Magnesium-to-Calcium Ratio and Mortality from COVID-19
Abstract
:1. Introduction
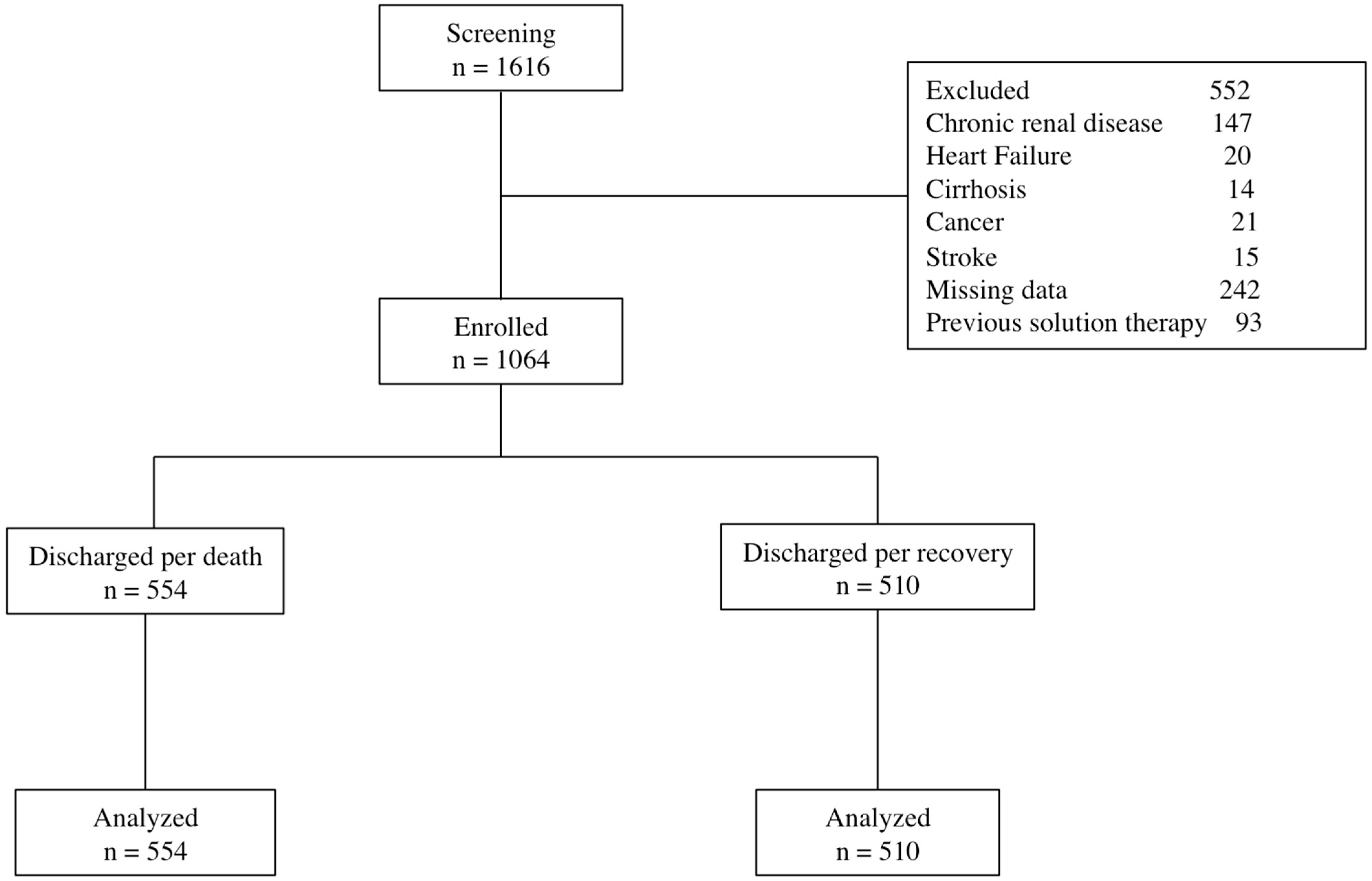
2. Materials and Methods
Statistical Analysis
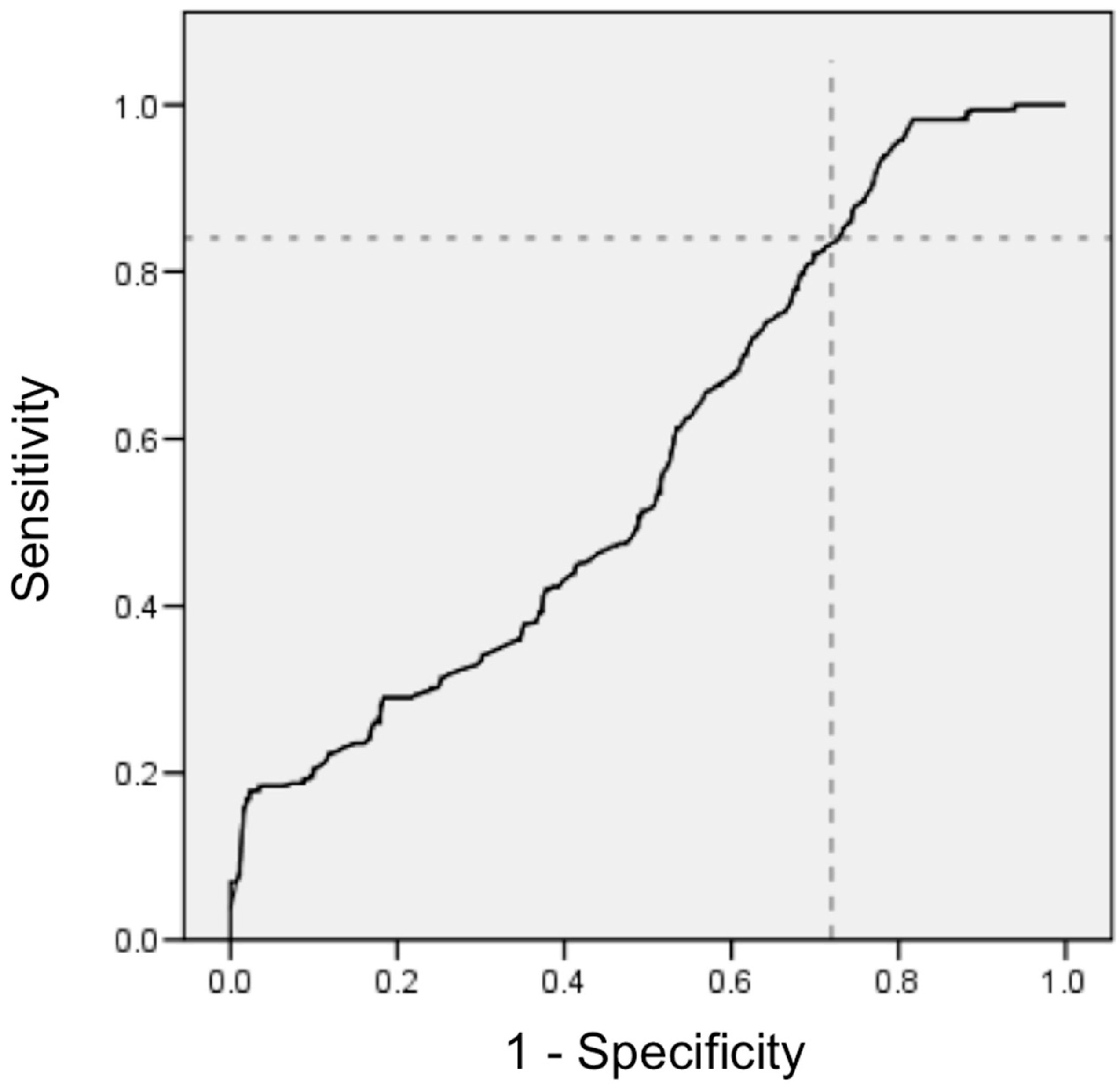
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Pollard, C.A.; Morran, M.P.; Nestor-Kalinoski, A.L. The COVID-19 pandemic: A global health crisis. Physiol. Genom. 2020, 52, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Mehta, O.P.; Bhandari, P.; Raut, A.; Kacimi, S.; Huy, N.T. Coronavirus Disease (COVID-19): Comprehensive Review of Clinical Presentation. Front. Public Health 2021, 8, 582932. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C. Combating COVID-19 and Building Immune Resilience: A Potential Role for Magnesium Nutrition? J. Am. Coll. Nutr. 2020, 39, 685–693. [Google Scholar] [CrossRef]
- Trapani, V.; Rosanoff, A.; Baniasadi, S.; Barbagallo, M.; Castiglioni, S.; Guerrero-Romero, F.; Iotti, S.; Mazur, A.; Micke, O.; Pourdowlat, G.; et al. The relevance of magnesium homeostasis in COVID-19. Eur. J. Nutr. 2022, 61, 625–636. [Google Scholar] [CrossRef]
- Zhu, L.; Bao, X.; Bi, J.; Lin, Y.; Shan, C.; Fan, X.; Bian, J.; Wang, X. Serum magnesium in patients with severe acute respiratory syndrome coronavirus 2 from Wuhan, China. Magnes Res. 2021, 34, 103–113. [Google Scholar] [CrossRef]
- Vabret, N.; Britton, G.J.; Gruber, C.; Hegde, S.; Kim, J.; Kuksin, M.; Levantovsky, R.; Malle, L.; Moreira, A.; Park, M.D.; et al. Immunology Review Project. Immunology of COVID-19: Current State of the Science. Immunity 2020, 52, 910–941. [Google Scholar] [CrossRef]
- Suhail, S.; Zajac, J.; Fossum, C.; Lowater, H.; McCracken, C.; Severson, N.; Laatsch, B.; Narkiewicz-Jodko, A.; Johnson, B.; Liebau, J.; et al. Role of Oxidative Stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) Infection: A Review. Protein J. 2020, 39, 644–656. [Google Scholar] [CrossRef]
- Ali, M.A.M.; Spinler, S.A. COVID-19 and thrombosis: From bench to bedside. Trends Cardiovasc. Med. 2021, 31, 143–160. [Google Scholar] [CrossRef]
