Parental Prepuberty Overweight and Offspring Lung Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
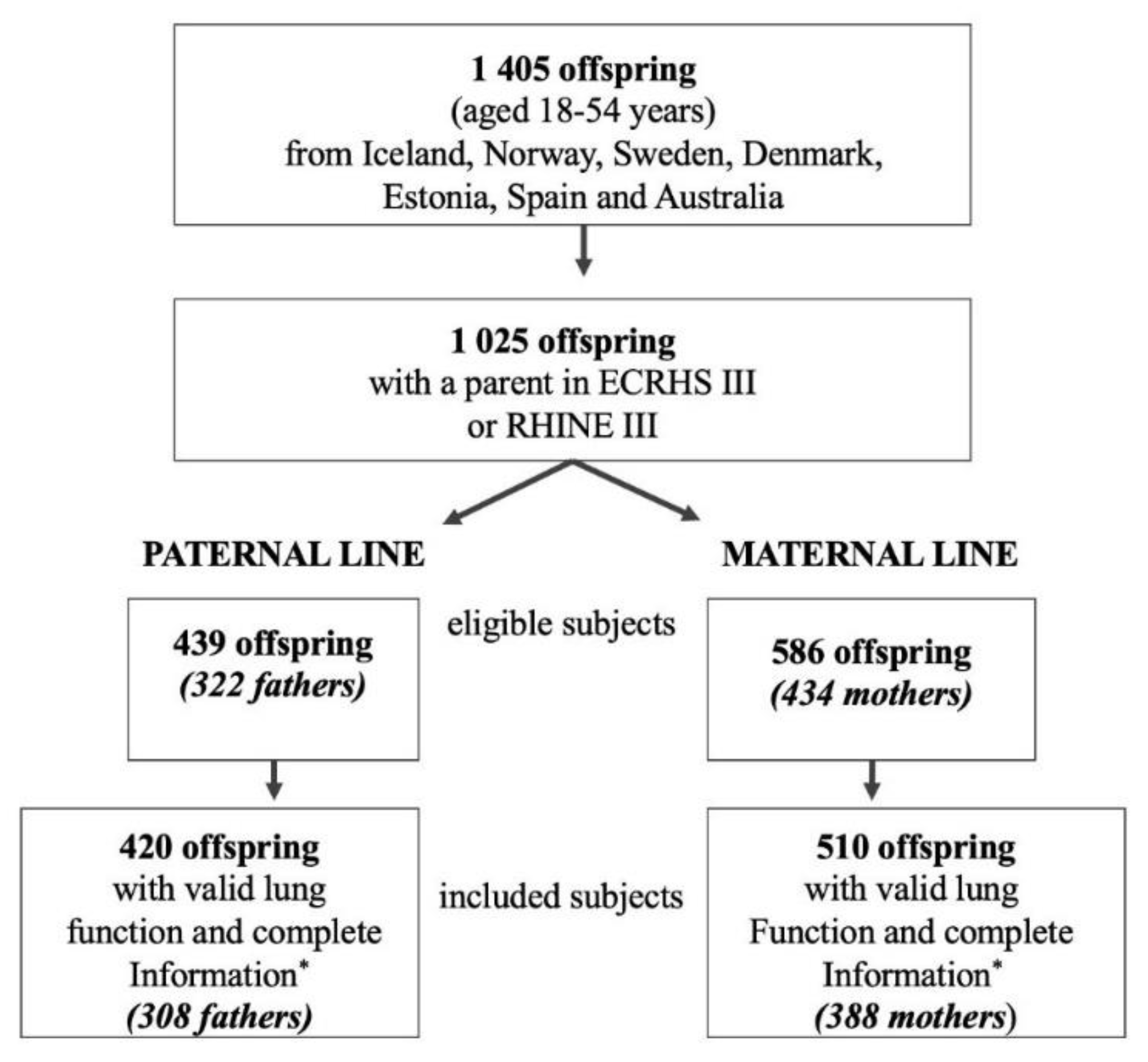
2.2. Study Population
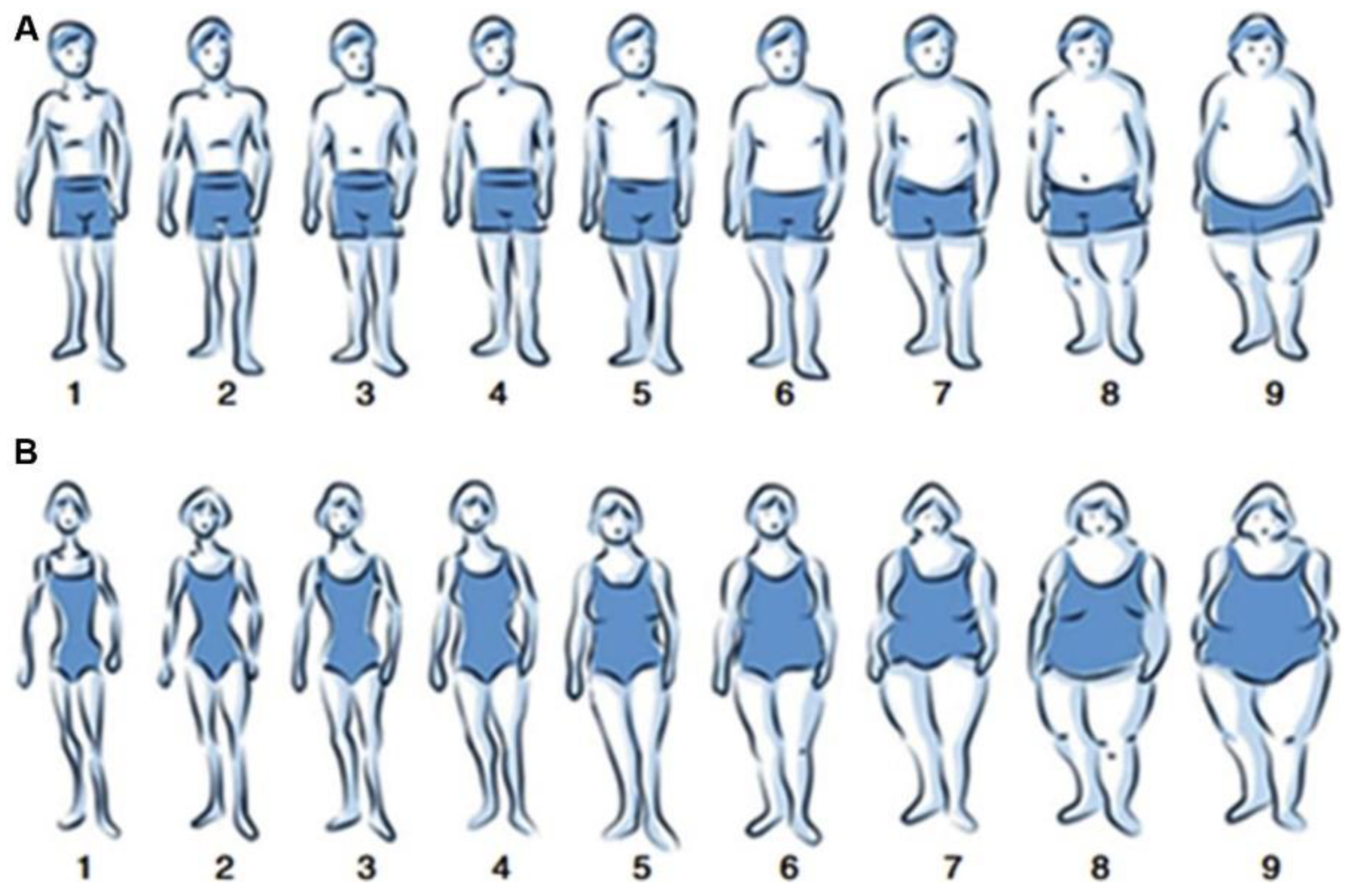
2.3. Lung Function and Definitions
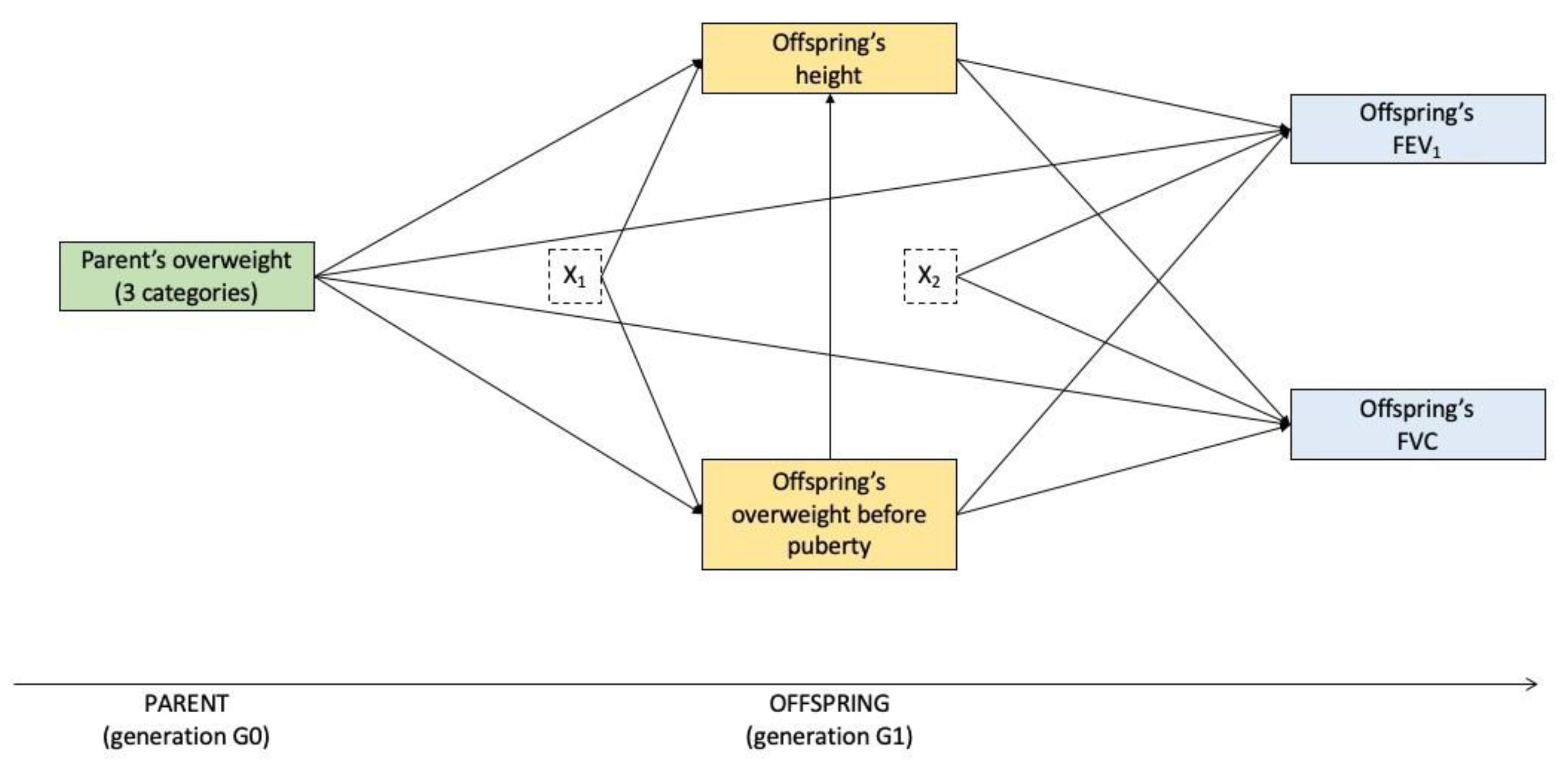
2.4. Statistical Analysis
Sensitivity Analyses
3. Results
3.1. Main Characteristics of the Study Subjects
3.2. Mediation Analysis
3.2.1. Paternal Line
3.2.2. Maternal Line
3.3. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soubry, A. Epigenetics as a Driver of Developmental Origins of Health and Disease: Did We Forget the Fathers? Bioessays 2018, 40, 1700113. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Suzuki, M. Parental obesity and overweight affect the body-fat accumulation in the offspring: The possible effect of a high-fat diet through epigenetic inheritance. Obes. Rev. 2006, 7, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.F.; Lin, R.C.; Laybutt, D.R.; Barres, R.; Owens, J.A.; Morris, M.J. Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature 2010, 467, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, V.; Maloney, C.A.; Wang, K.W.; Morris, M.J. Effects of paternal obesity on growth and adiposity of male rat offspring. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E117–E125. [Google Scholar] [CrossRef]
