Effect of Paternal Diet on Spermatogenesis and Offspring Health: Focus on Epigenetics and Interventions with Food Bioactive Compounds
Abstract
:1. Introduction
2. Diet and Male Reproductive Health
2.1. Effects of BFCs on Male Spermatogenesis
2.1.1. Vitamins
2.1.2. Trace Elements
2.1.3. Other BFCs
3. Diet and Male Reproductive Epigenetics
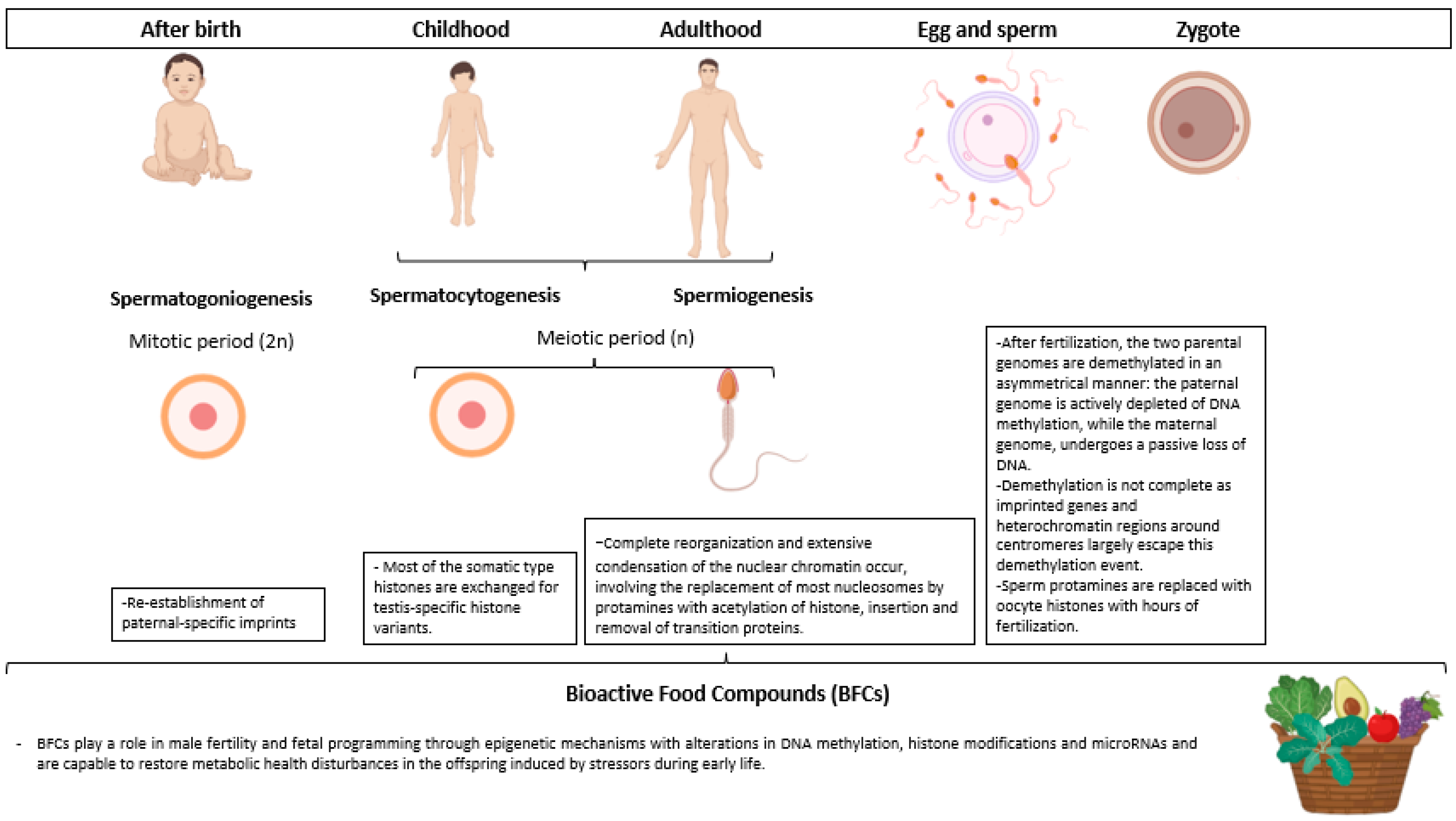
3.1. Sperm-Specific Epigenetics
3.2. BFCs Epigenetic Modulation in Male Germ Cells
4. Paternal Interventions with BFCs as a Potential Epigenetic Strategy to Improve Health and Prevent Chronic Disease in the Offspring
4.1. Nutrition and Paternal Origins of Health and Disease (POHaD)
4.2. Malnutrition and PoHAD
4.3. BFCs and PoHAD
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zegers-Hochschild, F.; Adamson, G.D.; de Mouzon, J.; Ishihara, O.; Mansour, R.; Nygren, K.; Sullivan, E.; van der Poel, S.; WHO. The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary on ART Terminology, 2009. Hum. Reprod. 2009, 24, 2683–2687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, A.D.; Majzoub, A.; Agawal, A. Metabolic syndrome and male fertility. World J. Men’s Health 2019, 37, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2014, 21, 411–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, I.D.; Fronczak, C.; Roth, L.; Meacham, R.B. Fertility and the aging male. Rev. Urol. 2011, 13, 184–190. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 16 February 2022).