- Gómez-Mesa, J.E.; Galindo-Coral, S.; Montes, M.C.; Muñoz Martin, A.J. Thrombosis and Coagulopathy in COVID-19. Curr. Probl. Cardiol. 2021, 46, 100742. [Google Scholar] [CrossRef]
- Nägele, M.P.; Haubner, B.; Tanner, F.C.; Ruschitzka, F.; Flammer, A.J. Endothelial dysfunction in COVID-19: Current findings and therapeutic implications. Atherosclerosis 2020, 314, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Babapoor-Farrokhran, S.; Gill, D.; Walker, J.; Rasekhi, R.T.; Bozorgnia, B.; Amanullah, A. Myocardial injury and COVID-19: Possible mechanisms. Life Sci. 2020, 253, 117723. [Google Scholar] [CrossRef] [PubMed]
- Gunay, S.; Caliskan, S.; Sigirli, D. Relationship of magnesemia with myocardial damage and mortality in patients with COVID-19. Magnes Res. 2021, 34, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Veronese, N.; Guerrero-Romero, F.; Barbagallo, M. Magnesium in Infectious Diseases in Older People. Nutrients 2021, 13, 180. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C. SARS-CoV-2 attachment to host cells is possibly mediated via RGD-integrin interaction in a calcium-dependent manner and suggests pulmonary EDTA chelation therapy as a novel treatment for COVID-19. Immunobiology 2021, 226, 152021. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef] [Green Version]
- Raesi, A.; Saedi Dezaki, E.; Moosapour, H.; Saeidifard, F.; Habibi, Z.; Rahmani, F.; Kheiri, S.; Taheri, E. Hypocalcemia in Covid-19: A Prognostic Marker for Severe Disease. Iran. J. Pathol. 2021, 16, 144–153. [Google Scholar] [CrossRef]
- Alemzadeh, E.; Alemzadeh, E.; Ziaee, M.; Abedi, A.; Salehiniya, H. The effect of low serum calcium level on the severity and mortality of COVID patients: A systematic review and meta-analysis. Immun. Inflamm. Dis. 2021, 9, 1219–1228. [Google Scholar] [CrossRef]
- Sun, J.K.; Zhang, W.H.; Zou, L.; Liu, Y.; Li, J.J.; Kan, X.H.; Dai, L.; Shi, Q.K.; Yuan, S.T.; Yu, W.K.; et al. Serum calcium as a biomarker of clinical severity and prognosis in patients with coronavirus disease 2019. Aging 2020, 12, 11287–11295. [Google Scholar] [CrossRef]