- Carone, B.R.; Fauquier, L.; Habib, N.; Shea, J.M.; Hart, C.E.; Li, R.; Bock, C.; Li, C.; Gu, H.; Zamore, P.D.; et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell 2010, 143, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Fullston, T.; Ohlsson Teague, E.M.; Palmer, N.O.; DeBlasio, M.J.; Mitchell, M.; Corbett, M.; Print, C.G.; Owens, J.A.; Lane, M. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J. 2013, 27, 4226–4243. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, C.R.; Wei, Y.P.; Zhao, Z.A.; Hou, Y.; Schatten, H.; Sun, Q.Y. Paternally induced transgenerational inheritance of susceptibility to diabetes in mammals. Proc. Natl. Acad. Sci. USA 2014, 111, 1873–1878. [Google Scholar] [CrossRef]
- Soubry, A.; Hoyo, C.; Jirtle, R.L.; Murphy, S.K. A paternal environmental legacy: Evidence for epigenetic inheritance through the male germ line. Bioessays 2014, 36, 359–371. [Google Scholar] [CrossRef]
- Soubry, A.; Murphy, S.K.; Wang, F.; Huang, Z.; Vidal, A.C.; Fuemmeler, B.F.; Kurtzberg, J.; Murtha, A.; Jirtle, R.L.; Schildkraut, J.M.; et al. Newborns of obese parents have altered DNA methylation patterns at imprinted genes. Int. J. Obes. 2015, 39, 650–657. [Google Scholar] [CrossRef]
