Anti-Inflammatory Effects Induced by a Polyphenolic Granular Complex from Olive (Olea europaea, Mainly Cultivar coratina): Results from In Vivo and Ex Vivo Studies in a Model of Inflammation and MIA-Induced Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ex Vivo Studies
2.2. In Vivo Studies
2.3. Formalin Test
2.4. Monoiodoacetate (MIA)-Induced Osteoarthritis
2.5. Paw Pressure Test
2.6. Incapacitance Test
2.7. Statistical Analysis
3. Results
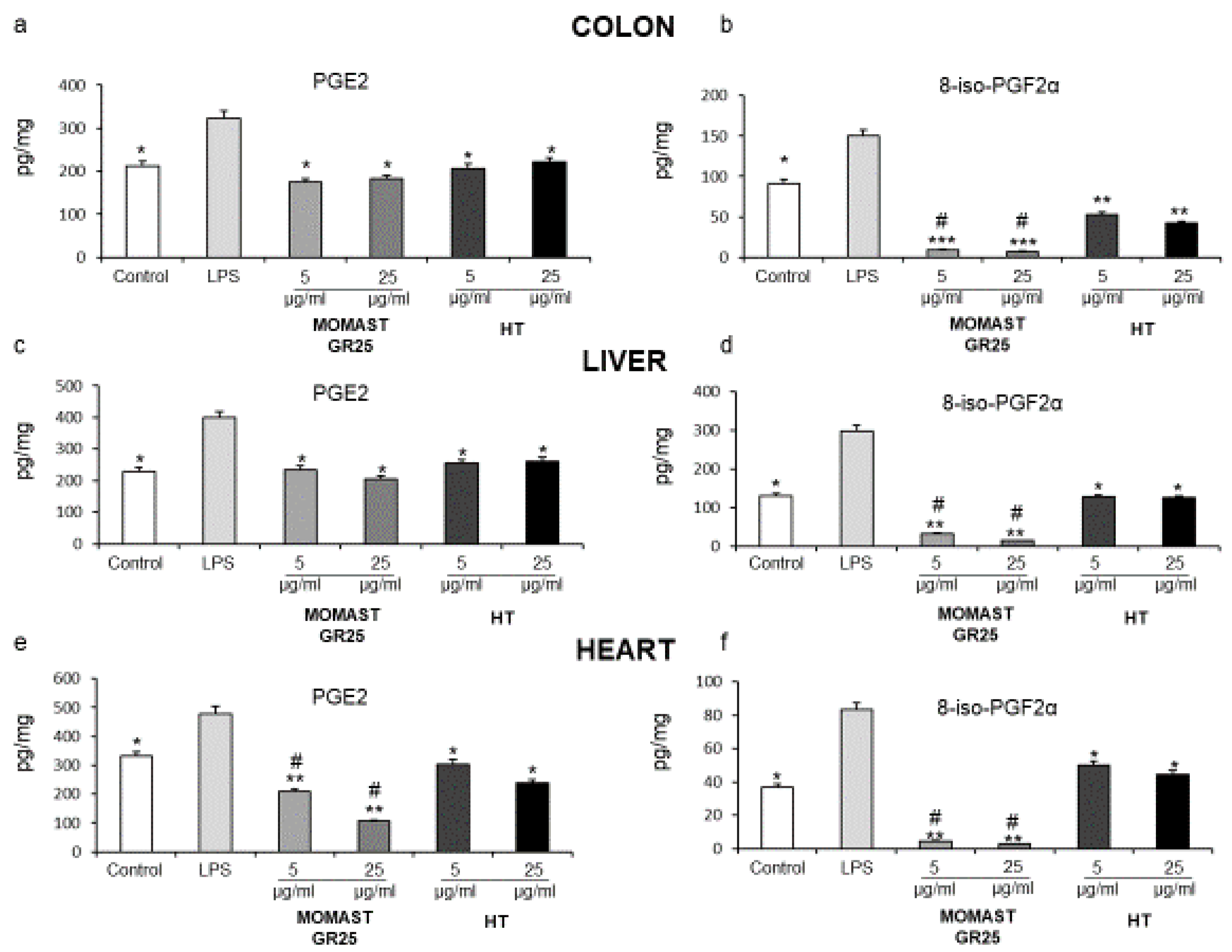
3.1. Inhibitory Effects of MOMAST® GR25 (5 and 25 µg/mL) on LPS-Induced PGE2 and 8-iso-PGF2α Levels in Colon, Liver and Heart Specimens
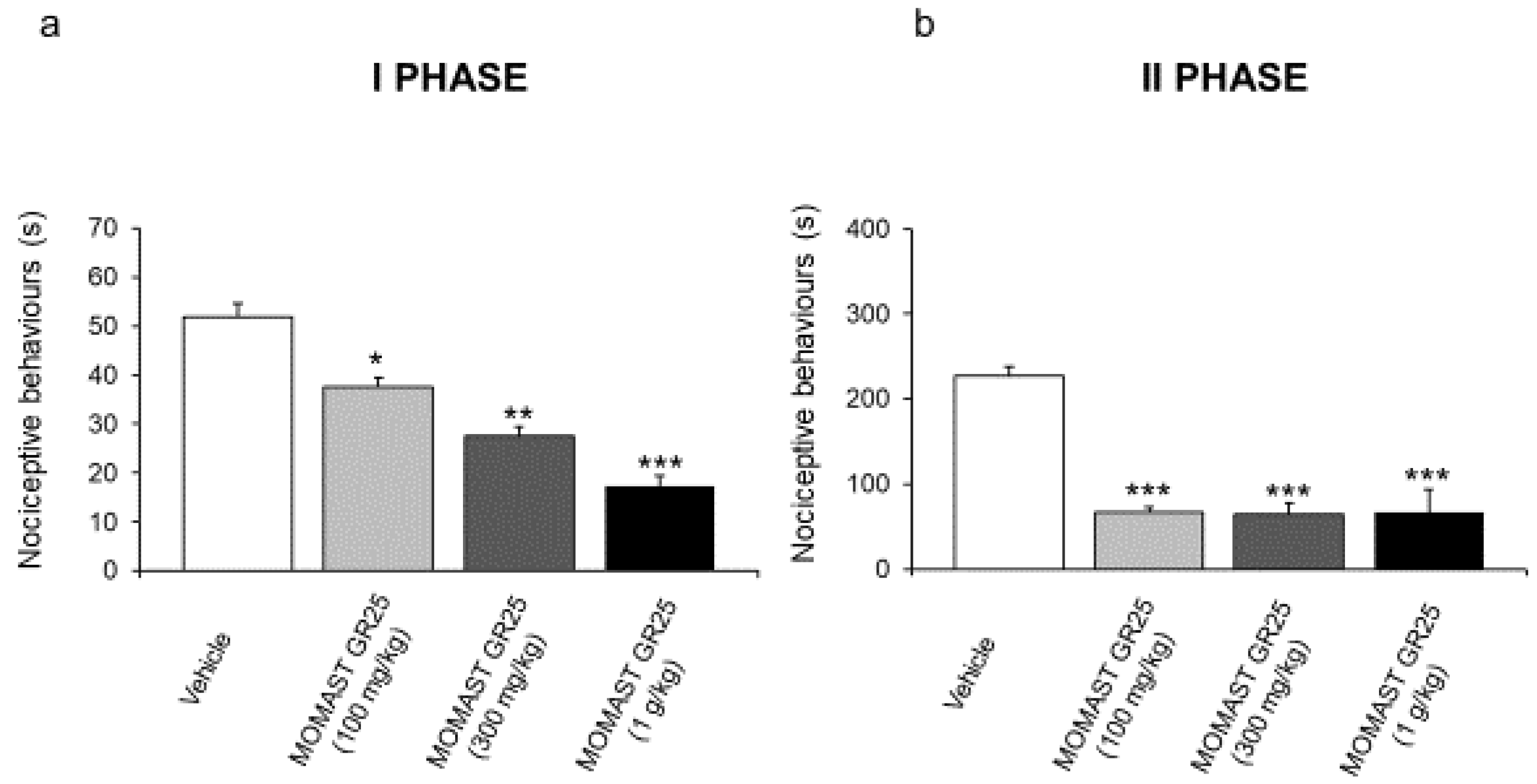
3.2. MOMAST® GR25 (100, 300 mg/kg and 1 g/kg) Reduced Responsiveness to Acute Inflammatory Stimuli
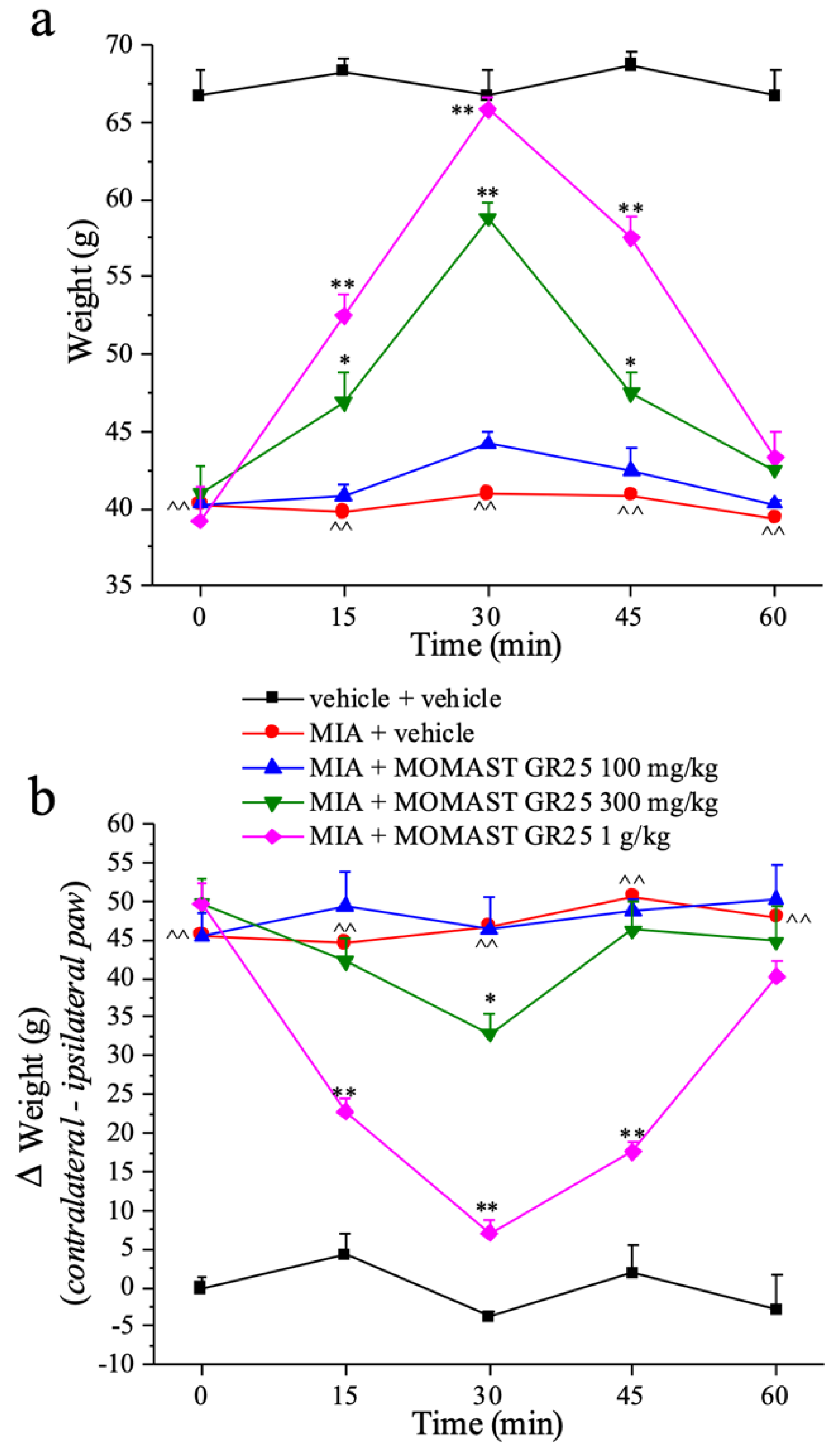
3.3. Effects of Acute Administration of MOMAST® GR25 (100, 300 mg/kg and 1 g/kg) in Experimental Osteoarthritis
3.4. Effect of Repeated Administration of MOMAST® GR25 (100, 300 mg/kg and 1 g/kg) in an Animal Model of Osteoarthritis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chebbi Mahjoub, R.; Khemiss, M.; Dhidah, M.; Dellai, A.; Bouraoui, A.; Khemiss, F. Chloroformic and methanolic extracts of Olea europaea L. leaves present anti-Inflammatory and analgesic activities. ISRN Pharmacol. 2011, 2011, 564972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omar, S.H. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masella, R.; Di Benedetto, R.; Varì, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005, 16, 577–586. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Statti, G.A.; Menichini, F. Biological and pharmacological activities of iridoids: Recent developments. Mini Rev. Med. Chem. 2008, 8, 399–420. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [Green Version]
- Angerosa, F.; d’Alessandro, N.; Corana, F.; Mellerio, G. Characterization of phenolic and secoiridoid aglycons present in virgin olive oil by gas chromatography-chemical ionization mass spectrometry. J. Chromatogr. 1996, 736, 195–203. [Google Scholar] [CrossRef]
- Cinquanta, L.; Esti, M.; La Notte, E. Evolution of phenolic compounds in virgin olive oil during storage. J. Am. Oil Chem. Soc. 1997, 74, 1259–1264. [Google Scholar] [CrossRef]
