Magnesium Orotate and the Microbiome–Gut–Brain Axis Modulation: New Approaches in Psychological Comorbidities of Gastrointestinal Functional Disorders
Abstract
:1. Introduction
2. Materials and Methods
3. General Aspects of Magnesium
3.1. Magnesium and Gastrointestinal (GI) Tract Health
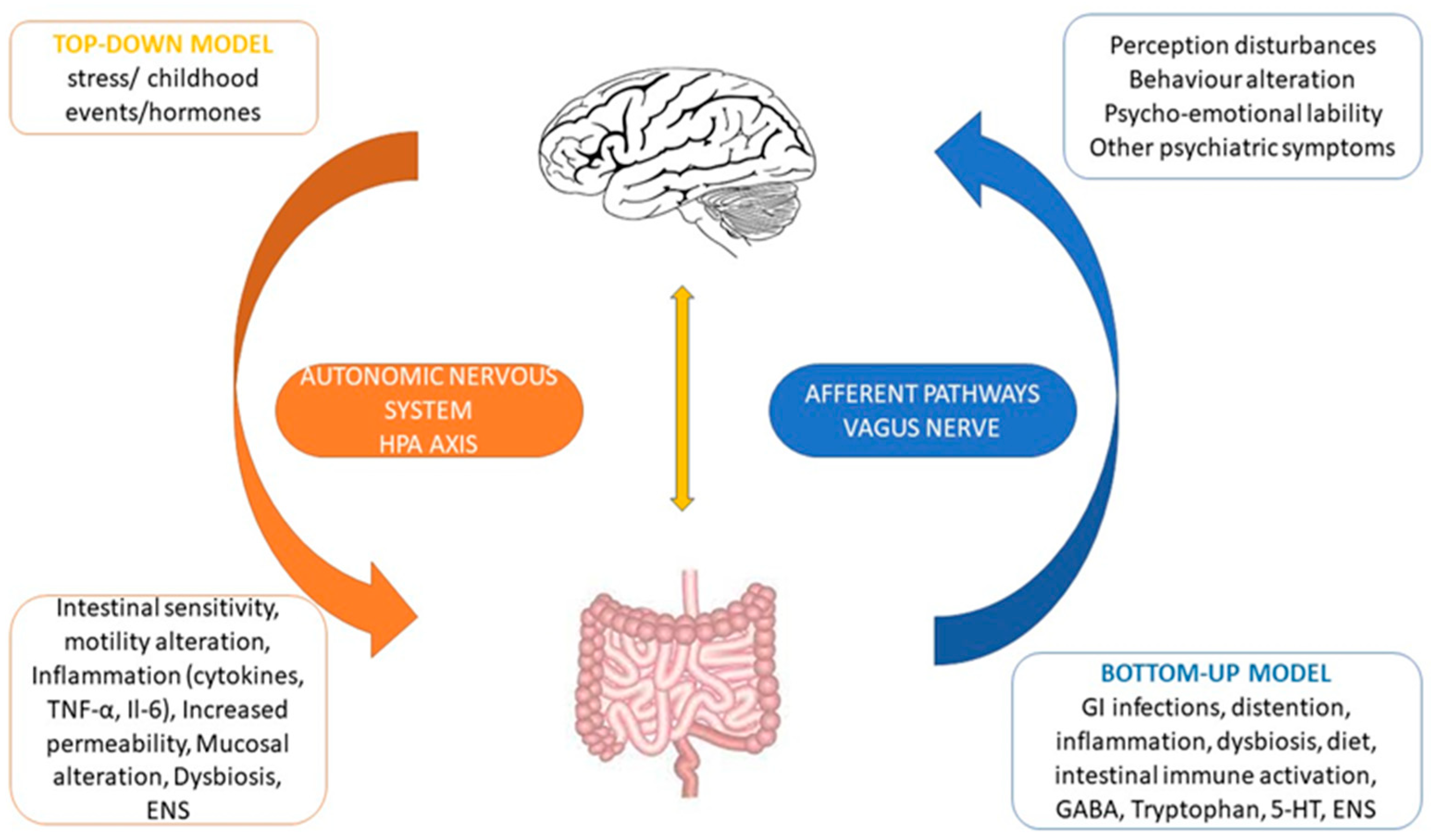
3.2. The Brain–Gut Axis
3.3. The Gut–Brain Axis and the Microbiome Physiology
3.4. Brain to Gut Connection
3.5. Gut to Brain Connection
4. Functional Gastrointestinal Disorders and the Microbiome–Gut–Brain Axis
5. Diet, Trace Elements and the Microbiome in the Gut–Brain Axis
Trace Elements
6. Magnesium, Magnesium Orotate: Where Does It Stand between the Gut and Brain?
6.1. Magnesium and Neurobiological Processes
6.2. Types of Magnesium Compounds and Their Effectiveness
6.3. Magnesium Orotate
7. Magnesium Orotate between Gut, Microbiome and Brain
7.1. S-Adenosylmethionine, Selective Serotonin Reuptake Inhibitors (SSRIs) and Magnesium Orotate
7.2. Magnesium Orotate, SSRI and Probiotics
7.3. Magnesium Orotate in Children
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Törnblom, H.; Drossman, D.A. Psychotropics, Antidepressants and Visceral Analgesics in Functional Gastrointestinal Disorders. Curr. Gastroenterol. Rep. 2018, 20, 58. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.I.; Sandhu, K.; Cryan, J.F.; Dinan, T.G. From isoniazid to psychobiotics: The gut microbiome as a new antidepressant target. Br. J. Hosp. Med. 2019, 80, 139. [Google Scholar] [CrossRef] [PubMed]
- Ezra-Nevo, G.; Henriques, S.F.; Ribeiro, C. The diet-microbiome tango: How nutrients lead the gut brain axis. Curr. Opin. Neurobiol. 2020, 62, 122–132. [Google Scholar] [CrossRef]
- Evrensel, A.; Ünsalver, B.; Ceylan, M.E. Neuroinflammation, Gut-Brain Axis and Depression. Psychiatry Investig. 2020, 17, 2. [Google Scholar] [CrossRef]
- Botturi, A.; Ciappolino, V.; DelVecchio, G.; Boscutti, A.; Viscardi, B.; Brambilla, P. The Role and the Effect of Magnesium in Mental Disorders: A Systematic Review. Nutrients 2020, 12, 1661. [Google Scholar] [CrossRef]
- Wang, J.; Um, P.; Dickerman, B.A.; Liu, J. Zinc, Magnesium, Selenium and Depression: A Review of the Evidence, Potential Mechanisms and Implications. Nutrients 2018, 10, 584. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Cui, B.-T.; Zhang, T.; Li, P.; Long, C.; Ji, G.-Z.; Zhang, F. Fecal microbiota transplantation cured epilepsy in a case with Crohn’s disease: The first report. World J. Gastroenterol. 2017, 23, 3565–3568. [Google Scholar] [CrossRef]