- Sales, V.M.; Ferguson-Smith, A.C.; Patti, M.E. Epigenetic Mechanisms of Transmission of Metabolic Disease across Generations. Cell Metab. 2017, 25, 559–571. [Google Scholar] [CrossRef]
- Wei, Y.; Schatten, H.; Sun, Q.Y. Environmental epigenetic inheritance through gametes and implications for human reproduction. Hum. Reprod. Update 2015, 21, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, A.; Lonnebotn, M.; Calciano, L.; Benediktsdottir, B.; Bertelsen, R.J.; Braback, L.; Dharmage, S.; Franklin, K.A.; Gislason, T.; Holm, M.; et al. Being overweight in childhood, puberty, or early adulthood: Changing asthma risk in the next generation? J. Allergy Clin. Immunol. 2020, 145, 791–799.e4. [Google Scholar] [CrossRef] [PubMed]
- Accordini, S.; Calciano, L.; Johannessen, A.; Portas, L.; Benediktsdottir, B.; Bertelsen, R.J.; Braback, L.; Carsin, A.E.; Dharmage, S.C.; Dratva, J.; et al. A three-generation study on the association of tobacco smoking with asthma. Int. J. Epidemiol. 2018, 47, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Svanes, C.; Koplin, J.; Skulstad, S.M.; Johannessen, A.; Bertelsen, R.J.; Benediktsdottir, B.; Braback, L.; Elie Carsin, A.; Dharmage, S.; Dratva, J.; et al. Father’s environment before conception and asthma risk in his children: A multi-generation analysis of the Respiratory Health In Northern Europe study. Int. J. Epidemiol. 2017, 46, 235–245. [Google Scholar] [CrossRef]
- Accordini, S.; Calciano, L.; Johannessen, A.; Benediktsdottir, B.; Bertelsen, R.J.; Braback, L.; Dharmage, S.C.; Forsberg, B.; Gomez Real, F.; Holloway, J.W.; et al. Prenatal and prepubertal exposures to tobacco smoke in men may cause lower lung function in future offspring: A three-generation study using a causal modelling approach. Eur. Respir. J. 2021, 58, 2002791. [Google Scholar] [CrossRef]
- Forno, E.; Han, Y.Y.; Mullen, J.; Celedon, J.C. Overweight, Obesity, and Lung Function in Children and Adults-A Meta-analysis. J. Allergy Clin. Immunol. Pract. 2018, 6, 570–581.e10. [Google Scholar] [CrossRef]
- Burney, P.G.; Hooper, R. Forced vital capacity, airway obstruction and survival in a general population sample from the USA. Thorax 2011, 66, 49–54. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Observatory (GHO) Data: Overweight and Obesity. Available online: http://www.who.int/gho/ncd/risk_factors/overweight/en/ (accessed on 21 April 2020).