- El, S.N.; Karakaya, S. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr. Rev. 2009, 67, 632–638. [Google Scholar] [CrossRef]
- Martínez, L.; Ros, G.; Nieto, G. Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat. Medicines 2018, 23, 13. [Google Scholar] [CrossRef] [Green Version]
- Fuccelli, R.; Fabiani, R.; Rosignoli, P. Hydroxytyrosol Exerts Anti-Inflammatory and Anti-Oxidant Activities in a Mouse Model of Systemic Inflammation. Molecules 2018, 23, 3212. [Google Scholar] [CrossRef] [Green Version]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological activities of the natural antioxidant oleuropein: Exceeding the expectation—A mini-review. J. Funct. Foods. 2015, 18, 926–994. [Google Scholar] [CrossRef]
- Filho, A.G.; Morel, A.F.; Adolpho, L.; Ilha, V.; Giralt, E.; Tarragó, T.; Dalcol, I.I. Inhibitory effect of verbascoside isolated from Buddleja brasiliensis Jacq. ex Spreng on prolyl oligopeptidase activity. Phytother. Res. 2012, 26, 1472–1475. [Google Scholar] [CrossRef] [PubMed]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Chunhua, M.; Shumin, W. Effects of acteoside on lipopolysaccharide-induced inflammation in acute lung injury via regulation of NF-kappaB pathway in vivo and in vitro. Toxicol. Appl. Pharmacol. 2015, 285, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Pesce, M.; Franceschelli, S.; Ferrone, A.; De Lutiis, M.A.; Patruno, A.; Grilli, A.; Felaco, M.; Speranza, L. Verbascoside down-regulates some pro-inflammatory signal transduction pathways by increasing the activity of tyrosine phosphatase SHP-1 in the U937 cell line. J. Cell. Mol. Med. 2015, 19, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, A.; Pati, S.; Minervini, F.; Linsalata, V.; Lattanzio, V. Verbascoside, isoverbascoside, and their derivates recovered from olive mill wastewater as possible food antioxidants. J. Agric. Food Chem. 2012, 60, 1822–1829. [Google Scholar] [CrossRef]
- Plotnikov, M.B.; Plotnikova, T.M. Tyrosol as a Neuroprotector: Strong Effects of a “Weak” Antioxidant. Curr. Neuropharmacol. 2021, 19, 434–448. [Google Scholar] [CrossRef]