- Sharma, R.; Biedenharn, K.R.; Fedor, J.M.; Agarwal, A. Lifestyle factors and reproductive health: Taking control of your fertility. Reprod. Biol. Endocrinol. 2013, 11, 66. [Google Scholar] [CrossRef] [Green Version]
- Nassan, F.L.; Chavarro, J.E.; Tanrikut, C. Diet and men’s fertility: Does diet affect sperm quality? Fertil. Steril. 2018, 110, 570–577. [Google Scholar] [CrossRef] [Green Version]
- Belan, M.; Carranza-Mamane, B.; Pesant, M.H.; AinMelk, Y.; Duval, K.; Jean-Denis, F.; Langlois, M.F.; Baillargeon, J.P. Male partners of subfertile couples in which the spouse is obese display adverse weight and lifestyle associated with reduced sperm quality. Obes. Res. Clin. Pract. 2019, 13, 226–232. [Google Scholar] [CrossRef]
- Bisconti, M.; Simon, J.F.; Grassi, S.; Leroy, B.; Martinet, B.; Arcolia, V.; Isachenko, V.; Hennebert, E. Influence of risk factors for male infertility on sperm protein composition. Int. J. Mol. Sci. 2021, 22, 13164. [Google Scholar] [CrossRef]
- Takeshima, T.; Usui, K.; Mori, K.; Asai, T.; Yasuda, K.; Kuroda, S.; Yumura, Y. Oxidative stress and male infertility. Reprod. Med. Biol. 2021, 20, 41–52. [Google Scholar] [CrossRef]
- Danielewicz, A.; Przybyłowicz, K.E.; Przybyłowicz, M. Dietary patterns and poor semen quality risk in men: A cross-sectional study. Nutrients 2018, 10, 1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazarin, C.B.B. Bioactive Food Components Activity in Mechanistic Approach; Academic Press: Cambridge, MA, USA, 2022; ISBN 9780128235690. [Google Scholar]
- Dias, T.R.; Alves, M.G.; Silva, B.M.; Oliveira, P.F. Nutritional Factors and Male Reproduction. In Encyclopedia of Reproduction; Elsevier: Amsterdam, The Netherlands, 2018; pp. 458–464. [Google Scholar]
- Salas-Huetos, A.; Bulló, M.; Salas-Salvadó, J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: A systematic review of observational studies. Hum. Reprod. Update 2017, 23, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; James, E.R.; Aston, K.I.; Jenkins, T.G.; Carrell, D.T. Diet and sperm quality: Nutrients, foods and dietary patterns. Reprod. Biol. 2019, 19, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Suliga, E.; Głuszek, S. The relationship between diet, energy balance and fertility in men. Int. J. Vitam. Nutr. Res. 2020, 90, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef] [Green Version]
- Homa, S.T.; Vessey, W.; Perez-Miranda, A.; Riyait, T.; Agarwal, A. Reactive Oxygen Species (ROS) in human semen: Determination of a reference range. J. Assist. Reprod. Genet. 2015, 32, 757–764. [Google Scholar] [CrossRef] [Green Version]
- Schagdarsurengin, U.; Steger, K. Epigenetics in male reproduction: Effect of paternal diet on sperm quality and offspring health. Nat. Rev. Urol. 2016, 13, 584–595. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Lanzillotti, C.; Mazziotta, C.; Tognon, M.; Martini, F. Epigenetics of Male Infertility: The Role of DNA Methylation. Front. Cell Dev. Biol. 2021, 9, 689624. [Google Scholar] [CrossRef]
- Craig, J.R.; Jenkins, T.G.; Carrell, D.T.; Hotaling, J.M. Obesity, male infertility, and the sperm epigenome. Fertil. Steril. 2017, 107, 848–859. [Google Scholar] [CrossRef] [Green Version]
- Fontelles, C.C.; da Cruz, R.S.; Hilakivi-Clarke, L.; de Assis, S.; Ong, T.P. Investigation of Paternal Programming of Breast Cancer Risk in Female Offspring in Rodent Models. Methods Mol. Biol. 2018, 1735, 207–220. [Google Scholar] [CrossRef]
- Soubry, A.; Hoyo, C.; Jirtle, R.L.; Murphy, S.K. A paternal environmental legacy: Evidence for epigenetic inheritance through the male germ line. BioEssays 2014, 36, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Amann, R.P.; Howards, S.S. Daily Spermatozoal Production and Epididymal Spermatozoal Reserves of the Human Male. J. Urol. 1980, 124, 211–215. [Google Scholar] [CrossRef]
- Stukenborg, J.-B.; Kjartansdóttir, K.R.; Reda, A.; Colon, E.; Albersmeier, J.P.; Söder, O. Male Germ Cell Development in Humans. Horm. Res. Paediatr. 2014, 81, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Oatley, J.M.; Brinster, R.L. Regulation of Spermatogonial Stem Cell Self-Renewal in Mammals. Annu. Rev. Cell Dev. Biol. 2008, 24, 263–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, W.H. Androgen Actions in the Testis and the Regulation of Spermatogenesis. In Molecular Mechanisms in Spermatogenesis; Springer Science+Business Media: Pittsburgh, PA, USA, 2021. [Google Scholar]
- Alves, M.G.; Rato, L.; Carvalho, R.A.; Moreira, P.I.; Socorro, S.; Oliveira, P.F. Hormonal control of Sertoli cell metabolism regulates spermatogenesis. Cell. Mol. Life Sci. 2013, 70, 777–793. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.B.; Walker, W.H. The regulation of spermatogenesis by androgens. Semin. Cell Dev. Biol. 2014, 30, 2–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.; Lee, S.O.; Wang, R.-S.; Yeh, S.; Chang, T.-M. Androgen Receptor (AR) Physiological Roles in Male and Female Reproductive Systems: Lessons Learned from AR-Knockout Mice Lacking AR in Selective Cells1. Biol. Reprod. 2013, 89, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Richardson, L.L.; Kleinman, H.K.; Dym, M. Basement Membrane Gene Expression by Sertoli and Peritubular Myoid Cells in Vitro in the Rat. Biol. Reprod. 1995, 52, 320–330. [Google Scholar] [CrossRef]
- Benatta, M.; Kettache, R.; Buchholz, N.; Trinchieri, A. The impact of nutrition and lifestyle on male fertility. Arch. Ital. Urol. Androl. 2020, 92, 121–131. [Google Scholar] [CrossRef]
- Arab, A.; Rafie, N.; Mansourian, M.; Miraghajani, M.; Hajianfar, H. Dietary patterns and semen quality: A systematic review and meta-analysis of observational studies. Andrology 2018, 6, 20–28. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Healthy Diet. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 16 August 2021).
