, 1. [Google Scholar] [CrossRef]
- Gu, H.F.; Mao, X.Y.; Du, M. Prevention of breast cancer by dietary polyphenols-role of cancer stem cells. Crit. Rev. Food Sci. Nutr. 2020, 60, 810–825. [Google Scholar] [CrossRef]
- Fu, T.; Guang, H.J.; Gao, X.Z. Percutaneous nerve electrical stimulation for fatigue caused by chemotherapy for cervical cancer. Medicine 2018, 97, e12020. [Google Scholar] [CrossRef]
- Das, T.; Anand, U.; Pandey, S.K.; Ashby, C.R., Jr.; Assaraf, Y.G.; Chen, Z.S.; Dey, A. Therapeutic strategies to overcome taxane resistance in cancer. Drug Resist. Updat. 2021, 55, 100754. [Google Scholar] [CrossRef]
- Macrì, R.; Musolino, V.; Gliozzi, M.; Carresi, C.; Maiuolo, J.; Nucera, S.; Scicchitano, M.; Bosco, F.; Scarano, F.; Ruga, S.; et al. Ferula L. Plant Extracts and Dose-Dependent Activity of Natural Sesquiterpene Ferutinin: From Antioxidant Potential to Cytotoxic Effects. Molecules 2020, 25, 5768. [Google Scholar] [CrossRef] [PubMed]
- Lepore, S.M.; Maggisano, V.; Lombardo, G.E.; Maiuolo, J.; Mollace, V.; Bulotta, S.; Russo, D.; Celano, M. Antiproliferative Effects of Cynaropicrin on Anaplastic Thyroid Cancer Cells. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 59–66. [Google Scholar] [CrossRef]
- Bulotta, S.; Corradino, R.; Celano, M.; Maiuolo, J.; D’Agostino, M.; Oliverio, M.; Procopio, A.; Filetti, S.; Russo, D. Antioxidant and antigrowth action of peracetylated oleuropein in thyroid cancer cells. J. Mol. Endocrinol. 2013, 51, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Capriglione, F.; Maiuolo, J.; Celano, M.; Damante, G.; Russo, D.; Bulotta, S.; Maggisano, V. Quercetin Protects Human Thyroid Cells against Cadmium Toxicity. Int. J. Mol. Sci. 2021, 22, 6849. [Google Scholar] [CrossRef] [PubMed]
- Celano, M.; Maggisano, V.; De Rose, R.F.; Bulotta, S.; Maiuolo, J.; Navarra, M.; Russo, D. Flavonoid Fraction of Citrus reticulata Juice Reduces Proliferation and Migration of Anaplastic Thyroid Carcinoma Cells. Nutr. Cancer 2015, 67, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Corasaniti, M.T.; Maiuolo, J.; Maida, S.; Fratto, V.; Navarra, M.; Russo, R.; Amantea, D.; Morrone, L.A.; Bagetta, G. Cell signaling pathways in the mechanisms of neuroprotection afforded by bergamot essential oil against NMDA-induced cell death in vitro. J. Pharmacol. 2007, 151, 518–529. [Google Scholar]
- Bulotta, S.; Corradino, R.; Celano, M.; D’Agostino, M.; Maiuolo, J.; Oliverio, M.; Procopio, A.; Iannone, M.; Rotiroti, D.; Russo, D. Antiproliferative and antioxidant effects on breast cancer cells of oleuropein and its semisynthetic peracetylated derivatives. Food Chem. 2011, 127, 1609–1614. [Google Scholar] [CrossRef]
| Botanical Characteristics | Genus Vaccinium L. [81] | Genus Citrus L. [123] | Genus Olea L. [124] | Genus Cynara L. [125,126,127,128,129,130,131,132,133,134,135,136,137] |
|---|---|---|---|---|
| Trunk/shrubs | Almost always small shrubs of modest size or even creeping. | Shrubs or evergreen trees with a height varying from 3 to 15 m. | Small evergreen trees, from 12 to 20 ft. high; Rigid branches and a grayish bark. | Herbaceous forms with height between 50 and 250 cm. The stems are erect, branched, and robust. |
| Leaf | Leathery, oval, and evergreen. | Ovoid or elliptic; coriaceous. | Opposite, evergreen, petiolate, and coriaceous. | Basal and cauline leaves; the lamina is pinnatisect and very thorny. |
| Flowers | United in clusters and terminal; white-pink; petals welded. | White or reddish; grows individually in leaf axils; consists of five petals. | Agglomerate, fasciculate, racemose, or decussate type with terminal or axillary posture. Hermaphrodite, actinomorphic, and tetracyclic. | Vast and globose terminal heads. Tubulose (actinomorphic), hermaphrodite, and fertile. Color is pink, purple, or violet. |
| Fruit | False fleshy berries of small and medium size. | Modified berry known as “hesperidium”. | Drupe, ovoid, globose, or oblong; mesocarp is fleshy; endocarp is hard. | Achenes with pappus. The shape of the achene is cylindrical and slightly angular. The pappus is formed of long deciduous or persistent feathery bristles. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiuolo, J.; Musolino, V.; Gliozzi, M.; Carresi, C.; Oppedisano, F.; Nucera, S.; Scarano, F.; Scicchitano, M.; Guarnieri, L.; Bosco, F.; et al. The Employment of Genera Vaccinium, Citrus, Olea, and Cynara Polyphenols for the Reduction of Selected Anti-Cancer Drug Side Effects. Nutrients 2022, 14, 1574. https://doi.org/10.3390/nu14081574
Maiuolo J, Musolino V, Gliozzi M, Carresi C, Oppedisano F, Nucera S, Scarano F, Scicchitano M, Guarnieri L, Bosco F, et al. The Employment of Genera Vaccinium, Citrus, Olea, and Cynara Polyphenols for the Reduction of Selected Anti-Cancer Drug Side Effects. Nutrients. 2022; 14(8):1574. https://doi.org/10.3390/nu14081574
Chicago/Turabian StyleMaiuolo, Jessica, Vincenzo Musolino, Micaela Gliozzi, Cristina Carresi, Francesca Oppedisano, Saverio Nucera, Federica Scarano, Miriam Scicchitano, Lorenza Guarnieri, Francesca Bosco, and et al. 2022. "The Employment of Genera Vaccinium, Citrus, Olea, and Cynara Polyphenols for the Reduction of Selected Anti-Cancer Drug Side Effects" Nutrients 14, no. 8: 1574. https://doi.org/10.3390/nu14081574
APA StyleMaiuolo, J., Musolino, V., Gliozzi, M., Carresi, C., Oppedisano, F., Nucera, S., Scarano, F., Scicchitano, M., Guarnieri, L., Bosco, F., Macrì, R., Ruga, S., Cardamone, A., Coppoletta, A. R., Ilari, S., Mollace, A., Muscoli, C., Cognetti, F., & Mollace, V. (2022). The Employment of Genera Vaccinium, Citrus, Olea, and Cynara Polyphenols for the Reduction of Selected Anti-Cancer Drug Side Effects. Nutrients, 14(8), 1574. https://doi.org/10.3390/nu14081574