- Chandramohan, R.; Pari, L. Anti-inflammatory effects of tyrosol in streptozotocin-induced diabetic Wistar rats. J. Funct. Food 2016, 27, 17–28. [Google Scholar] [CrossRef]
- International Olive Council. Determination of Biophenols in Olive by HPLC; COI/T.20/Doc. No. 29; International Olive Council: Madrid, Spain, 2009. [Google Scholar]
- Recinella, L.; Chiavaroli, A.; Orlando, G.; Menghini, L.; Ferrante, C.; Di Cesare Mannelli, L.; Ghelardini, C.; Brunetti, L.; Leone, S. Protective Effects Induced by Two Polyphenolic Liquid Complexes from Olive (Olea europaea, mainly Cultivar coratina) Pressing Juice in Rat Isolated Tissues Challenged with LPS. Molecules 2019, 24, 3002. [Google Scholar] [CrossRef] [Green Version]
- Recinella, L.; Chiavaroli, A.; Di Valerio, V.; Orlando, G.; Ferrante, C.; Gesmundo, R.; Granata, R.; Cai, W.; Sha, A.V.; Schally, R.; et al. Protective effects of growth hormone-releasing hormone analogs in DSS-induced colitis in mice. Sci. Rep. 2021, 11, 2530. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Micheli, L.; Zanardelli, M.; Ghelardini, C. Low dose native type II collagen prevents pain in a rat osteoarthritis model. BMC Musculoskelet. Disord. 2013, 14, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maresca, M.; Micheli, L.; Cinci, L.; Bilia, A.R.; Ghelardini, C.; Di Cesare Mannelli, L. Pain relieving and protective effects of Astragalus hydroalcoholic extract in rat arthritis models. J. Pharm. Pharmacol. 2017, 69, 1858–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leighton, G.E.; Rodriguez, R.E.; Hill, R.G.; Hughes, J. κ-Opioid agonist produce antinociception after i.v. and i.c.v. but not intrathecal administration in the rat. Br. J. Pharmacol. 1988, 93, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Bird, M.F.; Cerlesi, M.C.; Brown, M.; Malfacini, D.; Vezzi, V.; Molinari, P.; Micheli, L.; Di Cesare Mannelli, L.; Ghelardini, C.; Guerrini, R.; et al. Characterisation of the novel mixed mu-NOP peptide ligand dermorphin-N/OFQ (DeNo). PLoS ONE 2016, 11, e0156897. [Google Scholar] [CrossRef] [Green Version]
- Bove, S.E.; Calcaterra, S.L.; Brooker, R.M.; Huber, C.M.; Guzman, R.E.; Juneau, P.L.; Schrier, D.J.; Kilgore, K.S. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthr. Cartil. 2003, 11, 821–830. [Google Scholar] [CrossRef] [Green Version]