- Al-Azemi, M.; Omu, A.; Fatinikun, T.; Mannazhath, N.; Abraham, S. Factors contributing to gender differences in serum retinol and α-tocopherol in infertile couples. Reprod. Biomed. Online 2009, 19, 583–590. [Google Scholar] [CrossRef] [Green Version]
- Colagar, A.H.; Marzony, E.T. Ascorbic Acid in Human Seminal Plasma: Determination and Its Relationship to Sperm Quality. J. Clin. Biochem. Nutr. 2009, 45, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Akmal, M.; Qadri, J.Q.; Al-Waili, N.S.; Thangal, S.; Haq, A.; Saloom, K.Y. Improvement in Human Semen Quality after Oral Supplementation of Vitamin C. J. Med. Food 2006, 9, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Matorras, R.; Pérez-Sanz, J.; Corcóstegui, B.; Pérez-Ruiz, I.; Malaina, I.; Quevedo, S.; Aspichueta, F.; Crisol, L.; Martinez-Indart, L.; Prieto, B.; et al. Effect of vitamin E administered to men in infertile couples on sperm and assisted reproduction outcomes: A double-blind randomized study. F&S Rep. 2020, 1, 219–226. [Google Scholar] [CrossRef]
- Keskes-Ammar, L.; Feki-Chakroun, N.; Rebai, T.; Sahnoun, Z.; Ghozzi, H.; Hammami, S.; Zghal, K.; Fki, H.; Damak, J.; Bahloul, A. Sperm oxidative stress and the effect of an oral Vitamin E and selenium supplement on semen quality in infertile men. Arch. Androl. 2003, 49, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Blomberg Jensen, M.; Nielsen, J.E.; Jorgensen, A.; Rajpert-De Meyts, E.; Kristensen, D.M.; Jorgensen, N.; Skakkebaek, N.E.; Juul, A.; Leffers, H. Vitamin D receptor and Vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum. Reprod. 2010, 25, 1303–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinuta, K.; Tanaka, H.; Moriwake, T.; Aya, K.; Kato, S.; Seino, Y. Vitamin D Is an Important Factor in Estrogen Biosynthesis of Both Female and Male Gonads. Endocrinology 2000, 141, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Blomberg Jensen, M.; Bjerrum, P.J.; Jessen, T.E.; Nielsen, J.E.; Joensen, U.N.; Olesen, I.A.; Petersen, J.H.; Juul, A.; Dissing, S.; Jørgensen, N. Vitamin D is positively associated with sperm motility and increases intracellular calcium in human spermatozoa. Hum. Reprod. 2011, 26, 1307–1317. [Google Scholar] [CrossRef] [Green Version]
- Hoek, J.; Steegers-Theunissen, R.P.M.; Willemsen, S.P.; Schoenmakers, S. Paternal Folate Status and Sperm Quality, Pregnancy Outcomes, and Epigenetics: A Systematic Review and Meta-Analysis. Mol. Nutr. Food Res. 2020, 64, 1900696. [Google Scholar] [CrossRef] [Green Version]
- Boonyarangkul, A.; Vinayanuvattikhun, N.; Chiamchanya, C.; Visutakul, P. Comparative Study of the Effects of Tamoxifen Citrate and Folate on Semen Quality of the Infertile Male with Semen Abnormality. J. Med. Assoc. Thai 2015, 98, 1057–1063. [Google Scholar]
- Ahsan, U.; Kamran, Z.; Raza, I.; Ahmad, S.; Babar, W.; Riaz, M.H.; Iqbal, Z. Role of selenium in male reproduction—A review. Anim. Reprod. Sci. 2014, 146, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Safarinejad, M.R.; Safarinejad, S. Efficacy of Selenium and/or N-Acetyl-Cysteine for Improving Semen Parameters in Infertile Men: A Double-Blind, Placebo Controlled, Randomized Study. J. Urol. 2009, 181, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dong, X.; Hu, X.; Long, Z.; Wang, L.; Liu, Q.; Sun, B.; Wang, Q.; Wu, Q.; Li, L. Zinc levels in seminal plasma and their correlation with male infertility: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 22386. [Google Scholar] [CrossRef] [PubMed]
- Colagar, A.H.; Marzony, E.T.; Chaichi, M.J. Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr. Res. 2009, 29, 82–88. [Google Scholar] [CrossRef]
- Ciftci, H.; Verit, A.; Savas, M.; Yeni, E.; Erel, O. Effects of N-acetylcysteine on Semen Parameters and Oxidative/Antioxidant Status. Urology 2009, 74, 73–76. [Google Scholar] [CrossRef]
- Safarinejad, M.R. Efficacy of Coenzyme Q10 on Semen Parameters, Sperm Function and Reproductive Hormones in Infertile Men. J. Urol. 2009, 182, 237–248. [Google Scholar] [CrossRef]
- Balercia, G.; Buldreghini, E.; Vignini, A.; Tiano, L.; Paggi, F.; Amoroso, S.; Ricciardo-Lamonica, G.; Boscaro, M.; Lenzi, A.; Littarru, G. Coenzyme Q10 treatment in infertile men with idiopathic asthenozoospermia: A placebo-controlled, double-blind randomized trial. Fertil. Steril. 2009, 91, 1785–1792. [Google Scholar] [CrossRef]
- Safarinejad, M.R.; Safarinejad, S.; Shafiei, N.; Safarinejad, S. Effects of the Reduced Form of Coenzyme Q 10 (Ubiquinol) on Semen Parameters in Men with Idiopathic Infertility: A Double-Blind, Placebo Controlled, Randomized Study. J. Urol. 2012, 188, 526–531. [Google Scholar] [CrossRef] [Green Version]