- Reinehr, T.; Roth, C.L. Is there a causal relationship between obesity and puberty? Lancet Child. Adolesc. Health 2019, 3, 44–54. [Google Scholar] [CrossRef]
- Alotaibi, M.F. Physiology of puberty in boys and girls and pathological disorders affecting its onset. J. Adolesc. 2019, 71, 63–71. [Google Scholar] [CrossRef]
- Mahmoud, O.; Granell, R.; Tilling, K.; Minelli, C.; Garcia-Aymerich, J.; Holloway, J.W.; Custovic, A.; Jarvis, D.; Sterne, J.; Henderson, J. Association of Height Growth in Puberty with Lung Function. A Longitudinal Study. Am. J. Respir. Crit. Care Med. 2018, 198, 1539–1548. [Google Scholar] [CrossRef]
- Yousefi, M.; Karmaus, W.; Zhang, H.; Roberts, G.; Matthews, S.; Clayton, B.; Arshad, S.H. Relationships between age of puberty onset and height at age 18 years in girls and boys. World J. Pediatr. 2013, 9, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Burney, P.G.; Luczynska, C.; Chinn, S.; Jarvis, D. The European Community Respiratory Health Survey. Eur. Respir. J. 1994, 7, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Janson, C.; Chinn, S.; Jarvis, D.; Burney, P. Determinants of cough in young adults participating in the European Community Respiratory Health Survey. Eur. Respir. J. 2001, 18, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, A.; Verlato, G.; Benediktsdottir, B.; Forsberg, B.; Franklin, K.; Gislason, T.; Holm, M.; Janson, C.; Jogi, R.; Lindberg, E.; et al. Longterm follow-up in European respiratory health studies-patterns and implications. BMC Pulm. Med. 2014, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, D.; Newson, R.; Janson, C.; Corsico, A.; Heinrich, J.; Anto, J.M.; Abramson, M.J.; Kirsten, A.M.; Zock, J.P.; Bono, R.; et al. Prevalence of asthma-like symptoms with ageing. Thorax 2018, 73, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Dratva, J.; Bertelsen, R.; Janson, C.; Johannessen, A.; Benediktsdottir, B.; Braback, L.; Dharmage, S.C.; Forsberg, B.; Gislason, T.; Jarvis, D.; et al. Validation of self-reported figural drawing scales against anthropometric measurements in adults. Public Health Nutr. 2016, 19, 1944–1951. [Google Scholar] [CrossRef]
- Hill, M. Social Policy: A Comparative Analysis; Prentice-Hall/Harvester Wheatsheaf: London, UK, 1996. [Google Scholar]
- Muthén, B.O.; Muthén, L.K.; Asparouhov, T. Regression and Mediation Analysis Using Mplus; Muthén & Muthén: Los Angeles, CA, USA, 2016. [Google Scholar]
- Preacher, K.J.; Rucker, D.D.; Hayes, A.F. Addressing Moderated Mediation Hypotheses: Theory, Methods, and Prescriptions. Multivar. Behav. Res. 2007, 42, 185–227. [Google Scholar] [CrossRef]
- Ryu, E.; Cheong, J. Comparing Indirect Effects in Different Groups in Single-Group and Multi-Group Structural Equation Models. Front. Psychol. 2017, 8, 747. [Google Scholar] [CrossRef]
- Muthén, B. Applications of Causally Defined Direct and Indirect Effects in Mediation Analysis Using SEM in Mplus. 2011. Available online: https://www.statmodel.com/download/causalmediation.pdf. (accessed on 10 August 2020).
- Northstone, K.; Golding, J.; Davey Smith, G.; Miller, L.L.; Pembrey, M. Prepubertal start of father’s smoking and increased body fat in his sons: Further characterisation of paternal transgenerational responses. Eur. J. Hum. Genet. 2014, 22, 1382–1386. [Google Scholar] [CrossRef]
