Magnesium as an Important Factor in the Pathogenesis and Treatment of Migraine—From Theory to Practice
Abstract
:1. Introduction
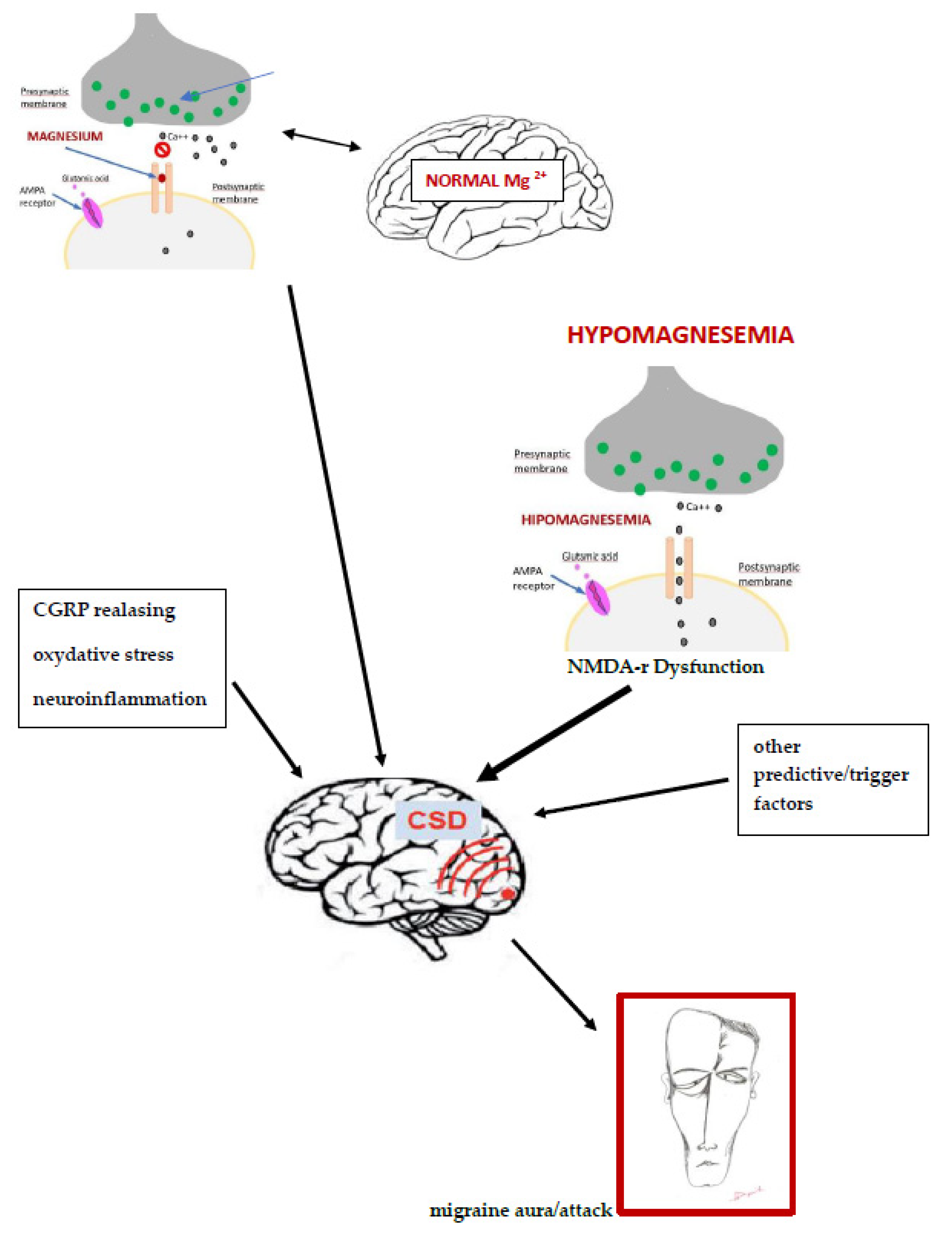
2. The Pathogenesis of Migraine and the Magnesium Role
3. The Role of Magnesium in Migraine Pathogenesis
4. The Rationale for Magnesium Treatment for Migraine
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnold, M. Headache Classification Committee of the International Headache Society (IHS) the International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar]
- Linde, M.; Gustavsson, A.; Stovner, L.J.; Steiner, T.J.; Barre, J.; Katsarava, Z.; Lainez, J.M.; Lampl, C.; Lanteri-Minet, M.; Rastenyte, D.; et al. The cost of headachedisorders in Europe: The Eurolight project. Eur. J. Neurol. 2012, 19, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef] [Green Version]
- de Dhaem, O.B.; Gharedaghi, M.H.; Bain, P.; Hettie, G.; Loder, E.; Burch, R. Identification of work accommodations and interventions associated with work productivity in adults with migraine: A scoping review. Cephalalgia 2021, 41, 760–773. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 954–976. [Google Scholar] [CrossRef] [Green Version]
- Antonaci, F.; Dumitrache, C.; De Cillis, I.; Allena, M. A review of current European treatment guidelines for migraine. J. Headache Pain 2010, 11, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Holland, S.; Silberstein, S.D.; Freitag, F.; Dodick, D.W.; Argoff, C.; Ashman, E. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2012, 78, 1346–1353. [Google Scholar] [CrossRef] [Green Version]
- Sarchielli, P.; Granella, F.; Prudenzano, M.P.; Pini, L.A.; Guidetti, V.; Bono, G.; Pinessi, L.; Alessandri, M.; Antonaci, F.; Fanciullacci, M.; et al. Italian guidelines for primary headaches: 2012 revised version. J. Headache Pain 2012, 13, 31–70. [Google Scholar] [CrossRef] [Green Version]
- Stępień, A.; Kozubski, W.; Rożniecki, J.J.; Domitrz, I. Migraine treatment recommendations developed by an Expert Group of the Polish Headache Society, the Headache Section of the Polish Neurological Society, and the Polish Pain Society. Neurol. Neurochir. Polska 2021, 55, 33–51. [Google Scholar] [CrossRef]
- Chądzyński, P.; Kacprzak, A.; Domitrz, W.; Domitrz, I. Migraine headache facilitators in a population of Polish women and their association with migraine occurrence—Preliminary results. Neurol. Neurochir. Polska 2018, 53, 377–383. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Fernández-Muñoz, J.J.; Palacios-Ceña, M.; Parás-Bravo, P.; Cigarán-Méndez, M.; Navarro-Pardo, E. Sleep disturbances in tension-type headache and migraine. Ther. Adv. Neurol. Disord. 2018, 11, 1756285617745444. [Google Scholar] [CrossRef]
- Moon, H.-J.; Seo, J.-G.; Park, S.-P. Perceived stress in patients with migraine: A case-control study. J. Headache Pain 2017, 18, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fila, M.; Chojnacki, C.; Chojnacki, J.; Blasiak, J. Nutrients to Improve Mitochondrial Function to Reduce Brain Energy Deficit and Oxidative Stress in Migraine. Nutrients 2021, 13, 4433. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Charles, A. Glutamate and Its Receptors as Therapeutic Targets for Migraine. Neurotherapeutics 2018, 15, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goadsby, P.J.; Holland, P.R. Pathophysiology of Migraine: An Update. Neurol. Clin. 2019, 37, 651–671. [Google Scholar] [CrossRef]