- Maresca, M.; Micheli, L.; Di Cesare Mannelli, L.; Tenci, B.; Innocenti, M.; Khatib, M.; Mulinacci, N.; Ghelardini, C. Acute effect of Capparis spinosa root extracts on rat articular pain. J. Ethnopharmacol. 2016, 193, 456–465. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef] [Green Version]
- Ivanavicius, S.P.; Ball, A.D.; Heapy, C.G.; Westwood, F.R.; Murray, F.; Read, S.J. Structural pathology in a rodent model of osteoarthritis is associated with neuropathic pain: Increased expression of ATF-3 and pharmacological characterisation. Pain 2007, 128, 272–282. [Google Scholar] [CrossRef]
- Visioli, F.; Bellomo, G.; Galli, C. Free radical-scavenging properties of olive oil polyphenols. Biochem. Biophys. Res. Commun. 1998, 247, 60–64. [Google Scholar] [CrossRef]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Carrasco-Pancorbo, A.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Del Carlo, M.; Gallina-Toschi, T.; Lercker, G.; Compagnone, D.; Fernández-Gutiérrez, A.J. Evaluation of the antioxidant capacity of individual phenolic compounds in virgin olive oil. Agric. Food Chem. 2005, 53, 8918–8925. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B.; Whiteman, M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sgarbossa, A.; Dal Bosco, M.; Pressi, G.; Cuzzocrea, S.; Dal Toso, R.; Menegazzi, M. Phenylethanoid glycosides from plant cell cultures induce heme oxygenase 1 gene expression in a human keratinocyte cell line by affecting the balance of NRF2 and BACH1 transcription factors. Chem. Biol. Interact. 2012, 199, 87–95. [Google Scholar] [CrossRef]
- Fridovich, I. Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen? Ann. N. Y. Acad. Sci. 1999, 893, 13–18. [Google Scholar] [CrossRef]
- Fang, Y.Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes. Res. Clin. Pract. 2013, 7, e330–e341. [Google Scholar] [CrossRef]
- Gong, D.; Geng, C.; Jiang, L.; Cao, J.; Yoshimura, H.; Zhong, L. Effects of hydroxytyrosol-20 on carrageenan-induced acute inflammation and hyperalgesia in rats. Phytother. Res. 2009, 23, 646–650. [Google Scholar] [CrossRef]