- Turner, R.J.; Vink, R. Magnesium in the central nervous system. In New Perspectives in Magnesium Research: Nutrition and Health; Springer: London, UK, 2007. [Google Scholar] [CrossRef] [Green Version]
- Glasdam, S.-M.; Glasdam, S.; Peters, G.H. The Importance of Magnesium in the Human Body: A Systematic Literature Review. Adv. Clin. Chem. 2016, 73, 169–193. [Google Scholar] [CrossRef] [Green Version]
- Van Vuuren, J.J.; Pillay, S.; Van Vuuren, C.J. Relationship between magnesium and lipids in patients with diabetes mellitus. J. Endocrinol. Metab. Diabetes S. Afr. 2019, 24, 46–49. [Google Scholar] [CrossRef]
- Anastassopoulou, J.; Theophanides, T. Magnesium-DNA interactions and the possible relation of magnesium to carcinogenesis. Irradiation and free radicals. Crit. Rev. Oncol. Hematol. 2002, 42, 79–91. [Google Scholar] [CrossRef]
- Zheltova, A.A.; Kharitonova, M.V.; Iezhitsa, I.N.; Spasov, A.A. Magnesium deficiency and oxidative stress: An update. Biomedicine 2016, 6, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, J.P.; Hahn, A. Intestinal Absorption and Factors Influencing Bioavailability of Magnesium-An Update. Curr. Nutr. Food Sci. 2017, 13, 260. [Google Scholar] [CrossRef] [PubMed]
- Bothe, G.; Coh, A.; Auinger, A. Efficacy and safety of a natural mineral water rich in magnesium and sulphate for bowel function: A double-blind, randomized, placebo-controlled study. Eur. J. Nutr. 2017, 56, 491–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffin, B.; Bortolloti, C.; Bourgeois, O.; Denicourt, L. Efficacy of a simethicone, activated charcoal and magnesium oxide combination (Carbosymag®) in functional dyspepsia: Results of a general practice-based randomized trial. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 494–499. [Google Scholar] [CrossRef]
- Omori, K.; Miyakawa, H.; Watanabe, A.; Nakayama, Y.; Lyu, Y.; Ichikawa, N.; Sasaki, H.; Shibata, S. The Combined Effects of Magnesium Oxide and Inulin on Intestinal Microbiota and Cecal Short-Chain Fatty Acids. Nutrients 2021, 13, 152. [Google Scholar] [CrossRef]
- Crowley, E.K.; Long-Smith, C.M.; Murphy, A.; Patterson, E.; Murphy, K.; O’Gorman, D.M.; Stanton, C.; Nolan, Y.M. Dietary Supplementation with a Magnesium-Rich Marine Mineral Blend Enhances the Diversity of Gastrointestinal Microbiota. Mar. Drugs. 2018, 16, 216. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, B.P.; Winther, G.; Kihl, P.; Nielsen, D.S.; Wegener, G.; Hansen, A.K.; Sørensen, D.B. Dietary magnesium deficiency affects gut microbiota and anxiety-like behaviour in C57BL/6N mice. Acta Neuropsychiatr. 2015, 27, 307–311. [Google Scholar] [CrossRef]