- Kaur, K.; Hernandez, V.; Al Hajaj, S.W.; Ebrahim, A.M.; Razack, M.; ElSharief, M.W.; Dragas, D. The Efficacy of Herbal Supplements and Nutraceuticals for Prevention of Migraine: Can They Help? Cureus 2021, 13, e14868. [Google Scholar] [CrossRef]
- Zaki, E.; Freilinger, T.; Klopstock, T.; Baldwin, E.E.; Heisner, K.R.U.; Adams, K.; Dichgans, M.; Wagler, S.; Boles, R.G. Two Common Mitochondrial DNA Polymorphisms are Highly Associated with Migraine Headache and Cyclic Vomiting Syndrome. Cephalalgia 2009, 29, 719–728. [Google Scholar] [CrossRef]
- Eising, E.; De Vries, B.; Ferrari, M.D.; Terwindt, G.M.; Maagdenberg, A.M.V.D. Pearls and pitfalls in genetic studies of migraine. Cephalalgia 2013, 33, 614–625. [Google Scholar] [CrossRef] [Green Version]
- Eising, E.; Huisman, S.M.H.; Mahfouz, A.; Vijfhuizen, L.S.; Anttila, V.; Winsvold, B.S.; Kurth, T.; Ikram, M.A.; Freilinger, T.; Kaprio, J.; et al. Gene co-expression analysis identifies brain regions and cell types involved in migraine pathophysiology: A GWAS-based study using the Allen Human Brain Atlas. Hum. Genet. 2016, 135, 425–439. [Google Scholar] [CrossRef] [Green Version]
- Olesen, J.; Larsen, B.; Lauritzen, M. Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann. Neurol. 1981, 9, 344–352. [Google Scholar] [CrossRef]
- Andersen, A.R.; Friberg, L.; Olsen, T.S.; Olesen, J. Delayed Hyperemia Following Hypoperfusion in Classic Migraine. Single Photon Emission Computed Tomographic Demonstration. Arch. Neurol. 1988, 45, 154–159. [Google Scholar] [CrossRef]
- Choudhuri, R.; Cui, L.; Young, C.; Bower, S.; Klein, R.M.; Welch, K.M.; Berman, N. Cortical Spreading Depression and Gene Regulation: Revalance to Migraine. Ann. Neurol. 2002, 51, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Zanette, E.M.; Angoli, A.; Roberti, C.; Chiarotti, F.; Cerbo, R.; Fieschi, C. Transcranial Doppler in Spontaneus Attacks of Migraine. Stroke 1992, 23, 680–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, R.P.; Iacoboni, M.; Mazziotta, J.C. Bilateral Spreading Cerebral Hypoperfusion during Spontaneous Migraine Headache. N. Engl. J. Med. 1994, 331, 1689–1692. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.D. Spreading cerebral hypoperfusion during migraine headache. N. Engl. J. Med. 1995, 332, 1516–1518. [Google Scholar]
- Lindahl, A.J.; Allder, S.; Jefferson, D.A.; Moody, A.R.; Martel, A.L. Prolonged hemiplegic migraine associated with unilateral hyperperfusion on perfusion weighted magnetic resonance imaging. J. Neurol. Neurosurg. Psychiatry 2002, 73, 202–203. [Google Scholar] [CrossRef] [Green Version]
- Vinogradova, L.V. Initiation of spreading depression by synaptic and network hyperactivity: Insights into trigger mechanisms of migraine aura. Cephalalgia 2018, 38, 1177–1187. [Google Scholar] [CrossRef]
- Van Harreveld, A.; Stamm, J.S.; Christensen, E. Spreading Depression in Rabbit, Cat and Monkey. Am. J. Physiol. 1956, 184, 312–320. [Google Scholar] [CrossRef] [Green Version]