- Nadjarzadeh, A.; Sadeghi, M.R.; Amirjannati, N.; Vafa, M.R.; Motevalian, S.A.; Gohari, M.R.; Akhondi, M.A.; Yavari, P.; Shidfar, F. Coenzyme Q10 improves seminal oxidative defense but does not affect on semen parameters in idiopathic oligoasthenoteratozoospermia: A randomized double-blind, placebo controlled trial. J. Endocrinol. Investig. 2011, 34, e224–e228. [Google Scholar] [CrossRef]
- Safarinejad, M.R.; Hosseini, S.Y.; Dadkhah, F.; Asgari, M.A. Relationship of omega-3 and omega-6 fatty acids with semen characteristics, and anti-oxidant status of seminal plasma: A comparison between fertile and infertile men. Clin. Nutr. 2010, 29, 100–105. [Google Scholar] [CrossRef]
- Safarinejad, M.R. Effect of omega-3 polyunsaturated fatty acid supplementation on semen profile and enzymatic anti-oxidant capacity of seminal plasma in infertile men with idiopathic oligoasthenoteratospermia: A double-blind, placebo-controlled, randomised study. Andrologia 2011, 43, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Attaman, J.A.; Toth, T.L.; Furtado, J.; Campos, H.; Hauser, R.; Chavarro, J.E. Dietary fat and semen quality among men attending a fertility clinic. Hum. Reprod. 2012, 27, 1466–1474. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.-X.; Yuan, D.-J.; Wang, Q.-L.; Jiang, F.; Guo, J.; Tang, Y.-G.; Zheng, L.-X.; Kang, J.X. Association of decreased spermatozoa omega-3 fatty acid levels and increased oxidative DNA damage with varicocele in infertile men: A case control study. Reprod. Fertil. Dev. 2016, 28, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Soto, J.C.; Domingo, J.C.; Cordobilla, B.; Nicolás, M.; Fernández, L.; Albero, P.; Gadea, J.; Landeras, J. Dietary supplementation with docosahexaenoic acid (DHA) improves seminal antioxidant status and decreases sperm DNA fragmentation. Syst. Biol. Reprod. Med. 2016, 62, 387–395. [Google Scholar] [CrossRef] [Green Version]
- Balercia, G.; Regoli, F.; Armeni, T.; Koverech, A.; Mantero, F.; Boscaro, M. Placebo-controlled double-blind randomized trial on the use of l-carnitine, l-acetylcarnitine, or combined l-carnitine and l-acetylcarnitine in men with idiopathic asthenozoospermia. Fertil. Steril. 2005, 84, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, A.; Sgrò, P.; Salacone, P.; Paoli, D.; Gilio, B.; Lombardo, F.; Santulli, M.; Agarwal, A.; Gandini, L. A placebo-controlled double-blind randomized trial of the use of combined l-carnitine and l-acetyl-carnitine treatment in men with asthenozoospermia. Fertil. Steril. 2004, 81, 1578–1584. [Google Scholar] [CrossRef]
- Gudas, L.J.; Wagner, J.A. Retinoids regulate stem cell differentiation. J. Cell. Physiol. 2011, 226, 322–330. [Google Scholar] [CrossRef] [Green Version]
- Ross, S.A.; McCaffery, P.J.; Drager, U.C.; De Luca, L.M. Retinoids in Embryonal Development. Physiol. Rev. 2000, 80, 1021–1054. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.-H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an Antioxidant: Evaluation of Its Role in Disease Prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Ener, K.; Aldemir, M.; Işık, E.; Okulu, E.; Özcan, M.F.; Uğurlu, M.; Tangal, S.; Özayar, A. The impact of vitamin E supplementation on semen parameters and pregnancy rates after varicocelectomy: A randomised controlled study. Andrologia 2016, 48, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.; Hadi, A.; Moosavian, S.P.; Askari, G.; Nasirian, M. The association between serum vitamin D, fertility and semen quality: A systematic review and meta-analysis. Int. J. Surg. 2019, 71, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Amini, L.; Mohammadbeigi, R.; Vafa, M.; Haghani, H.; Vahedian-Azimi, A.; Karimi, L.; Jahanfar, S.; Jamialahmadi, T.; Talebi, A.; Sahebkar, A. Evaluation of the effect of Vitamin D3 supplementation on quantitative and qualitative parameters of spermograms and hormones in infertile men: A Randomized controlled trial. Complement. Ther. Med. 2020, 53, 102529. [Google Scholar] [CrossRef] [PubMed]
- Naderi, N.; House, J.D. Recent Developments in Folate Nutrition. In Advances in Food and Nutrition Research; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 83, pp. 195–213. [Google Scholar]
- Chan, Y.-M.; Bailey, R.; O’Connor, D.L. Folate. Adv. Nutr. 2013, 4, 123–125. [Google Scholar] [CrossRef]
- Schisterman, E.F.; Sjaarda, L.A.; Clemons, T.; Carrell, D.T.; Perkins, N.J.; Johnstone, E.; Lamb, D.; Chaney, K.; Van Voorhis, B.J.; Ryan, G.; et al. Effect of Folic Acid and Zinc Supplementation in Men on Semen Quality and Live Birth among Couples Undergoing Infertility Treatment: A Randomized Clinical Trial. J. Am. Med. Assoc. 2020, 323, 35–48. [Google Scholar] [CrossRef]
- Czeizel, A.E.; Dudás, I.; Vereczkey, A.; Bánhidy, F. Folate deficiency and folic acid supplementation: The prevention of neural-tube defects and congenital heart defects. Nutrients 2013, 5, 4760–4775. [Google Scholar] [CrossRef] [Green Version]