- García-Legorreta, A.; Soriano-Pérez, L.A.; Flores-Buendía, A.M.; Medina-Campos, O.N.; Noriega, L.G.; Granados-Portillo, O.; Nambo-Venegas, R.; Tovar, A.R.; Mendoza-Vargas, A.; Barrera-Oviedo, D.; et al. Effect of Dietary Magnesium Content on Intestinal Microbiota of Rats. Nutrients 2020, 12, 2889. [Google Scholar] [CrossRef]
- Pachikian, B.D.; Neyrinck, A.M.; Deldicque, L.; De Backer, F.C.; Catry, E.; Dewulf, E.M.; Sohet, F.M.; Bindels, L.B.; Everard, A.; Francaux, M.; et al. Changes in Intestinal Bifidobacteria Levels Are Associated with the Inflammatory Response in Magnesium-Deficient Mice. J. Nutr. 2010, 140, 509–514. [Google Scholar] [CrossRef] [Green Version]
- Winther, G.; Jørgensen, B.M.P.; Elfving, B.; Nielsen, D.S.; Kihl, P.; Lund, S.; Sørensen, D.B.; Wegener, G. Dietary magnesium deficiency alters gut microbiota and leads to depressive-like behaviour. Acta Neuropsychiatr. 2015, 27, 168–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kisters, K.; Gremmler, B.; Schmidt, J.; Gröber, U.; Tokmak, F. Positive Effect of Magnesium Orotate Therapy in Hypertensive Heart Disease. Metabolomics 2017, 7, 195. [Google Scholar]
- Syrkin, A.L.; Salagaev, G.I.; Syrkina, E.A.; Lysenko, A. Advantages of magnesium orotate for correction of magnesium deficiency in patients with various heart rhythm disturbances. Kardiol. I Serdechno Sosud. Khirurgiya 2019, 12, 308. [Google Scholar] [CrossRef]
- Karachentsev, Y.I.; Kravchun, N.A.; Chernyaeva, A.A.; Dunaeva, I.P.; Kholodny, A.V.; Efimenko, T.I.; Ashurov, E.M. Place of magnesium orotate in the complex therapy of patients with type 2 diabetes mellitus with hyperuricemia. Probl. Endokr. Patol. 2020, 71, 23–29. [Google Scholar] [CrossRef]
- Kalacheva, A.G.; Gromova, O.A.; Grishina, T.R.; Bogacheva, T.E.; Demidov, V.I.; Torshin, I.Y.; Tomilova, I.K. Investigation of the effects of magnesium orotate in a model of primary generalized seizures. Nevrol. Neiropsikhiatriya Psikhosomatika 2017, 9, 61–66. [Google Scholar] [CrossRef]
- Perlmutter, D. The Microbiome and the Brain; CRC Press: Boca Raton, FL, USA; p. 233. Available online: https://www.perlego.com/book/1546614/the-microbiome-and-the-brain-pdf (accessed on 11 December 2021).