- Martens-Mantai, T.; Speckmann, E.-J.; Gorji, A. Propagation of cortical spreading depression into the hippocampus: The role of the entorhinal cortex. Synapse 2014, 68, 574–584. [Google Scholar] [CrossRef]
- Boska, M.D.; Welch, K.M.; Barker, P.B.; Nelson, J.A.; Schultz, L. Contrasts in cortical magnesium, phospholipid and energy metabolism between migraine syndromes. Neurology 2002, 58, 1227–1233. [Google Scholar] [CrossRef]
- Mishima, K.; Takeshima, T.; Shimomura, T.; Okada, H.; Kitano, A.; Takahashi, K.; Nakashima, K. Platelet ionized magnesium, cyclic AMP, and cyclic GMP levels in migraine and tension-type headache. Headache J. Head Face Pain 1997, 37, 561–564. [Google Scholar] [CrossRef]
- Chang, C.L.; Donaghy, M.; Poulter, N. Migraine and stroke in young women: Case-control study. BMJ 1999, 318, 13–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkland, A.; Sarlo, G.L.; Holton, K.F. The Role of Magnesium in Neurological Disorders. Nutrients 2018, 10, 730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welch, K.M.; Ramadan, N.M. Mitochondria, magnesium and migraine. J. Neurol. Sci. 1995, 134, 9–14. [Google Scholar] [CrossRef]
- Aloisi, P.; Marrelli, A.; Porto, C.; Tozzi, E.; Cerone, G. Visual Evoked Potentials and Serum Magnesium Levels in Juvenile Migraine Patients. Headache J. Head Face Pain 1997, 37, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Mauskop, A.; Altura, B.T.; Altura, B.M. Serum Ionized Magnesium Levels and Serum Ionized Calcium/Ionized Magnesium Ratios in Women with Menstrual Migraine. Headache 2002, 42, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.F.; Ding, H.; Jiao, R.Q.; Wu, X.X.; Kong, L.D. Possibility of magnesium supplementation for supportive treatment in patients with COVID-19. Eur. J. Pharmacol. 2020, 886, 173546. [Google Scholar] [CrossRef]
- Haanes, K.A.; Edvinsson, L. Pathophysiological Mechanisms in Migraine and the Identification of New Therapeutic Targets. CNS Drugs. 2019, 33, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Kalia, S.K.; Salter, M.W. NMDA receptors in clinical neu-rology: Excitatory times ahead. Lancet Neurol. 2008, 7, 742–755. [Google Scholar] [CrossRef] [Green Version]
- Lauritzen, M. Cortical spreading depression in migraine. Cephalalgia 2001, 21, 757–760. [Google Scholar] [CrossRef]
- Sanchez del Rio, M.; Olesen, J.; Diener, H.C. Hemodynamics and neuroimaging of migraines. In The Headaches, 3rd ed.; Olesen, J., Goadsby, P.J., Ramadan, N.M., Tfel-Hansen, P., Welch, K.M.A., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; pp. 351–361. [Google Scholar]
- Dolati, S.; Rikhtegar, R.; Mehdizadeh, A.; Yousefi, M. The Role of Magnesium in Pathophysiology and Migraine Treatment. Biol. Trace Elem. Res. 2019, 196, 375–383. [Google Scholar] [CrossRef]
- Brennan, K.C.; Beltrán-Parrazal, L.; López-Valdés, H.E.; Theriot, J.; Toga, A.W.; Charles, A.C. Distinct Vascular Conduction with Cortical Spreading Depression. J. Neurophysiol. 2007, 97, 4143–4151. [Google Scholar] [CrossRef] [PubMed]