- Vanderhout, S.M.; Panah, M.R.; Garcia-Bailo, B.; Grace-Farfaglia, P.; Samsel, K.; Dockray, J.; Jarvi, K.; El-Sohemy, A. Nutrition, genetic variation and male fertility. Transl. Androl. Urol. 2021, 10, 1410–1431. [Google Scholar] [CrossRef]
- Wong, W.Y.; Merkus, H.M.W.; Thomas, C.M.; Menkveld, R.; Zielhuis, G.A.; Steegers-Theunissen, R.P. Effects of folic acid and zinc sulfate on male factor subfertility: A double-blind, randomized, placebo-controlled trial. Fertil. Steril. 2002, 77, 491–498. [Google Scholar] [CrossRef]
- Da Silva, T.M.; Maia, M.C.S.; Arruda, J.T.; Approbato, F.C.; Mendonça, C.R.; Approbato, M.S. Folic acid does not improve semen parametrs in subfertile men: A double-blin, randomized, placebo-controlled study. JBRA Assist. Reprod. 2013, 17, 123–125. [Google Scholar] [CrossRef]
- Raigani, M.; Yaghmaei, B.; Amirjannti, N.; Lakpour, N.; Akhondi, M.M.; Zeraati, H.; Hajihosseinal, M.; Sadeghi, M.R. The micronutrient supplements, zinc sulphate and folic acid, did not ameliorate sperm functional parameters in oligoasthenoteratozoospermic men. Andrologia 2014, 46, 956–962. [Google Scholar] [CrossRef]
- Rodríguez, A.L.; Rijsselaere, T.; Beek, J.; Vyt, P.; Van Soom, A.; Maes, D. Boar seminal plasma components and their relation with semen quality. Syst. Biol. Reprod. Med. 2013, 59, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Mirnamniha, M.; Faroughi, F.; Tahmasbpour, E.; Ebrahimi, P.; Beigi Harchegani, A. An overview on role of some trace elements in human reproductive health, sperm function and fertilization process. Rev. Environ. Health 2019, 34, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Boitani, C.; Puglisi, R. Selenium, a Key Element in Spermatogenesis and Male Fertility. In Molecular Mechanisms in Spermatogenesis; Springer: New York, NY, USA, 2009; pp. 65–73. [Google Scholar]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.; MacPherson, A.; Yates, R.W.; Hussain, B.; Dixon, J. The effect of oral selenium supplementation on human sperm motility. BJU Int. 1998, 82, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Hawkes, W.C.; Alkan, Z.; Wong, K. Selenium Supplementation Does Not Affect Testicular Selenium Status or Semen Quality in North American Men. J. Androl. 2009, 30, 525–533. [Google Scholar] [CrossRef]
- Qazi, I.H.; Angel, C.; Yang, H.; Zoidis, E.; Pan, B.; Wu, Z.; Ming, Z.; Zeng, C.J.; Meng, Q.; Han, H.; et al. Role of selenium and selenoproteins in male reproductive function: A review of past and present evidences. Antioxidants 2019, 8, 268. [Google Scholar] [CrossRef] [Green Version]
- Foresta, C.; Garolla, A.; Cosci, I.; Menegazzo, M.; Ferigo, M.; Gandin, V.; De Toni, L. Role of zinc trafficking in male fertility: From germ to sperm. Hum. Reprod. 2014, 29, 1134–1145. [Google Scholar] [CrossRef] [Green Version]
- Askari, M.; Faryabi, R.; Mozaffari, H.; Darooghegi Mofrad, M. The effects of N-Acetylcysteine on serum level of inflammatory biomarkers in adults. Findings from a systematic review and meta-analysis of randomized clinical trials. Cytokine 2020, 135, 155239. [Google Scholar] [CrossRef]
- Littarru, G.P.; Tiano, L. Bioenergetic and Antioxidant Properties of Coenzyme Q10: Recent Developments. Mol. Biotechnol. 2007, 37, 31–37. [Google Scholar] [CrossRef]
- Giahi, L.; Mohammadmoradi, S.; Javidan, A.; Sadeghi, M.R. Nutritional modifications in male infertility: A systematic review covering 2 decades. Nutr. Rev. 2016, 74, 118–130. [Google Scholar] [CrossRef] [Green Version]
- Díaz, R.; Torres, M.A.; Bravo, S.; Sanchez, R.; Sepúlveda, N. Determination of fatty acid profile in ram spermatozoa and seminal plasma. Andrologia 2016, 48, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, M.; Laus, F.; Spaterna, A.; Marchegiani, A. Use of nutraceuticals in the stallion: Effects on semen quality and preservation. Reprod. Domest. Anim. 2021, 56, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Smits, R.M.; Mackenzie-Proctor, R.; Yazdani, A.; Stankiewicz, M.T.; Jordan, V.; Showell, M.G. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2019, 2019, CD007411. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Said, T.M. Carnitines and male infertility. Reprod. Biomed. Online 2004, 8, 376–384. [Google Scholar] [CrossRef]
- Mongioi, L.; Calogero, A.E.; Vicari, E.; Condorelli, R.A.; Russo, G.I.; Privitera, S.; Morgia, G.; La Vignera, S. The role of carnitine in male infertility. Andrology 2016, 4, 800–807. [Google Scholar] [CrossRef]
- Kopets, R.; Kuibida, I.; Chernyavska, I.; Cherepanyn, V.; Mazo, R.; Fedevych, V.; Gerasymov, S. Dietary supplementation with a novel L-carnitine multi-micronutrient in idiopathic male subfertility involving oligo-, astheno-, teratozoospermia: A randomized clinical study. Andrology 2020, 8, 1184–1193. [Google Scholar] [CrossRef]