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Ford, A.C.; Lacy, B.E.; Harris, L.A.; Quigley, E.M.M.; Moayyedi, P. Effect of Antidepressants and Psychological Therapies in Irritable Bowel Syndrome. Am. J. Gastroenterol. 2019, 114, 1350–1365. [Google Scholar] [CrossRef]
- Cedeño, M.M.C.; Moreira, J.F.C.; Diaz, M.J.C.; Chavez, P.E.P.; Marquinez, S.P.M.; Espinoza, A.M.F.; Veliz, A.B.B.; Alava, R.A.M.; Mendoza, A.A.G. Use of antidepressant drugs in the treatment of irritable bowel syndrome. Arch. Venez. Farmacol. Ter. 2019, 38, 6. [Google Scholar]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 11. [Google Scholar] [CrossRef]
- Gómez-Eguílaz, M.; Ramón-Trapero, J.L.; Pérez-Martínez, L.; Blanco, J.R. El eje microbiota-intestino-cerebro y sus grandes proyecciones. Rev. Neurol. 2019, 68, 111–117. [Google Scholar] [CrossRef]
- de Palma, G.; Collins, S.M.; Bercik, P.; Verdu, E.F. The microbiota-gut-brain axis in gastrointestinal disorders: Stressed bugs, stressed brain or both? J. Physiol. 2014, 592, 2989–2997. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-W.; Sha, J.; Jiaying, H. Gut-brain axis: Probiotic, Bacillus subtilis, prevents aggression via the modification of the central serotonergic system. In Oral Health by Using Probiotic Products; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Constante, M.; De Palma, G.; Lu, J.; Jury, J.; Rondeau, L.; Caminero, A.; Collins, S.M.; Verdu, E.F.; Bercik, P. Saccharomyces boulardii CNCM I-745 modulates the microbiota–gut–brain axis in a humanized mouse model of Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2021, 33, e13985. [Google Scholar] [CrossRef] [PubMed]
- Roy Sarkar, S.; Mitra Mazumder, P.; Chatterjee, K.; Sarkar, A.; Adhikary, M.; Mukhopadhyay, K.; Banerjee, S. Saccharomyces boulardii ameliorates gut dysbiosis associated cognitive decline. Physiol. Behav. 2021, 236, 113411. [Google Scholar] [CrossRef] [PubMed]
- Villageliú, D.; Mark, L. Dopamine production in Enterococcus faecium: A microbial endocrinology-based mechanism for the selection of probiotics based on neurochemical-producing potential. PLoS ONE 2018, 13, e0207038. [Google Scholar] [CrossRef]
- Srikantha, P.; Mohajeri, M.H. The possible role of the microbiota-gut-brain-axis in autism spectrum disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef] [Green Version]
- Tamana, S.K.; Tun, H.M.; Konya, T.; Chari, R.S.; Field, C.J.; Guttman, D.S.; Becker, A.B.; Moraes, T.J.; Turvey, S.E.; Subbarao, P.; et al. Bacteroides-dominant gut microbiome of late infancy is associated with enhanced neurodevelopment. Gut Microbes 2021, 13, 1930875. [Google Scholar] [CrossRef]
- Zhu, X.; Han, Y.; Du, J.; Liu, R.; Jin, K.; Yi, W. Microbiota-gut-brain axis and the central nervous system. Oncotarget 2017, 8, 53829. [Google Scholar] [CrossRef] [Green Version]
- Kapourchali, F.R.; Cresci, G.A. Cresci Early-Life Gut Microbiome—The Importance of Maternal and Infant Factors in Its Establishment. Nutr. Clin. Pract. 2020, 35, 386–405. [Google Scholar] [CrossRef]
- Agans, R.; Rigsbee, L.; Kenche, H.; Michail, S.; Khamis, H.J.; Paliy, O. Distal gut microbiota of adolescent children is different from that of adults. FEMS Microbiol. Ecol. 2011, 77, 404–412. [Google Scholar] [CrossRef]
- Simkin, D.R. Microbiome and Mental Health, Specifically as It Relates to Adolescents. Curr. Psychiatry Rep. 2019, 21, 93. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Kashyap, P.C. Beyond phylotyping: Understanding the impact of gut microbiota on host biology. Neurogastroenterol. Motil. 2013, 25, 358–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasheras, I.; Seral, P.; Latorre, E.; Barroso, E.; Gracia-García, P.; Santabárbara, J. Microbiota and gut-brain axis dysfunction in autism spectrum disorder: Evidence for functional gastrointestinal disorders. Asian J. Psychiatr. 2020, 47, 101874. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.K.; Davies, P.S. Davies Pre- and probiotics in the management of children with autism and gut issues: A review of the current evidence. Eur. J. Clin. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Soni, N.K.; Qamra, A.; Trivedi, H.H.; Sanjay, K.; Prakash, A.; Roy, S.; Mukherjee, S. A Review of Digestive Enzyme and Probiotic Supplementation for Functional Gastrointestinal Disorders. Indian Pract. 2020, 73, 3. Available online: http://articles.theindianpractitioner.com/index.php/tip/article/download/944/897 (accessed on 8 December 2021).