- Sontia, B.; Touyz, R.M. Magnesium transport in hypertension. Pathophysiology 2007, 14, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Yogi, A.; Callera, G.E.; Antunes, T.T.; Tostes, R.C.; Touyz, R.M. Vascular biology of magnesium and its transporters in hypertension. Magnes Res. 2010, 23, S207–S215. [Google Scholar] [CrossRef] [PubMed]
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe Covid-19. N. Engl. J. Med. 2020, 383, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Shu, X.O.; Deng, X.; Xiang, Y.B.; Li, H.; Yang, G.; Shrubsole, M.J.; Ji, B.; Cai, H.; Chow, W.H.; et al. Modifying effect of calcium/magnesium intake ratio and mortality: A population-based cohort study. BMJ Open 2013, 3, e002111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahmoun, A.E.; Singh, B.B. Does a higher ratio of serum calcium to magnesium increase the risk for postmenopausal breast cancer? Med. Hypotheses 2010, 75, 315–318. [Google Scholar] [CrossRef] [Green Version]
- Wen, F.; Zhou, Y.; Wang, W.; Hu, Q.C.; Liu, Y.T.; Zhang, P.F.; Du, Z.D.; Dai, J.; Li, Q. Ca/Mg infusions for the prevention of oxaliplatin-related neurotoxicity in patients with colorectal cancer: A meta-analysis. Ann. Oncol. 2013, 24, 171–178. [Google Scholar] [CrossRef]
- Parohan, M.; Yaghoubi, S.; Seraji, A.; Javanbakht, M.H.; Sarraf, P.; Djalali, M. Risk factors for mortality in patients with Coronavirus disease 2019 (COVID-19) infection: A systematic review and meta-analysis of observational studies. Aging Male 2020, 23, 1416–1424. [Google Scholar] [CrossRef]
- Mina, A.; van Besien, K.; Platanias, L.C. Hematological manifestations of COVID-19. Leuk. Lymphoma 2020, 61, 2790–2798. [Google Scholar] [CrossRef]
- Viana-Llamas, M.C.; Arroyo-Espliguero, R.; Silva-Obregón, J.A.; Uribe-Heredia, G.; Núñez-Gil, I.; García-Magallón, B.; Torán-Martínez, C.G.; Castillo-Sandoval, A.; Díaz-Caraballo, E.; Rodríguez-Guinea, I.; et al. Hypoalbuminemia on admission in COVID-19 infection: An early predictor of mortality and adverse events. A retrospective observational study. Med. Clin. 2021, 156, 428–436. [Google Scholar] [CrossRef]