- Buhling, K.; Schumacher, A.; zu Eulenburg, C.; Laakmann, E. Influence of oral vitamin and mineral supplementation on male infertility: A meta-analysis and systematic review. Reprod. Biomed. Online 2019, 39, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Champroux, A.; Cocquet, J.; Henry-Berger, J.; Drevet, J.R.; Kocer, A. A decade of exploring the mammalian sperm epigenome: Paternal epigenetic and transgenerational inheritance. Front. Cell Dev. Biol. 2018, 6, 50. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Twinn, D.S.; Hjort, L.; Novakovic, B.; Ozanne, S.E.; Saffery, R. Intrauterine programming of obesity and type 2 diabetes. Diabetologia 2019, 62, 1789–1801. [Google Scholar] [CrossRef] [Green Version]
- Marcho, C.; Oluwayiose, O.A.; Pilsner, J.R. The preconception environment and sperm epigenetics. Andrology 2020, 8, 924–942. [Google Scholar] [CrossRef]
- Ozkocer, S.E.; Konac, E. The current perspective on genetic and epigenetic factors in sperm maturation in the epididymis. Andrologia 2021, 53, e13989. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, A.; Miyauchi, N.; Hamada, H.; Hiura, H.; Chiba, H.; Okae, H.; Sato, A.; John, R.M.; Arima, T. Epigenetic alterations in sperm associated with male infertility. Congenit. Anom. 2015, 55, 133–144. [Google Scholar] [CrossRef] [PubMed]
- McSwiggin, H.M.; O’Doherty, A.M. Epigenetic reprogramming during spermatogenesis and male factor infertility. Reproduction 2018, 156, 2454. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Yuan, S. Epigenetic regulations in mammalian spermatogenesis: RNA-m6A modification and beyond. Cell. Mol. Life Sci. 2021, 78, 4893–4905. [Google Scholar] [CrossRef] [PubMed]
- Radford, E.J. Exploring the extent and scope of epigenetic inheritance. Nat. Rev. Endocrinol. 2018, 14, 345–355. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, H.; Wu, H.; Jin, L.; Chen, B.; Pang, H.; Ming, Z.; Cheng, Y.; Zhou, C.; Guo, M.; et al. Diet-Induced Paternal Obesity Impairs Cognitive Function in Offspring by Mediating Epigenetic Modifications in Spermatozoa. Obesity 2018, 26, 1749–1757. [Google Scholar] [CrossRef]
- Li, N.; Shen, Q.; Hua, J. Epigenetic remodeling in male germline development. Stem Cells Int. 2016, 2016, 3152173. [Google Scholar] [CrossRef] [Green Version]
- Ong, T.P.; Moreno, F.S.; Ross, S.A. Targeting the epigenome with bioactive food components for cancer prevention. J. Nutrigenet. Nutrigenom. 2012, 4, 275–292. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Chu, M.; Chu, C.; Du, Y.; Shi, J.; Liu, X.; Liu, Y.; Zhang, H.; Zhang, Z.; Yan, N. Wild rice (Zizania spp.): A review of its nutritional constituents, phytochemicals, antioxidant activities, and health-promoting effects. Food Chem. 2020, 331, 127293. [Google Scholar] [CrossRef]
- Davis, C.D.; Hord, N.G. Nutritional “omics” technologies for elucidating the role(s) of bioactive food components in colon cancer prevention. J. Nutr. 2005, 135, 2694–2697. [Google Scholar] [CrossRef] [Green Version]
- Schulz, M.; Seraglio, S.K.T.; Brugnerotto, P.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Composition and potential health effects of dark-colored underutilized Brazilian—A review. Food Res. Int. 2020, 137, 109744. [Google Scholar] [CrossRef] [PubMed]
- Lambrot, R.; Xu, C.; Saint-Phar, S.; Chountalos, G.; Cohen, T.; Paquet, M.; Suderman, M.; Hallett, M.; Kimmins, S. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat. Commun. 2013, 4, 2889. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, Z.; Yang, D.; Jiang, X.; Sun, J.; Tian, L.; Hu, J.; Wu, B.; Bai, W. Cyanidin-3-O-glucoside restores spermatogenic dysfunction in cadmium-exposed pubertal mice via histone ubiquitination and mitigating oxidative damage. J. Hazard. Mater. 2020, 387, 121706. [Google Scholar] [CrossRef] [PubMed]
- Mandy, M.; Nyirenda, M. Developmental Origins of Health and Disease: The relevance to developing nations. Int. Health 2018, 10, 66–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, D. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986, 327, 1077–1081. [Google Scholar] [CrossRef]
- Bianco-Miotto, T.; Craig, J.M.; Gasser, Y.P.; Van Dijk, S.J.; Ozanne, S.E. Epigenetics and DOHaD: From basics to birth and beyond. J. Dev. Orig. Health Dis. 2017, 8, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Vaag, A.A.; Grunnet, L.G.; Arora, G.P.; Brøns, C. The thrifty phenotype hypothesis revisited. Diabetologia 1992, 55, 595–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGowan, P.O.; Matthews, S.G. Prenatal Stress, Glucocorticoids, and Developmental Programming of the Stress Response. Endocrinology 2018, 159, 69–82. [Google Scholar] [CrossRef]