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional components in Western diet versus Mediterranean diet at the gut microbiota–immune system interplay. Implications for health and disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Keightley, P.C.; Koloski, N.A.; Talley, N.J. Pathways in gut-brain communication: Evidence for distinct gut-to-brain and brain-to-gut syndromes. Aust. N. Z. J. Psychiatry 2015, 49, 207–214. [Google Scholar] [CrossRef]
- Sadeghi, O.; Keshteli, A.H.; Afshar, H.; Esmaillzadeh, A.; Adibi, P. Adherence to Mediterranean dietary pattern is inversely associated with depression, anxiety and psychological distress. Nutr. Neurosci. 2021, 24, 248–259. [Google Scholar] [CrossRef]
- Nagpal, R.; Shively, C.A.; Register, T.C.; Craft, S.; Yadav, H. Gut microbiome-Mediterranean diet interactions in improving host health. F1000Research 2019, 8, 699. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Rodríguez, A.; Rubio-Arias, J.; Ramos-Campo, D.J.; Reche-García, C.; Leyva-Vela, B.; Nadal-Nicolás, Y. Psychological and Sleep Effects of Tryptophan and Magnesium-Enriched Mediterranean Diet in Women with Fibromyalgia. Int. J. Environ. Res. Public Health 2020, 17, 2227. [Google Scholar] [CrossRef] [Green Version]
- Jaeggi, T.; Kortman, G.A.M.; Moretti, D.; Chassard, C.; Holding, P.; Dostal, A.; Boekhorst, J.; Timmerman, H.M.; Swinkels, D.W.; Tjalsma, H.; et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2015, 64, 731–742. [Google Scholar] [CrossRef]
- Fang, S.; Zhuo, Z.; Yu, X.; Wang, H.; Feng, J. Oral administration of liquid iron preparation containing excess iron induces intestine and liver injury, impairs intestinal barrier function and alters the gut microbiota in rats. J. Trace Elem. Med. Biol. 2018, 47, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Chassard, C.; Rohner, F.; N’Goran, E.K.; Nindjin, C.; Dostal, A.; Utzinger, J.; Ghattas, H.; Lacroix, C.; Hurrell, R.F. The effects of iron fortification on the gut microbiota in African children: A randomized controlled trial in Côte d’Ivoire. Am. J. Clin. Nutr. 2010, 92, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Seura, T.; Yoshino, Y.; Fukuwatari, T. The relationship between habitual dietary intake and gut microbiota in young Japanese women. J. Nutr. Sci. Vitaminol. 2017, 63, 396–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zackular, J.P.; Moore, J.; Jordan, A.T.; Juttukonda, L.J.; Noto, M.J.; Nicholson, M.; Crews, J.D.; Semler, M.W.; Zhang, Y.; Ware, L.B.; et al. Dietary zinc alters the microbiota and decreases resistance to Clostridium difficile infection. Nat. Med. 2016, 22, 1330–1334. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, A.; Parra, P.; Laraichi, S.; Serra, F.; Palou, A. Calcium supplementation modulates gut microbiota in a prebiotic manner in dietary obese mice. Mol. Nutr. Food Res. 2016, 60, 468–480. [Google Scholar] [CrossRef]