- Daniel, O.; Mauskop, A. Nutraceuticals in Acute and Prophylactic Treatment of Migraine. Curr. Treat. Options Neurol. 2016, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.; Grider, M.H. Physiology, Adenosine Triphosphate. In StatPearls; StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Maier, J.A.; Pickering, G.; Giacomoni, E.; Cazzaniga, A.; Pellegrino, P. Headaches and Magnesium: Mechanisms, Bioavailability, Therapeutic Efficacy and Potential Advantage of Magnesium Pidolate. Nutrients 2020, 12, 2660. [Google Scholar] [CrossRef] [PubMed]
- Myrdal, U.; Leppert, J.; Edvinsson, L.; Ekman, R.; Hedner, T.; Nilsson, H.; Ringqvist, I. Magnesium sulphate infusion decreases circulating calcitonin gene-related peptide (CGRP) in women with primary Raynaud’s phenomenon. Clin. Physiol. 1994, 14, 539–546. [Google Scholar] [CrossRef]
- Sun-Edelstein, C.; Mauskop, A. Role of magnesium in the pathogenesis and treatment of migraine. Expert Rev. Neurother. 2009, 9, 369–379. [Google Scholar] [CrossRef]
- Tajti, J.; Szok, D.; Nyári, A.; Vécsei, L. CGRP and CGRP-receptor as targets of migraine therapy: Brain Prize-2021. CNS Neurol. Disord. Drug Targets 2021, 20, 1. [Google Scholar] [CrossRef]
- Vandervorst, F.; Van Deun, L.; Van Dycke, A.; Paemeleire, K.; Reuter, U.; Schoenen, J.; Versijpt, J. CGRP monoclonal antibodies in migraine: An efficacy and tolerability comparison with standard prophylactic drugs. J. Headache Pain 2021, 22, 128. [Google Scholar] [CrossRef]
- Elin, R.J. Assessment of magnesium status for diagnosis and therapy. Magnes. Res. 2010, 23, 194–198. [Google Scholar]
- Ramadan, N.; Halvorson, H.; Vande-Linde, A.; Levine, S.R.; Helpern, J.; Welch, K. Low Brain Magnesium in Migraine. Headache J. Head Face Pain 1989, 29, 416–419. [Google Scholar] [CrossRef]
- Thomas, J.; Millot, J.-M.; Sebille, S.; Delabroise, A.-M.; Thomas, E.; Manfait, M.; Arnaud, M.J. Free and total magnesium in lymphocytes of migraine patients—Effect of magnesium-rich mineral water intake. Clin. Chim. Acta 2000, 295, 63–75. [Google Scholar] [CrossRef]
- Trauninger, A.; Pfund, Z.; Koszegi, T.; Czopf, J. Oral Magnesium Load Test in Patients with Migraine. Headache J. Head Face Pain 2002, 42, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Oskouei, D.S.; Farhoudi, M.; Mohammadzade, S.; Ghaemmaghamihezaveh, S.; Hasani, A.; Hamdi, A. Relation between serum magnesium level and migraine attacks. Neurosciences 2011, 16, 320–323. [Google Scholar] [PubMed]
- Samaie, A.; Asghari, N.; Ghorbani, R.; Arda, J. Blood Magnesium levels in migraineurs within and between the headache attacks: A case control study. Pan Afr. Med. J. 2012, 11, 46. [Google Scholar]
- Assarzadegan, F.; Asgarzadeh, S.; Hatamabadi, H.R.; Shahrami, A.; Tabatabaey, A.; Asgarzadeh, M. Serum concentration of magnesium as an independent risk factor in migraine attacks: A matched case-control study and review of the literature. Int. Clin. Psychopharm. 2016, 31, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.R.; Bhattacharjee, M.; Islam, M.S.; Banerjee, S.; Hossain, S.; Hossain, M.I.; Haidar, M.R. Relation between Serum Magnesium Level and Migraine. Mymensingh Med. J 2021, 30, 301–306. [Google Scholar]
- Cegielska, J.; Szmidt-Sałkowska, E.; Domitrz, W.; Gaweł, M.; Radziwoń-Zaleska, M.; Domitrz, I. Migraine and Its Association with Hyperactivity of Cell Membranes in the Course of Latent Magnesium Deficiency—Preliminary Study of the Importance of the Latent Tetany Presence in the Migraine Pathogenesis. Nutrients 2021, 13, 2701. [Google Scholar] [CrossRef]