- Ornellas, F.; Carapeto, P.V.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Obese fathers lead to an altered metabolism and obesity in their children in adulthood: Review of experimental and human studies. J. Pediatr. 2017, 93, 551–559. [Google Scholar] [CrossRef]
- Silva, L.B.A.R.; Pinheiro-Castro, N.; Novaes, G.M.; de Freitas Laiber Pascoal, G.; Ong, T.P. Bioactive food compounds, epigenetics and chronic disease prevention: Focus on early-life interventions with polyphenols. Food Res. Int. 2019, 125, 108646. [Google Scholar] [CrossRef]
- Yeshurun, S.; Short, A.K.; Bredy, T.W.; Pang, T.Y.; Hannan, A.J. Paternal environmental enrichment transgenerationally alters affective behavioral and neuroendocrine phenotypes. Psychoneuroendocrinology 2017, 77, 225–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontelles, C.C.; Guido, L.N.; Rosim, M.P.; Andrade, F.D.O.; Jin, L.; Inchauspe, J.; Pires, V.C.; de Castro, I.A.; Hilakivi-Clarke, L.; de Assis, S.; et al. Paternal programming of breast cancer risk in daughters in a rat model: Opposing effects of animal- and plant-based high-fat diets. Breast Cancer Res. 2016, 18, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soubry, A.; Murphy, S.K.; Wang, F.; Huang, Z.; Vidal, A.C.; Fuemmeler, B.F.; Kurtzberg, J.; Murtha, A.; Jirtle, R.L.; Schildkraut, J.M.; et al. Newborns of obese parents have altered DNA methylation patterns at imprinted genes. Int. J. Obes. 2015, 39, 650–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soubry, A.; Schildkraut, J.M.; Murtha, A.; Wang, F.; Huang, Z.; Bernal, A.; Kurtzberg, J.; Jirtle, R.L.; Murphy, S.K.; Hoyo, C. Paternal obesity is associated with IGF2 hypomethylation in newborns: Results from a Newborn Epigenetics Study (NEST) cohort. BMC Med. 2013, 11, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkins, A.J.; Dias, I.; Tsuro, H.; Allen, D.; Emes, R.D.; Moreton, J.; Wilson, R.; Ingram, R.J.M.; Sinclair, K.D. Paternal diet programs offspring health through sperm- and seminal plasma-specific pathways in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 10064–10069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soubry, A. POHaD: Why we should study future fathers. Environ. Epigenet. 2018, 4, dvy007. [Google Scholar] [CrossRef]
- Ong, T.P.; Ozanne, S.E. Developmental programming of type 2 diabetes: Early nutrition and epigenetic mechanisms. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 354–360. [Google Scholar] [CrossRef]
- Segars, J. Assisted Reproductive Technologies and the Developmental Origins of Health and Disease. Semin. Reprod. Med. 2018, 36, 173–174. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Rassoulzadegan, M.; Tuorto, F.; Chen, Q. Sperm RNA code programmes the metabolic health of offspring. Nat. Rev. Endocrinol. 2019, 15, 489–498. [Google Scholar] [CrossRef] [Green Version]
- Hur, S.S.J.; Cropley, J.E.; Suter, C.M. Paternal epigenetic programming: Evolving metabolic disease risk. J. Mol. Endocrinol. 2017, 58, R159–R168. [Google Scholar] [CrossRef]
- McPherson, N.O.; Fullston, T.; Aitken, R.J.; Lane, M. Paternal obesity, interventions, and mechanistic pathways to impaired health in offspring. Ann. Nutr. Metab. 2014, 64, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Bodden, C.; Hannan, A.J.; Reichelt, A.C. Diet-Induced Modification of the Sperm Epigenome Programs Metabolism and Behavior. Trends Endocrinol. Metab. 2020, 31, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.L.; Paganopoulou, P.; Akhtar, S.; Urquhart, N.; Philomin, R.; Dickinson, Y.; Watkins, A.J. Paternal diet impairs F1 and F2 offspring vascular function through sperm and seminal plasma specific mechanisms in mice. J. Physiol. 2020, 598, 699–715. [Google Scholar] [CrossRef] [PubMed]
- Watkins, A.J.; Sirovica, S.; Stokes, B.; Isaacs, M.; Addison, O.; Martin, R.A. Paternal low protein diet programs preimplantation embryo gene expression, fetal growth and skeletal development in mice. Biochim. Biophys. Acta—Mol. Basis Dis. 2017, 1863, 1371–1381. [Google Scholar] [CrossRef]
- Vanhees, K.; Vonhögen, I.G.C.; Van Schooten, F.J.; Godschalk, R.W.L. You are what you eat, and so are your children: The impact of micronutrients on the epigenetic programming of offspring. Cell. Mol. Life Sci. 2014, 71, 271–285. [Google Scholar] [CrossRef]
- Da Cruz, R.S.; Carney, E.J.; Clarke, J.; Cao, H.; Cruz, M.I.; Benitez, C.; Jin, L.; Fu, Y.; Cheng, Z.; Wang, Y.; et al. Paternal malnutrition programs breast cancer risk and tumor metabolism in offspring. Breast Cancer Res. 2018, 20, 99. [Google Scholar] [CrossRef] [Green Version]
- Bernhardt, L.; Dittrich, M.; El-Merahbi, R.; Saliba, A.E.; Müller, T.; Sumara, G.; Vogel, J.; Nichols-Burns, S.; Mitchell, M.; Haaf, T.; et al. A genome-wide transcriptomic analysis of embryos fathered by obese males in a murine model of diet-induced obesity. Sci. Rep. 2021, 11, 3–9. [Google Scholar] [CrossRef]