- Trautvetter, U.; Camarinha-Silva, A.; Jahreis, G.; Lorkowski, S.; Glei, M. High phosphorus intake and gut-related parameters-Results of a randomized placebo-controlled human intervention study. Nutr. J. 2018, 17, 23. [Google Scholar] [CrossRef]
- Shen, H.; Han, J.; Li, Y.; Lu, C.; Zhou, J.; Li, Y.; Su, X. Different host-specific responses in thyroid function and gut microbiota modulation between diet-induced obese and normal mice given the same dose of iodine. Appl. Microbiol. Biotechnol. 2019, 103, 3537–3547. [Google Scholar] [CrossRef]
- Oriach, C.S.; Robertson, R.C.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Food for thought: The role of nutrition in the microbiota-gut-brain axis. Clin. Nutr. Exp. 2016, 6, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Möykkynen, T.; Uusi-Oukari, M.; Heikkilä, J.; Lovinger, D.M.; Lüddens, H.; Korpi, E.R. Magnesium potentiation of the function of native and recombinant GABAA receptors. Neuroreport 2001, 12, 2175–2179. [Google Scholar] [CrossRef]
- Nechifor, M. Magnesium in major depression. Magnes. Res. 2009, 22, 163–166. [Google Scholar] [CrossRef] [Green Version]
- Kirkland, A.E.; Sarlo, G.L.; Holton, K.F. The role of magnesium in neurological disorders. Nutrients 2018, 10, 730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, A.B.; Lutsey, P.L.; Gottesman, R.F.; Tin, A.; Alonso, A. Low serum magnesium is associated with incident dementia in the ARIC-NCS cohort. Nutrients 2020, 12, 3074. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Hu, L.; Dong, Y.; Xu, J.; Wei, Y.; Yu, D.; Xu, J.; Zhang, W. The effect of magnesium intake on stroke incidence: A systematic review and meta-analysis with trial sequential analysis. Front. Neurol. 2019, 10, 852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousain-Bosc, M.; Siatka, C.; Bali, J.P. Magnesium, hyperactivity and autism in children. In Magnesium in the Central Nervous System [Internet]; University of Adelaide Press: Adelaide, Australia, 2011; pp. 283–302. [Google Scholar] [CrossRef]
- Koo, C.-H.; Koo, B.; Han, J.; Lee, H.; Lim, D.; Shin, H. The effects of intraoperative magnesium sulfate administration on emergence agitation and delirium in pediatric patients: A systematic review and meta-analysis of randomized controlled trials. Paediatr. Anaesth. 2022, 32, 522–530. [Google Scholar] [CrossRef]
- Portnoy, J.; McGouldrick, S.H.; Raine, A.; Zemel, B.S.; Tucker, K.L.; Liu, J. Lower dietary intake of magnesium is associated with more callous–unemotional traits in children. Nutr. Neurosci. 2021. [Google Scholar] [CrossRef]
- Na, H.S.; Ryu, J.H.; Do, S.H. The role of magnesium in pain. In Magnesium in the Central Nervous System [Internet]; University of Adelaide Press: Adelaide, Ausralia, 2011; pp. 157–166. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507264/pdf/Bookshelf_NBK507264.pdf (accessed on 15 December 2021).