- Office of Dietary Supplements—Magnesium, n.d. Available online: https://ods.od.nih.gov/factsheets/MagnesiumHealthProfessional/ (accessed on 27 November 2020).
- Pfaffenrath, V.; Wessely, P.; Meyer, C.; Isler, H.R.; Evers, S.; Grotemeyer, K.H.; Taneri, Z.; Soyka, D.; G”Bel, H.; Fischer, M. Magnesium in the Prophylaxis of Migraine—A Double-Blind, Placebo-Controlled Study. Cephalalgia 1996, 16, 436–440. [Google Scholar] [CrossRef]
- Walker, A.F.; Marakis, G.; Christie, S.; Byng, M. Mg citrate found more bioavailable than other Mg preparations in a randomised, double-blind study. Magnes. Res. 2003, 16, 183–191. [Google Scholar]
- Coudray, C.; Rambeau, M.; Feillet-Coudray, C.; Gueux, E.; Tressol, J.C.; Mazur, A.; Rayssiguier, Y. Study of magnesium bioavailability from ten organic and inorganic Mg salts in Mg-depleted rats using a stable isotope approach. Magnes. Res. 2005, 18, 215–223. [Google Scholar]
- Karimi, N.; Razian, A.; Heidari, M. The efficacy of magnesium oxide and sodium valproate in prevention of migraine headache: A randomized, controlled, double-blind, crossover study. Acta Neurol. Belg. 2021, 121, 167–173. [Google Scholar] [CrossRef]
- Wang, F.; Eeden, S.K.V.D.; Ackerson, L.M.; Salk, S.E.; Reince, R.H.; Elin, R.J. Oral Magnesium Oxide Prophylaxis of Frequent Migrainous Headache in Children: A Randomized, Double-Blind, Placebo-Controlled Trial. Headache J. Head Face Pain 2003, 43, 601–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morel, V.; Pickering, M.-E.; Goubayon, J.; Djobo, M.; Macian, N.; Pickering, G. Magnesium for Pain Treatment in 2021? State of the Art. Nutrients 2021, 13, 1397. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, J.S.; Zobitz, M.M.; Poindexter, J.R.; Pak, C.Y.C. Magnesium bioavailability from magnesium citrate and magnesium oxide. J. Am. Coll. Nutr. 1990, 99, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Muehlbauer, B.; Schwenk, M.; Coram, W.M.; Antonin, K.H.; Etienne, P.; Bieck, P.R.; Douglas, F.L. Magnesium-L-aspartate-HCl and magnesium-oxide: Bioavailability in healthy volunteers. Eur. J. Clin. Pharmacol. 1991, 40, 437–438. [Google Scholar] [CrossRef] [PubMed]
- Khani, S.; Hejazi, S.A.; Yaghoubi, M.; Sharifipour, E. Comparative study of magnesium, sodium valproate, and concurrent magnesium-sodium valproate therapy in the prevention of migraine headaches: A randomized controlled double-blind trial. J. Headache Pain 2021, 22, 21. [Google Scholar] [CrossRef]
- Burch, R. Epidemiology and Treatment of Menstrual Migraine and Migraine during Pregnancy and Lactation: A Narrative Review. Headache J. Head Face Pain 2020, 60, 200–216. [Google Scholar] [CrossRef]
- Gallelli, L.; Avenoso, T.; Falcone, D.; Palleria, C.; Peltrone, F.; Esposito, M.; De Sarro, G.; Carotenuto, M.; Guidetti, V. Effects of Acetaminophen and Ibuprofen in Children with Migraine Receiving Preventive Treatment with Magnesium. Headache J. Head Face Pain 2013, 54, 313–324. [Google Scholar] [CrossRef]
- Slavin, M.; Li, H.; Khatri, M.; Frankenfeld, C. Dietary magnesium and migraine in adults: A cross-sectional analysis of the National Health and Nutrition Examination Survey 2001–2004. Headache J. Head Face Pain 2021, 61, 276–286. [Google Scholar] [CrossRef]