- Li, X.; Xu, S.; Yu, M.; Wang, K.; Tao, Y.; Zhou, Y.; Shi, J.; Zhou, M.; Wu, B.; Yang, Z.; et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020, 146, 110–118. [Google Scholar] [CrossRef]
- Oh, S.M.; Skendelas, J.P.; Macdonald, E.; Bergamini, M.; Goel, S.; Choi, J.; Segal, K.R.; Vivek, K.; Nair, S.; Leff, J. On-admission anemia predicts mortality in COVID-19 patients: A single center, retrospective cohort study. Am. J. Emerg. Med. 2021, 48, 40–147. [Google Scholar] [CrossRef] [PubMed]
- Simadibrata, D.M.; Calvin, J.; Wijaya, A.D.; Ibrahim, N. Neutrophil-to-lymphocyte ratio on admission to predict the severity and mortality of COVID-19 patients: A meta-analysis. Am. J. Emerg. Med. 2021, 42, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; de Oliveira, M.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chem. Lab. Med. 2020, 58, 1021–1028. [Google Scholar] [CrossRef] [Green Version]
- Iotti, S.; Wolf, F.; Mazur, A.; Maier, J.A. The COVID-19 pandemic: Is there a role for magnesium? Hypotheses and perspectives. Magnes Res. 2020, 33, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhu, X.; Rosanoff, A.; Costello, R.B.; Yu, C.; Ness, R.; Seidner, D.L.; Murff, H.J.; Roumie, C.L.; Shrubsole, M.J.; et al. Magnesium Depletion Score (MDS) Predicts Risk of Systemic Inflammation and Cardiovascular Mortality among US Adults. J. Nutr. 2021, 151, 2226–2235. [Google Scholar] [CrossRef]
- Akobeng, A.K. Understanding diagnostic tests 1: Sensitivity, specificity and predictive values. Acta Paediatr. 2007, 96, 338–341. [Google Scholar] [CrossRef] [PubMed]
| Hospital Discharged | |||
|---|---|---|---|
| Death | Recovery | ||
| N | 554 | 510 | p Value |
| Age, years | 64.4 ± 14.7 | 57.5 ± 15.8 | 0.0001 |
| Oxygen saturation, % | 85.2 ± 5.9 | 89.4 ± 1.2 | 0.16 |
| Body mass index, kg/m2 | 29.9 ± 7.3 | 29.6 ± 7.1 | 0.66 |
| Obesity, n (%) | 209 (37.7) | 184 (36.1) | 0.62 |
| Diabetes, n (%) | 221 (39.9) | 201 (39.4) | 0.92 |
| Hypertension, n (%) | 260 (46.9) | 223 (43.7) | 0.32 |
| Chronic obstructive pulmonary disease, n (%) | 19 (3.4) | 31 (6.1) | 0.22 |
| Hemoglobin | 13.6 ± 2.8 | 14.1 ± 2.4 | 0.02 |