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, nutrition, detection, social impact of diseases linked to its deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef]
- Coudray, C.; Rambeau, M.; Feillet-Coudray, C.; Gueux, E.; Tressol, J.C.; Mazur, A.; Rayssiguier, Y. Study of magnesium bioavailability from ten organic and inorganic Mg salts in Mg-depleted rats using a stable isotope approach. Magnes. Res. 2005, 18, 215–223. [Google Scholar]
- Ates, M.; Kizildag, S.; Yuksel, O.; Hosgorler, F.; Yuce, Z.; Guvendi, G.; Kandis, S.; Karakilic, A.; Koc, B.; Uysal, N. Dose-dependent absorption profile of different magnesium compounds. Biol. Trace Elem. Res. 2019, 192, 244–251. [Google Scholar] [CrossRef]
- Eremenko, N.N.; Shikh, E.V.; Serebrova, S.Y.; Sizova, Z.M. Comparative study of the bioavailability of magnesium salts. Drug Metab. Pers. Ther. 2019, 34, 21–27. [Google Scholar] [CrossRef]
- Löffler, M.; Carrey, E.A.; Zameitat, E. Orotate (orotic acid): An essential and versatile molecule. Nucleosides. Nucleotides Nucleic Acids 2016, 35, 566–577. [Google Scholar] [CrossRef]
- Okonkwo, P.; Kinsella, J.E. Orotic Acid in Yoghurt. J. Dairy Sci. 1969, 52, 1861–1862. [Google Scholar] [CrossRef]
- Zeana, C. Magnesium orotate in myocardial and neuronal protection. Rom. J. Intern. Med. 1999, 37, 91–97. Available online: https://pubmed.ncbi.nlm.nih.gov/15523949/ (accessed on 10 December 2021). [PubMed]
- Manna, B.L.; Hauge, S.M. A possible relationship of vitamin B13 to orotic acid. J. Biol. Chem. 1953, 202, 91–96. [Google Scholar] [CrossRef]
- Moisa, C.; Bungau, S.; Behl, T.; Banica, F.; Purza, L.; Moleriu, R.D.; Cadar, O.; Fratila, O.; Tit, D.M. Aspects Regarding the Relationship between the Stability of Six Magnesium Compounds and their Cellular Uptake in Mice. Rev. Chim. 2000, 71, 193–204. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Mischoulon, D.; Shyu, I.; Alpert, J.E.; Fava, M. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: A double-blind, randomized clinical trial. Am. J. Psychiatry 2010, 167, 942–948. [Google Scholar] [CrossRef]
- Dileepan, K.N.; Kennedy, J. Undefined 1983 Rapid Conversion of Newly-Synthesized Orotate to Uridine-5-Monophosphate by Rat Liver Cytosolic Enzymes. FEBS Lett. 1983, 153, 1–5. Available online: https://www.sciencedirect.com/science/article/pii/0014579383801070 (accessed on 10 December 2021). [CrossRef] [Green Version]
- Bambling, M.; Parham, S.C.; Coulson, S.; Vitetta, L. S-adenosylmethionine (SAMe) and Magnesium Orotate as adjunctives to SSRIs in sub-optimal treatment response of depression in adults: A. pilot study. Adv. Integr. Med. 2015, 2, 56–62. [Google Scholar] [CrossRef]
- Bambling, M.; Edwards, S.C.; Hall, S.; Vitetta, L. A combination of probiotics and magnesium orotate attenuate depression in a small SSRI resistant cohort: An intestinal anti-inflammatory response is suggested. Inflammopharmacology 2017, 25, 271–274. [Google Scholar] [CrossRef]
- Viktorova, I.A.; Kiseliova, D.S.; Kalitskaya, I.G.; Korableva, L.M.; Suvorova, S.G. Clinical features and characteristics of vegetative status in children and adolescents with connective tissue dysplasia. Vopr. Sovrem. Pediatr. 2008, 7, 27–33. [Google Scholar]
- Bardanzellu, F.; Puddu, M.; Peroni, D.G.; Fanos, V. The Human Breast Milk Metabolome in Overweight and Obese Mothers. Front. Immunol. 2020, 11, 1533. [Google Scholar] [CrossRef]
- Sukmajaya, A.C.; Lusida, M.I.; Soetjipto; Setiawati, Y. Systematic review of gut microbiota and attention-deficit hyperactivity disorder (ADHD). Ann. Gen. Psychiatry 2021, 20, 12. [Google Scholar] [CrossRef]