- Swaminathan, R. Magnesium metabolism and its disorders. Clin. Biochem. Rev. 2003, 24, 47–66. [Google Scholar]
- Ahmed, F.; Mohammed, A. Magnesium: The Forgotten Electrolyte—A Review on Hypomagnesemia. Med. Sci. 2019, 7, 56. [Google Scholar] [CrossRef] [Green Version]
| Reference | Method of Study | Patients No. | Diagnosis | Outcome | Conclusions |
|---|---|---|---|---|---|
| Thomas et al., 2000 [53] | case control | 29 + 18 | migraine + control | significantly lower concentrations of total Mg in erythrocytes and lymphocytes in migraine | Mg in lymphocytes appears to be the most sensitive index of Mg deficiency |
| Trauninger et.al. 2002 [54] | case control | 20 + 20 | migraine + healthy | no significant difference between the groups in the baseline serum and urine Mg concentrations, although the latter tended to be lower in the migraine | Mg retention occurs in patients with migraine after oral loading, suggesting a systemic Mg deficiency |
| Talebi et al., 2011 [55] | case control | 140 + 140 | migraine + healthy | the average serum Mg level in migraine was significantly lower, no significant difference between the mean level of serum Mg in migraine with aura and without aura | serum Mg in migraine patients was related to the frequency of migraine attacks, supporting the use of Mg in prevention and treatment of migraine |
| Samaie et al., 2012 [56] | case control | 50 + 50 | migraine + healthy | no significant differences, but serum total Mg level was notably lower in the migraine | assessing serum Mg level might predict migraine attacks and help to determine optimal dose of administered Mg for achieving appropriate therapeutic outcome |
| Assarzadegan et al., 2016 [57] | case control | 40 + 40 | migraine + healthy | significant lower Mg serum levels during the migraine attacks and between the attacks compared with healthy individuals | the serum level of Mg is an independent factor for migraine |
| Karim et al., 2021 [58] | cross-sectional analytical | 70 | migraine | serum Mg level lower in severe migraine in comparison to mild to moderate headache | in all migraine groups Mg within normal range |
| Pfaffenrath et al., 1996 [61] | double-blind placebo-controlled study | 150 | migraine | with regard to the number of migraine days or migraine attacks there was no benefit with Mg compared to placebo | there were no centre-specific differences, and the final assessments of treatment efficacy by the doctor and patient were largely equivocal |
| Walker et al., 2003 [62] | randomised double-blind placebo-controlled parallel | 46 | healthy | supplementation of the organic forms of Mg citrate and amino-acid chelate showed greater absorption than Mg oxide | supplementation with Mg citrate shows superior bioavailability |
| Karimi et al., 2021 [63] | single-center, randomized, controlled, double-blind, crossover | 31 + 32 | migraine + control | Mg oxide vs valproate sodium did not show statistically significant difference in the efficacy of both drugs in migraine preventive | Mg oxide can be equally effective in the prevention of migraine attacks as valproate sodium, additionally without significant side effects |