| Leukocyte | 11.15 (8.0–16.0) | 9.21 (0.68–12.6) | 0.001 |
| Neutrophils | 9.8 (6.6–14.1) | 7.35 (4.90–10.7) | 0.0001 |
| Lymphocytes | 0.79 (0.54–1.21) | 0.90 (0.74–1.45) | 0.0001 |
| Platelets | 250 (185–336) | 254 (195–326) | 0.371 |
| Fasting glucose, mg/dL | 203.7 ± 156.1 | 163.1 ± 99.9 | 0.0001 |
| Serum creatinine, mg/dL | 1.7 ± 0.4 | 1.4 ± 0.5 | 0.08 |
| Albumin, g/L | 3.2 ± 0.6 | 3.5 ± 0.6 | 0.0001 |
| Magnesium, mg/dL | 1.91 ± 0.31 | 1.97 ± 0.23 | 0.01 |
| Calcium, mg/dL | 8.3 ± 0.9 | 8.1 ± 1.2 | 0.39 |
| Magnesium/calcium ratio | 0.23 ± 0.05 | 0.26 ± 0.10 | 0.02 |
| D-dimer, mg/dL † | 0.94 (0.31–0.84) | 0.66 (0.32–0.81) | 0.37 |
| hsC-reactive protein | 21.6 (8.2–31.4) | 12.9 (5.5–24.1) | 0.0001 |
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Hospital Discharged | Hospital Discharged | |||||
| Death | Recovery | Death | Recovery | |||
| N | 276 | 225 | p Value | 278 | 285 | p Value |
| Age, years | 65.6 ± 13.8 | 59.01 ± 15.5 | 0.001 | 63.5 ± 15.0 | 55.3 ± 15.9 | 0.0001 |
| Diabetes, n (%) | 91 (32.9) | 139 (61.8) | 0.0001 | 96 (34.5) | 95 (33.3) | 0.83 |
| Hypertension | 112 (40.6) | 135 (60.6) | 0.0002 | 118 (42.4) | 116 (40.7) | 0.73 |
| Obesity, n (%) | 84 (30.4) | 124 (55.1) | 0.0001 | 71 (25.5) | 114 (40.0) | 0.0003 |
| CPOD *, n (%) | 9 (3.3) | 18 (8.0) | 0.41 | 10 (3.6) | 12 (4.2) | 0.95 |
| Body mass index, kg/m2 | 31.2 ± 7.5 | 30.8 ± 7.7 | 0.63 | 28.8 ± 7.1 | 28.6 ± 6.5 | 0.67 |
| Hemoglobin, | 12.7 ± 2.6 | 13.2 ± 2.2 | 0.09 | 14.1 ± 2.7 | 14.8 ± 2.3 | 0.03 |
| Leukocytes † | 10.9 (7.7–14.7) | 9.10 (6.6–12.1) | 0.001 | 11.26 (8.2–16.2) | 9.60 (7.1–13.9) | 0.002 |
| Neutrophils † | 9.3 (6.4–12.3) | 6.98 (4.7–9.66) | 0.001 | 10.22 (6.7–14.2) | 7.6 (5.1–11.6) | 0.0001 |
| Lymphocytes † | 0.95 (0.57–1.42) | 1.11 (0.81–1.67) | 0.03 | 0.74 (0.52–0.99) | 0.98 (0.69–1.37) | 0.001 |
| Platelets | 252 (182–339) | 281 (204–340) | 0.43 | 247 (192–322) | 239 (187–317) | 0.67 |
| Fasting glucose, mg/dL | 209.7 ± 161.2 | 174.9 ± 110.6 | 0.06 | 200.8 ± 154.0 | 153.8 ± 92.0 | 0.001 |
| Creatinine, mg/dL | 2.1 ± 0.3 | 1.4 ± 0.2 | 0.03 | 1.99 ± 0.3 | 1.65 ± 0. 3 | 0.24 |