- Bygren, L.O.; Kaati, G.; Edvinsson, S. Longevity determined by paternal ancestors’ nutrition during their slow growth period. Acta Biotheor. 2001, 49, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Soubry, A. Epigenetic inheritance and evolution: A paternal perspective on dietary influences. Prog. Biophys. Mol. Biol. 2015, 118, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Marcovecchio, M.L.; Chiarelli, F. Obesity and growth during childhood and puberty. World Rev. Nutr. Diet. 2013, 106, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Dodd, J.M.; Du Plessis, L.E.; Deussen, A.R.; Grivell, R.M.; Yelland, L.N.; Louise, J.; McPhee, A.J.; Robinson, J.S.; Owens, J.A. Paternal obesity modifies the effect of an antenatal lifestyle intervention in women who are overweight or obese on newborn anthropometry. Sci. Rep. 2017, 7, 1557. [Google Scholar] [CrossRef]
- Campbell, B.; Simpson, J.A.; Bui, D.S.; Lodge, C.J.; Lowe, A.J.; Matheson, M.C.; Bowatte, G.; Burgess, J.A.; Hamilton, G.S.; Leynaert, B.; et al. Early menarche is associated with lower adult lung function: A longitudinal cohort study from the first to sixth decade of life. Respirology 2020, 25, 289–297. [Google Scholar] [CrossRef]
- Martinez, F.D. Early-Life Origins of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2016, 375, 871–878. [Google Scholar] [CrossRef]
- Minelli, C.; Dean, C.H.; Hind, M.; Alves, A.C.; Amaral, A.F.; Siroux, V.; Huikari, V.; Soler Artigas, M.; Evans, D.M.; Loth, D.W.; et al. Association of Forced Vital Capacity with the Developmental Gene NCOR2. PLoS ONE 2016, 11, e0147388. [Google Scholar] [CrossRef]
- Barker, D.J. The fetal and infant origins of adult disease. BMJ 1990, 301, 1111. [Google Scholar] [CrossRef]
- Krauss-Etschmann, S.; Bush, A.; Bellusci, S.; Brusselle, G.G.; Dahlen, S.E.; Dehmel, S.; Eickelberg, O.; Gibson, G.; Hylkema, M.N.; Knaus, P.; et al. Of flies, mice and men: A systematic approach to understanding the early life origins of chronic lung disease. Thorax 2013, 68, 380–384. [Google Scholar] [CrossRef][Green Version]
- Saad, N.J.; Patel, J.; Burney, P.; Minelli, C. Birth Weight and Lung Function in Adulthood: A Systematic Review and Meta-analysis. Ann. Am. Thorac. Soc. 2017, 14, 994–1004. [Google Scholar] [CrossRef]
- Binder, N.K.; Beard, S.A.; Kaitu’u-Lino, T.J.; Tong, S.; Hannan, N.J.; Gardner, D.K. Paternal obesity in a rodent model affects placental gene expression in a sex-specific manner. Reproduction 2015, 149, 435–444. [Google Scholar] [CrossRef] [PubMed]
- McPherson, N.O.; Fullston, T.; Aitken, R.J.; Lane, M. Paternal obesity, interventions, and mechanistic pathways to impaired health in offspring. Ann. Nutr. Metab. 2014, 64, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Lonnebotn, M.; Svanes, C.; Igland, J.; Franklin, K.A.; Accordini, S.; Benediktsdottir, B.; Bentouhami, H.; Blanco, J.A.G.; Bono, R.; Corsico, A.; et al. Body silhouettes as a tool to reflect obesity in the past. PLoS ONE 2018, 13, e0195697. [Google Scholar] [CrossRef] [PubMed]
| (a) | |||||
| Paternal Line | |||||
| Generation | Total | Sons | Daughter’s | p-Value ‡ | |
| Father (G0) | Number of fathers | 308 | 165 | 143 | - |
| Age (years), median (range) | 56 (40–66) | 55 (40–66) | 56 (40–65) | 0.311 | |
| Low education level †, % (n) | 8.4 (26) | 8.5 (14) | 8.4 (12) | 0.571 | |
| Overweight status, % (n) | |||||
| before puberty | 12.0 (37) | 12.1 (20) | 11.9 (17) | 0.747 | |
| at age 30 years but not before puberty | 10.4 (32) | 9.1 (15) | 11.9 (17) | ||
| never | 77.6 (239) | 78.8 (130) | 76.2 (109) | ||
| Offspring (G1) | Number of adult offspring | 420 | 197 | 223 | - |
| Age (years), median (range) | 29 (18–51) | 30 (18–47) | 28 (18–51) | 0.122 | |
| Height (cm), mean (sd) | 174.1 (8.98) | 181 (6.5) | 168 (6.3) | <0.001 | |
| Pre-bronchodilator FEV1 (mL), mean (sd) | 3798 (761) | 4387 (629) | 3277 (405) | <0.001 | |