| Wang et al., 2003 [65] | randomized, double-blind, placebo-controlled, parallel-group | 58 + 60 children of ages 3 to 17 years | migraine + control | a statistically significant downward trend in the frequency and severity of migraine pain was found in the group treated with Mg oxide but not in the placebo group | the study is inconclusive |
| Morel et al., 2021 [66] | systematic review of RCT | 81 RCTs on Mg treatment in pain (18 RCTs in migraine) | different types of pain, including migraine | the greatest number of RCTs covering this issue was found in post-operative pain and migraine treated Mg | additional, programmed clinical trials are needed to achieve a sufficient level of scientific evidence to recommend and optimize the use of magnesium in the treatment of pain, mainly chronic pain |
| Lindberg et al., 1990 [67] | observational | 17 | healthy | the level of Mg in urine after an oral loading with two Mg salts - higher after Mg citrate than Mg oxide | Mg citrate is more soluble and bioavailable than Mg oxide |
| Muehlbauer et al., 1991 [68] | observational | 24 | healthy | Mg-oxide showed significantly lower absorption than Mg-l-aspartate-HCI (granules/tablets) | Mg-l-aspartate-HCI appear to be the first choice for Mg substitution |
| Khani et al., 2021 [69] | randomized single-center double-blind parallel-group controlled | 82 + 70 + 70 (sodium valproate + magnesium with sodium valproate + magnesium) | migraine | significant reduction of migraine in valproate with Mg group | Mg enhance the antimigraine properties of sodium valproate in combination therapy and reduce the required valproate dose for migraine prophylaxis |
| Gallelli et al., 2014 [71] | single-blinded, balanced-recruitment, parallel-group, single-center | 116 children of ages 5–16 | migraine | Mg, acetaminophen, ibuprofen decreased pain intensity, but did not modify its frequency; in both acetaminophen and ibuprofen groups, magnesium significantly reduced the pain frequency | Mg increased the efficacy of ibuprofen and acetaminophen with not age-related effects |
| Slavin et al., 2021 [72] | cross-sectional | 3626 | migraine or sever headache | Mg consumption associated with lower odds of migraine | evaluate the role of dietary Mg intake on migraine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domitrz, I.; Cegielska, J. Magnesium as an Important Factor in the Pathogenesis and Treatment of Migraine—From Theory to Practice. Nutrients 2022, 14, 1089. https://doi.org/10.3390/nu14051089
Domitrz I, Cegielska J. Magnesium as an Important Factor in the Pathogenesis and Treatment of Migraine—From Theory to Practice. Nutrients. 2022; 14(5):1089. https://doi.org/10.3390/nu14051089
Chicago/Turabian StyleDomitrz, Izabela, and Joanna Cegielska. 2022. "Magnesium as an Important Factor in the Pathogenesis and Treatment of Migraine—From Theory to Practice" Nutrients 14, no. 5: 1089. https://doi.org/10.3390/nu14051089
APA StyleDomitrz, I., & Cegielska, J. (2022). Magnesium as an Important Factor in the Pathogenesis and Treatment of Migraine—From Theory to Practice. Nutrients, 14(5), 1089. https://doi.org/10.3390/nu14051089