- Küpeli, E.; Sahin, F.P.; Yesilada, E.; Calis, I.; Eze, N. In vivo anti-inflammatory and antinociceptive activity of phenolic compounds from Sideritis stricta. Z. Naturforsch. C. J. Biosci. 2007, 62, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.L.; Woo, E.-R.; Kang, K.W. Inhibition of lipopolysaccharide-inducible nitric oxide synthase expression by acteoside through blocking of AP-1 activation. J. Ethnopharmacol. 2005, 97, 561–566. [Google Scholar] [CrossRef]
- Guingamp, C.; Gegout-Pottie, P.; Philippe, L.; Terlain, B.; Netter, P.; Gillet, P. Mono-iodoacetate-induced experimental osteoarthritis: A dose-response study of loss of mobility, morphology, and biochemistry. Arthritis Rheum. 1997, 40, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Guzman, R.E.; Evans, M.G.; Bove, S.; Morenko, B.; Kilgore, K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: An animal model of osteoarthritis. Toxicol. Pathol. 2003, 31, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Sabri, M.I.; Ochs, S. Inhibition of glyceraldehyde-3-phosphate dehydrogenase in mammalian nerve by iodoacetic acid. J. Neurochem. 1971, 18, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- van der Kraan, P.M.; Vitters, E.L.; van de Putte, L.B.; van den Berg, W.B. Development of osteoarthritic lesions in mice by ‘metabolic’ and ‘mechanical’ alterations in the knee joints. Am. J. Pathol. 1989, 135, 1001–1014. [Google Scholar]
- Janusz, M.J.; Hookfin, E.B.; Heitmeyer, S.A.; Woessner, J.F.; Freemont, A.J.; Hoyland, J.A.; Brown, K.K.; Hsieh, L.C.; Almstead, N.C.; De, B.; et al. Moderation of iodoacetate-induced experimental osteoarthritis in rats by matrix metalloproteinase inhibitors. Osteoarthr. Cartil. 2001, 9, 751–760. [Google Scholar] [CrossRef]
- Schiene, K.; De Vry, J.; Tzschentke, T.M. Antinociceptive and antihyperalgesic effects of tapentadol in animal models of inflammatory pain. J. Pharmacol. Exp. Ther. 2011, 339, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Di Cesare Mannelli, L.; Bani, D.; Bencini, A.; Brandi, M.L.; Calosi, L.; Cantore, M.; Carossino, A.M.; Ghelardini, C.; Valtancoli, B.; Failli, P. Therapeutic Effects of the Superoxide Dismutase Mimetic Compound MnIIMe2DO2A on Experimental Articular Pain in Rats. Mediat. Inflamm. 2013, 2013, 905360. [Google Scholar] [CrossRef] [Green Version]