- Stanescu, D.E.; Hughes, N.; Patel, P.; De León, D.D. A novel mutation in GATA6 causes pancreatic agenesis. Pediatr. Diabetes 2015, 16, 67–70. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.-Y.; Cheng, Y.; Jin, L.-Y.; Zhou, Y.; Pang, H.-Y.; Zhu, H.; Yan, C.-C.; Yan, Y.-S.; Yu, J.-E.; Sheng, J.-Z.; et al. Paternal obesity impairs hepatic gluconeogenesis of offspring by altering Igf2/H19 DNA methylation. Mol. Cell. Endocrinol. 2021, 529, 111264. [Google Scholar] [CrossRef]
- Fontelles, C.C.; Carney, E.; Clarke, J.; Nguyen, N.M.; Yin, C.; Jin, L.; Cruz, M.I.; Ong, T.P.; Hilakivi-Clarke, L.; de Assis, S. Paternal overweight is associated with increased breast cancer risk in daughters in a mouse model. Sci. Rep. 2016, 6, 28602. [Google Scholar] [CrossRef] [Green Version]
- Romanus, S.; Neven, P.; Soubry, A. Extending the Developmental Origins of Health and Disease theory: Does paternal diet contribute to breast cancer risk in daughters? Breast Cancer Res. 2016, 18, 18–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steluti, J.; Palchetti, C.Z.; Miranda, A.M.H.; Fisberg, R.M.; Marchioni, D.M. DNA methylation and one-carbon metabolism related nutrients and polymorphisms: Analysis after mandatory flour fortification with folic acid. Br. J. Nutr. 2020, 123, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Clare, C.E.; Brassington, A.H.; Kwong, W.Y.; Sinclair, K.D. One-Carbon Metabolism: Linking Nutritional Biochemistry to Epigenetic Programming of Long-Term Development. Annu. Rev. Anim. Biosci. 2019, 7, 263–287. [Google Scholar] [CrossRef] [PubMed]
- Chleilat, F.; Schick, A.; Deleemans, J.M.; Reimer, R.A. Paternal methyl donor supplementation in rats improves fertility, physiological outcomes, gut microbial signatures and epigenetic markers altered by high fat/high sucrose diet. Int. J. Mol. Sci. 2021, 22, 689. [Google Scholar] [CrossRef]
- Morgan, H.L.; Aljumah, A.; Rouillon, C.; Watkins, A.J. Paternal low protein diet and the supplementation of methyl-donors impact fetal growth and placental development in mice. Placenta 2021, 103, 124–133. [Google Scholar] [CrossRef]
- McPherson, N.O.; Fullston, T.; Kang, W.X.; Sandeman, L.Y.; Corbett, M.A.; Owens, J.A.; Lane, M. Paternal under-nutrition programs metabolic syndrome in offspring which can be reversed by antioxidant/vitamin food fortification in fathers. Sci. Rep. 2016, 6, 27010. [Google Scholar] [CrossRef] [Green Version]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef]
- Guido, L.N.; Fontelles, C.C.; Rosim, M.P.; Pires, V.C.; Cozzolino, S.M.F.; Castro, I.A.; Bolaños-Jiménez, F.; Barbisan, L.F.; Ong, T.P. Paternal selenium deficiency but not supplementation during preconception alters mammary gland development and 7,12-dimethylbenz[a]anthracene-induced mammary carcinogenesis in female rat offspring. Int. J. Cancer 2016, 139, 1873–1882. [Google Scholar] [CrossRef]
- Pascoal, G.F.L.; Novaes, G.M.; Sobrinho, M.D.P.; Hirayama, B.; Castro, I.A.; Ong, T.P. Selenium Supplementation during Puberty and Young Adulthood Mitigates Obesity-Induced Metabolic, Cellular and Epigenetic Alterations in Male Rat Physiology. Antioxidants 2022, 11, 895. [Google Scholar] [CrossRef]
| Nutritional Factor | Major Outcomes | References |
|---|---|---|
| Vitamin A |
| [36] |
| Vitamin C |
| [37,38] |
| Vitamin E |
| [36,39,40] |
| Vitamin D |
| [41,42,43] |
| Vitamin B9 |
| [44,45] |
| Selenium |
| [46,47] |
| Zinc |
| [48,49] |
| N-acetylcysteine |
| [47,50] |
| Coenzyme Q10 |
| [51,52,53,54] |
| Omega-3 polyunsaturated fatty acid |
| [55,56,57,58,59] |
| Carnitines |
| [60,61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascoal, G.d.F.L.; Geraldi, M.V.; Maróstica, M.R., Jr.; Ong, T.P. Effect of Paternal Diet on Spermatogenesis and Offspring Health: Focus on Epigenetics and Interventions with Food Bioactive Compounds. Nutrients 2022, 14, 2150. https://doi.org/10.3390/nu14102150
Pascoal GdFL, Geraldi MV, Maróstica MR Jr., Ong TP. Effect of Paternal Diet on Spermatogenesis and Offspring Health: Focus on Epigenetics and Interventions with Food Bioactive Compounds. Nutrients. 2022; 14(10):2150. https://doi.org/10.3390/nu14102150
Chicago/Turabian StylePascoal, Gabriela de Freitas Laiber, Marina Vilar Geraldi, Mário Roberto Maróstica, Jr., and Thomas Prates Ong. 2022. "Effect of Paternal Diet on Spermatogenesis and Offspring Health: Focus on Epigenetics and Interventions with Food Bioactive Compounds" Nutrients 14, no. 10: 2150. https://doi.org/10.3390/nu14102150
APA StylePascoal, G. d. F. L., Geraldi, M. V., Maróstica, M. R., Jr., & Ong, T. P. (2022). Effect of Paternal Diet on Spermatogenesis and Offspring Health: Focus on Epigenetics and Interventions with Food Bioactive Compounds. Nutrients, 14(10), 2150. https://doi.org/10.3390/nu14102150