| Pre-bronchodilator FVC (mL), mean (sd) | 4692 (969) | 5490 (727) | 3987 (492) | <0.001 | |
| Pre-bronchodilator FEV1/FVC%, mean (sd) | 81.3 (6.0) | 80.0 (6.0) | 82.4 (5.8) | <0.001 | |
| Post-bronchodilator FEV1 * (mL), mean (sd) | 3918 (790) | 4531 (648) | 3359 (394) | <0.001 | |
| Post -bronchodilator FVC * (mL), mean (sd) | 4673 (978) | 5460 (750) | 3954 (481) | <0.001 | |
| Post-bronchodilator FEV1/FVC% *, mean (sd) | 84.2 (5.7) | 83.1 (5.6) | 85.2 (5.6) | <0.001 | |
| Overweight before puberty, % (n) | 21.7 (91) | 12.7 (25) | 29.6 (66) | <0.001 | |
| Ever smoking, % (n) | 25.2 (106) | 28.4 (56) | 22.4 (50) | 0.177 | |
| (b) | |||||
| Maternal Line | |||||
| Generation | Total | Sons | Daughter’s | p-Value ‡ | |
| Mother (G0) | Number of mothers | 388 | 197 | 191 | - |
| Age (years), median (range) | 55 (40–66) | 55 (40–66) | 55 (40–66) | 0.869 | |
| Low education level †, % (n) | 10.1 (39) | 8.6 (17) | 11.5 (22) | 0.219 | |
| Overweight status, % (n) | |||||
| before puberty | 25.0 (97) | 24.9 (49) | 25.1 (48) | 0.910 | |
| at age 30 years but not before puberty | 22.9 (89) | 23.9 (47) | 22.0 (42) | ||
| never | 52.1 (202) | 51.2 (101) | 52.9 (101) | ||
| Offspring (G1) | Number of adult offspring | 510 | 232 | 278 | - |
| Age (years), median (range) | 31 (18–54) | 31 (18–46) | 30 (18–54) | 0.797 | |
| Height (cm), mean (sd) | 173.9 (9.44) | 181 (6.4) | 168 (6.6) | <0.001 | |
| Pre-bronchodilator FEV1 (mL), mean (sd) | 3780 (814) | 4430 (620) | 3238 (500) | <0.001 | |
| Pre-bronchodilator FVC (mL), mean (sd) | 4675 (1028) | 5537 (761) | 3957 (568) | <0.001 | |
| Pre-bronchodilator FEV1/FVC%, mean (sd) | 81.1 (5.9) | 80.2 (5.9) | 81.9 (5.9) | <0.001 | |
| Post-bronchodilator FEV1 * (mL), mean (sd) | 3915 (832) | 4577 (630) | 3339 (488) | <0.001 | |
| Post -bronchodilator FVC * (mL), mean (sd) | 4679 (1039) | 5526 (785) | 3942 (561) | <0.001 | |
| Post-bronchodilator FEV1/FVC% *, mean (sd) | 84.0 (5.5) | 83.1 (5.6) | 84.8 (5.4) | <0.001 | |
| Overweight before puberty, % (n) | 20.4 (104) | 17.7 (41) | 22.7 (63) | 0.186 | |
| Ever smoking, % (n) | 32.6 (166) | 30.2 (70) | 34.5 (96) | 0.299 | |
| Offspring’s Overweight before Puberty ‡ Beta (95% CI) | Offspring’s Adult Height (cm) Beta (95% CI) | Offspring’s FEV1 (mL) Beta (95% CI) | Offspring’s FVC (mL) Beta (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Sons | Daughters | Sons | Daughters | Sons | Daughters | Sons | Daughters | |
| Fathers’ overweight (vs. never) | ||||||||
| before puberty | 0.56 (−0.19, 1.19) | 0.83 (0.32, 1.45) | −3.42 (−6.18, −0.57) | −2.11 (−4.58, 0.65) | −164 (−355, 45) | 26 (−155, 187) | −262 † (−501, −9) | 78 † (−138, 283) |
| at age 30 years but not before | ||||||||
| puberty | 0.07 (−4.26, 0.88) | −0.02 (−0.79, 0.57) | −0.17 (−3.56, 6.85) | −0.2 (−2.72, 1.87) | −15 (−313, 504) | 51 (−101, 209) | −43 (−363, 632) | 64 (−109, 252) |
| Offspring’s overweight before puberty (vs. absent) | - | - | 0.37 (−1.16, 1.99) | −1.17 (−2.28, −0.09) | 36 (−64, 139) | 32 (−35, 97) | 54 (−51, 170) | 48 (−29, 122) |
| Offspring’s height in adulthood (cm) | - | - | - | - | 42 (31, 53) | 30 (21, 40) | 61 † (48, 74) | 41 † (31, 53) |
| Offspring’s FEV1 (mL) Beta (95% CI) | Offspring’s FVC (mL) Beta (95% CI) | ||||
|---|---|---|---|---|---|
| Indirect Effects | Sons | Daughters | Sons | Daughters | |
| Father’s overweight (vs. never) | |||||
| before puberty | via offspring’s overweight | 20 (−41, 111) | 26 (−30, 99) | 30 (−30, 143) | 40 (−24, 126) |
| via offspring’s height | −144 (−272, −23) | −64 (−146, 19) | −210 (−380, −34) | −87 (−202, 26) | |