- Horcajada, M.N.; Sanchez, C.; Membrez Scalfo, F.; Drion, P.; Comblain, F.; Taralla, S.; Donneau, A.F.; Offord, E.A.; Henrotin, Y. Oleuropein or rutin consumption decreases the spontaneous development of osteoarthritis in the Hartley guinea pig. Osteoarthr. Cartil. 2015, 23, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Di Cesare Mannelli, L.; Corti, F.; Micheli, L.; Zanardelli, M.; Ghelardini, C. Delay of morphine tolerance by palmitoylethanolamide. BioMed Res. Int. 2015, 2015, 894732. [Google Scholar] [CrossRef] [Green Version]
- Di Cesare Mannelli, L.; Micheli, L.; Lucarini, E.; Ghelardini, C. Ultramicronized N-Palmitoylethanolamine Supplementation for Long-Lasting, Low-Dosed Morphine Antinociception. Front. Pharmacol. 2018, 9, 473. [Google Scholar] [CrossRef] [Green Version]
- Carcolé, M.; Kummer, S.; Gonçalves, L.; Zamanillo, D.; Merlos, M.; Dickenson, A.H.; Fernández-Pastor, B.; Cabañero, D.; Maldonado, R. Sigma-1 receptor modulates neuroinflammation associated with mechanical hypersensitivity and opioid tolerance in a mouse model of osteoarthritis pain. Br. J. Pharmacol. 2019, 176, 3939–3955. [Google Scholar] [CrossRef] [PubMed]
- McQuay, H. Opioids in pain management. Lancet 1999, 353, 2229–2232. [Google Scholar] [CrossRef]
- Kallenborn-Gerhardt, W.; Schröder, K.; Geisslinger, G.; Schmidtko, A. NOXious signaling in pain processing. Pharmacol. Ther. 2013, 137, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Q.; Porreca, F.; Cuzzocrea, S.; Galen, K.; Lightfoot, R.; Masini, E.; Muscoli, C.; Mollace, V.; Ndengele, M.; Ischiropoulos, H.; et al. A newly identified role for superoxide in inflammatory pain. J. Pharmacol. Exp. Ther. 2004, 309, 869–878. [Google Scholar] [CrossRef]
- Cetrullo, S.; D’Adamo, S.; Guidotti, S.; Borzì, R.M.; Flamigni, F. Hydroxytyrosol prevents chondrocyte death under oxidative stress by induc-ing autophagy through sirtuin 1-dependent and -independentmechanisms. Biochim. Biophys. Acta 2016, 1860, 1181–1191. [Google Scholar] [CrossRef]