| Albumin, g/L | 3.2 ± 0.5 | 3.6 ± 0.6 | 0.001 | 3.3 ± 0.7 | 3.5 ± 0.6 | 0.001 |
| Serum Calcium, mg/dL | 8.1± 1.2 | 7.5 ± 2.1 | 0.002 | 8.4 ± 0.7 | 8.1 ± 0.9 | 0.01 |
| Serum Magnesium, mg/dL | 1.90 ± 0.29 | 1.98 ± 0.24 | 0.04 | 1.91 ± 0.32 | 2.01 ± 0.22 | 0.006 |
| Magnesium/calcium ratio | 0.24 ± 0.03 | 0.28 ± 0.01 | 0.0001 | 0.23 ± 0.04 | 0.26 ± 0.01 | 0.0001 |
| D-dimer, mg/dL † | 1.34 (0.53–5.24) | 0.43 (0.33–0.81) | 0.38 | 0.60 (0.28–2.10) | 0.48 (0.28–0.87) | 0.43 |
| C-reactive protein † | 20.1 (6.8–27.4) | 9.0 (5.5–21.9) | 0.008 | 21.9 (9.0–20.7) | 16.0 (5.5–26.1) | 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero-Romero, F.; Mercado, M.; Rodriguez-Moran, M.; Ramírez-Renteria, C.; Martínez-Aguilar, G.; Marrero-Rodríguez, D.; Ferreira-Hermosillo, A.; Simental-Mendía, L.E.; Remba-Shapiro, I.; Gamboa-Gómez, C.I.; et al. Magnesium-to-Calcium Ratio and Mortality from COVID-19. Nutrients 2022, 14, 1686. https://doi.org/10.3390/nu14091686
Guerrero-Romero F, Mercado M, Rodriguez-Moran M, Ramírez-Renteria C, Martínez-Aguilar G, Marrero-Rodríguez D, Ferreira-Hermosillo A, Simental-Mendía LE, Remba-Shapiro I, Gamboa-Gómez CI, et al. Magnesium-to-Calcium Ratio and Mortality from COVID-19. Nutrients. 2022; 14(9):1686. https://doi.org/10.3390/nu14091686
Chicago/Turabian StyleGuerrero-Romero, Fernando, Moises Mercado, Martha Rodriguez-Moran, Claudia Ramírez-Renteria, Gerardo Martínez-Aguilar, Daniel Marrero-Rodríguez, Aldo Ferreira-Hermosillo, Luis E. Simental-Mendía, Ilan Remba-Shapiro, Claudia I. Gamboa-Gómez, and et al. 2022. "Magnesium-to-Calcium Ratio and Mortality from COVID-19" Nutrients 14, no. 9: 1686. https://doi.org/10.3390/nu14091686
APA StyleGuerrero-Romero, F., Mercado, M., Rodriguez-Moran, M., Ramírez-Renteria, C., Martínez-Aguilar, G., Marrero-Rodríguez, D., Ferreira-Hermosillo, A., Simental-Mendía, L. E., Remba-Shapiro, I., Gamboa-Gómez, C. I., Albarrán-Sánchez, A., & Sanchez-García, M. L. (2022). Magnesium-to-Calcium Ratio and Mortality from COVID-19. Nutrients, 14(9), 1686. https://doi.org/10.3390/nu14091686