- Chernikova, M.A.; Flores, G.D.; Kilroy, E.; Labus, J.S.; Mayer, E.A.; Aziz-Zadeh, L. The Brain-Gut-Microbiome System: Pathways and Implications for Autism Spectrum Disorder. Nutrients 2021, 13, 4497. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Mazaletskaya, A.L.; Ajsuvakova, O.P.; Bjørklund, G.; Skalnaya, M.G.; Chernova, L.N.; Skalny, A.A.; Tinkov, A.A. Magnesium Status in Children with Attention-Deficit/Hyperactivity Disorder and/or Autism Spectrum Disorder. J. Korean Acad. Child Adolesc. Psychiatry 2020, 31, 41–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ailincai, D.; Porzio, W.; Marin, L. Hydrogels based on imino-chitosan amphiphiles as a matrix for drug delivery systems. Polymers 2020, 12, 2687. [Google Scholar] [CrossRef] [PubMed]

| Genera | Neurochemical Involvement | Deficiency | Probiotic Therapeutical Result | Reference/ Study |
|---|---|---|---|---|
| Lactobacillus | GABA 1, BDNF 2, Vagal stimulation | FGID 3, behavior disorders, affective symptoms | Decrease intestinal distension, excitability and inflammation; decrease visceral pain by expression of opioid/cannabinoid receptors; mood and affective symptoms’ improvement | [32] |
| Bifidobacterium | GABA, 5-HT 4 | Depression, anxiety, cognitive impairment, autism, ADHD 5, FGID | Behavioral symptom resolution, digestive symptoms clearing, neurodegenerative protection, visceral pain modulation | [33] |
| Bacillus | 5-HT | Increased intestinal wall permeability, inflammation, oxidative stress, cognitive impairment, behavior and affective disorders | Decrease gastrointestinal inflammation, mood regulation | [34] |
| Saccharomyces | Myeloperoxidase, acetylcholine esterase | Increases gut inflammation, oxidative stress, neuronal damage | Reduces inflammatory cytokine, neurodegenerative protection | [35,36] |
| Enterococcus, Lactococcus | Dopamine, Histamine | Pathogenic bacteria overgrowth, gut inflammation, eating and affective disorders | Inhibits pathogenic bacteria overgrowth, reduces inflammation, histologic changes’ improvement, visceral pain reduction, mood and eating behavior improvement | [37] |
| Streptococcus | 5-HT | Inflammatory response, depressive/anxiety symptoms, cognitive impairment, Autistic Spectrum Disorder (ASD 6) | Digestive symptoms relief, cognitive and affective improvement | [38] |
| Bacteroides | Currently under study | Apparent role in neurodevelopment disorders (ADHD 5/ASD 6), functional digestive imbalances | Suggested cognitive/behavioral improvement, gastrointestinal function improvement in children with ASD/ADHD. | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiopu, C.; Ștefănescu, G.; Diaconescu, S.; Bălan, G.G.; Gimiga, N.; Rusu, E.; Moldovan, C.A.; Popa, B.; Tataranu, E.; Olteanu, A.V.; et al. Magnesium Orotate and the Microbiome–Gut–Brain Axis Modulation: New Approaches in Psychological Comorbidities of Gastrointestinal Functional Disorders. Nutrients 2022, 14, 1567. https://doi.org/10.3390/nu14081567
Schiopu C, Ștefănescu G, Diaconescu S, Bălan GG, Gimiga N, Rusu E, Moldovan CA, Popa B, Tataranu E, Olteanu AV, et al. Magnesium Orotate and the Microbiome–Gut–Brain Axis Modulation: New Approaches in Psychological Comorbidities of Gastrointestinal Functional Disorders. Nutrients. 2022; 14(8):1567. https://doi.org/10.3390/nu14081567
Chicago/Turabian StyleSchiopu, Cristina, Gabriela Ștefănescu, Smaranda Diaconescu, Gheoghe G. Bălan, Nicoleta Gimiga, Elena Rusu, Cosmin Alec Moldovan, Bogdan Popa, Elena Tataranu, Andrei Vasile Olteanu, and et al. 2022. "Magnesium Orotate and the Microbiome–Gut–Brain Axis Modulation: New Approaches in Psychological Comorbidities of Gastrointestinal Functional Disorders" Nutrients 14, no. 8: 1567. https://doi.org/10.3390/nu14081567
APA StyleSchiopu, C., Ștefănescu, G., Diaconescu, S., Bălan, G. G., Gimiga, N., Rusu, E., Moldovan, C. A., Popa, B., Tataranu, E., Olteanu, A. V., Boloș, A., & Ștefănescu, C. (2022). Magnesium Orotate and the Microbiome–Gut–Brain Axis Modulation: New Approaches in Psychological Comorbidities of Gastrointestinal Functional Disorders. Nutrients, 14(8), 1567. https://doi.org/10.3390/nu14081567