- Granados-Principal, S.; El-Azem, N.; Pamplona, R.; Ramirez-Tortosa, C.; Pulido-Moran, M.; Vera-Ramirez, L.; Quiles, J.L.; Sanchez-Rovira, P.; Naudí, A.; Portero-Otin, M.; et al. Hydroxytyrosol ameliorates oxidative stress and mitochondrial dysfunction in doxorubicin-induced cardiotoxicity in rats with breast cancer. Biochem. Pharmacol. 2014, 90, 25–33. [Google Scholar] [CrossRef]
- Li, J.; Yang, G.; Wang, S.; Jiang, L.; Liu, X.; Geng, C.; Zhong, L.; Chen, M. The protective effects of hydroxytyrosol against ortho-phenylphenol-induced DNA damage in HepG2 cells. Toxicol. Mech. Methods 2012, 22, 432–437. [Google Scholar] [CrossRef]
- Campo, G.; Marchesini, J.; Bristot, L.; Monti, M.; Gambetti, S.; Pavasini, R.; Pollina, A.; Ferrari, R. The in vitro effects of verbascoside on human platelet aggregation. J. Thromb. Thrombolysis 2012, 34, 318–325. [Google Scholar] [CrossRef]
- Paola, R.D.; Oteri, G.; Mazzon, E.; Crisafulli, C.; Galuppo, M.; Toso, R.D.; Pressi, G.; Cordasco, G.; Cuzzocrea, S. Effects of verbascoside, biotechnologically purified by Syringa vulgaris plant cell cultures, in a rodent model of periodontitis. J. Pharm. Pharmacol. 2011, 63, 707–717. [Google Scholar] [CrossRef]
- Kahraman, C.; Tatli, I.I.; Orhan, I.E.; Akdemir, Z.S. Cholinesterase inhibitory and antioxidant properties of Verbascum mucronatum Lam. and its secondary metabolites. Z. Naturforsch. C. J. Biosci. 2010, 65, 667–674. [Google Scholar] [CrossRef]
- Isacchi, B.; Iacopi, R.; Bergonzi, M.C.; Ghelardini, C.; Galeotti, N.; Norcini, M.; Vivoli, E.; Vincieri, F.F.; Bilia, A.R. Antihyperalgesic activity of verbascoside in two models of neuropathic pain. J. Pharm. Pharmacol. 2011, 63, 594–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szychlinska, M.A.; Castrogiovanni, P.; Trovato, F.M.; Nsir, H.; Zarrouk, M.; Lo Furno, D.; Di Rosa, M.; Imbesi, R.; Musumeci, G. Physical activity and Mediterranean diet based on olive tree phenolic compounds from two different geographical areas have protective effects on early osteoarthritis, muscle atrophy and hepatic steatosis. Eur. J. Nutr. 2019, 58, 565–581. [Google Scholar] [CrossRef] [PubMed]
- FDA. 2005. Available online: https://www.fda.gov/media/72309/download (accessed on 20 January 2022).