| via offspring’s overweight and height | 9 (−31, 69) | −29 (−83, 1) | 13 (−43, 99) | −40 (−111, 2) | |
| at age 30 years but not before puberty | via offspring’s overweight | 3 (−426, 69) | −1 (−35, 33) | 4 (−555, 79) | −1 (−47, 43) |
| via offspring’s height | −7 (−152, 267) | −6 (−86, 58) | −10 (−218, 395) | −8 (−118, 77) | |
| via offspring’s overweight and height | 1 (−229, 47) | 1 (−26, 34) | 2 (−336, 66) | 1 (−36, 46) | |
| Offspring’s Overweight before Puberty ‡, Beta (95% CI) | Offspring’s Adult Height (cm) Beta (95% CI) | Offspring’s FEV1 (mL) Beta (95% CI) | Offspring’s FVC (mL) Beta (95%CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Sons | Daughters | Sons | Daughters | Sons | Daughters | Sons | Daughters | |
| Mothers’ overweight (vs. never) | ||||||||
| before puberty | 0.59 (−0.31, 0.82) | 0.52 (−0.09, 0.71) | 0.1 (−3.54, 1.13) | −0.12 (−3.25, 0.82) | 115 (−182, 199) | 160 (−39, 213) | 124 (−237, 229) | 110 (−117, 170) |
| at age 30 years but not before puberty | 0.54 (−0.34, 0.78) | 0.3 (−0.45, 0.5) | 1.41 (−2.33, 2.5) | 1.1 (−2.4, 2.04) | 98 (−228, 200) | 186 (−47, 244) | 116 (−263, 236) | 180 (−87, 253) |
| Offspring’s overweight before puberty (vs. absent) | - | - | 1.38 (−0.77, 1.96) | 0.57 (−1.31, 1.09) | 114 (−69, 165) | 82 (−36, 113) | 35 (−69, 192) | 83 (−51, 117) |
| Offspring’s height in adulthood (cm) | - | - | - | - | 53 (34, 59) | 42 (29, 46) | 75 † (53, 82) | 53 † (38, 58) |
| Offspring’s FEV1 (mL) Beta (95% CI) | Offspring’s FVC (mL) Beta (95% CI) | ||||
|---|---|---|---|---|---|
| Indirect Effects | Sons | Daughters | Sons | Daughters | |
| Mother’s overweight (vs. never) | |||||
| before puberty | via offspring’s overweight | 34 (−24, 70) | 27 (−10, 49) | 40 (−29, 81) | 28 (−14, 51) |
| via offspring’s height | 4 (−160, 48) | −4 (−117, 30) | 6 (−236, 69) | −5 (−149, 37) | |
| via offspring’s overweight and height | 19 (−12, 37) | 5 (−16, 12) | 27 (−18, 54) | 6 (−21, 15) | |
| at age 30 years but not before puberty | via offspring’s overweight | 26 (−29, 57) | 13 (−19, 29) | 32 (−33, 67) | 15 (−19, 34) |
| via offspring’s height | 61 (−104, 112) | 39 (−87, 74) | 91 (−151, 163) | 50 (−110, 93) | |
| via offspring’s overweight and height | 20 (−9, 42) | 9 (−8, 21) | 29 (−14, 60) | 12 (−11, 27) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lønnebotn, M.; Calciano, L.; Johannessen, A.; Jarvis, D.L.; Abramson, M.J.; Benediktsdóttir, B.; Bråbäck, L.; Franklin, K.A.; Godoy, R.; Holm, M.; et al. Parental Prepuberty Overweight and Offspring Lung Function. Nutrients 2022, 14, 1506. https://doi.org/10.3390/nu14071506
Lønnebotn M, Calciano L, Johannessen A, Jarvis DL, Abramson MJ, Benediktsdóttir B, Bråbäck L, Franklin KA, Godoy R, Holm M, et al. Parental Prepuberty Overweight and Offspring Lung Function. Nutrients. 2022; 14(7):1506. https://doi.org/10.3390/nu14071506
Chicago/Turabian StyleLønnebotn, Marianne, Lucia Calciano, Ane Johannessen, Deborah L. Jarvis, Michael J. Abramson, Bryndís Benediktsdóttir, Lennart Bråbäck, Karl A. Franklin, Raúl Godoy, Mathias Holm, and et al. 2022. "Parental Prepuberty Overweight and Offspring Lung Function" Nutrients 14, no. 7: 1506. https://doi.org/10.3390/nu14071506
APA StyleLønnebotn, M., Calciano, L., Johannessen, A., Jarvis, D. L., Abramson, M. J., Benediktsdóttir, B., Bråbäck, L., Franklin, K. A., Godoy, R., Holm, M., Janson, C., Jõgi, N. O., Kirkeleit, J., Malinovschi, A., Pereira-Vega, A., Schlünssen, V., Dharmage, S. C., Accordini, S., Gómez Real, F., & Svanes, C. (2022). Parental Prepuberty Overweight and Offspring Lung Function. Nutrients, 14(7), 1506. https://doi.org/10.3390/nu14071506