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.K.; Chung, H.J.; Pyee, Y.; Choi, T.J.; Park, H.J.; Hong, J.Y.; Shin, J.; Lee, J.H.; Ha, I.H.; Lee, S.K. Effects of intra-articular SHINBARO treatment on monosodium iodoacetate-induced osteoarthritis in rats. Chin. Med. 2016, 11, 17. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.Y.; Chang, Y.; Sun, X.J.; Dai, X.; Wei, W. The role of prostaglandin E2 receptor signaling of dendritic cells in rheumatoid arthritis. Int. Immunopharmacol. 2014, 23, 163–169. [Google Scholar] [CrossRef]

| Name: | MOMAST® GR25 |
|---|---|
| Description: | Polyphenolic active complex of hydroxytyrosol-(Olea europaea fruit extract) with total polyphenolic content of 25 g/kg |
| Source Type: | Mainly Cultivar coratina |
| Physical State: | Granular |
| Appearance: | Powder-light beige |
| Moisture: | N. A. |
| Ash: | Less than 10% (600 °C) |
| Total heavy metals (as Pb): | Less than 10 ppm |
| Total Plate Count: | Less than 100 cfu/g |
| Pesticides: | Absence |
| POLYPHENOLIC CONTENT | |
| Hydroxytyrosol (HPLC) | 1.0–2.5% |
| Verbascoside (HPLC) | 0.02–0.5% |
| Tyrosol (HPLC) | 0.1–1.0% |
| Oleuropein (HPLC) | <0.5% |
| Total Polyphenols (HPLC) | >2.5% |
| Paw Pressure Test Contralateral Paw Weight (g) | |||||
|---|---|---|---|---|---|
| Treatments | 0 min | 15 min | 30 min | 45 min | 60 min |
| vehicle + vehicle | 70.3 ± 1.7 | 65.8 ± 1.5 | 66.7 ± 1.7 | 67.3 ± 1.6 | 68.3 ± 1.7 |
| MIA + vehicle | 68.3 ± 0.8 | 67.0 ± 0.9 | 69.2 ± 0.8 | 70.1 ± 0.5 | 64.0 ± 1.3 |
| MIA + MOMAST® GR25 100 mg/kg | 66.7 ± 1.7 | 68.3 ± 1.7 | 68.3 ± 1.7 | 65.3 ± 0.3 | 65.2 ± 0.2 |
| MIA + MOMAST® GR25 300 mg/kg | 66.9 ± 1.0 | 67.5 ± 1.3 | 66.9 ± 1.0 | 66.3 ± 1.0 | 65.4 ± 0.3 |
| MIA + MOMAST® GR25 1 g/kg | 65.8 ± 0.8 | 66.7 ± 1.7 | 66.7 ± 1.7 | 66.7 ± 1.7 | 66.7 ± 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Recinella, L.; Micheli, L.; Chiavaroli, A.; Libero, M.L.; Orlando, G.; Menghini, L.; Acquaviva, A.; Di Simone, S.; Ferrante, C.; Ghelardini, C.; et al. Anti-Inflammatory Effects Induced by a Polyphenolic Granular Complex from Olive (Olea europaea, Mainly Cultivar coratina): Results from In Vivo and Ex Vivo Studies in a Model of Inflammation and MIA-Induced Osteoarthritis. Nutrients 2022, 14, 1487. https://doi.org/10.3390/nu14071487
Recinella L, Micheli L, Chiavaroli A, Libero ML, Orlando G, Menghini L, Acquaviva A, Di Simone S, Ferrante C, Ghelardini C, et al. Anti-Inflammatory Effects Induced by a Polyphenolic Granular Complex from Olive (Olea europaea, Mainly Cultivar coratina): Results from In Vivo and Ex Vivo Studies in a Model of Inflammation and MIA-Induced Osteoarthritis. Nutrients. 2022; 14(7):1487. https://doi.org/10.3390/nu14071487
Chicago/Turabian StyleRecinella, Lucia, Laura Micheli, Annalisa Chiavaroli, Maria Loreta Libero, Giustino Orlando, Luigi Menghini, Alessandra Acquaviva, Simonetta Di Simone, Claudio Ferrante, Carla Ghelardini, and et al. 2022. "Anti-Inflammatory Effects Induced by a Polyphenolic Granular Complex from Olive (Olea europaea, Mainly Cultivar coratina): Results from In Vivo and Ex Vivo Studies in a Model of Inflammation and MIA-Induced Osteoarthritis" Nutrients 14, no. 7: 1487. https://doi.org/10.3390/nu14071487
APA StyleRecinella, L., Micheli, L., Chiavaroli, A., Libero, M. L., Orlando, G., Menghini, L., Acquaviva, A., Di Simone, S., Ferrante, C., Ghelardini, C., Brunetti, L., & Leone, S. (2022). Anti-Inflammatory Effects Induced by a Polyphenolic Granular Complex from Olive (Olea europaea, Mainly Cultivar coratina): Results from In Vivo and Ex Vivo Studies in a Model of Inflammation and MIA-Induced Osteoarthritis. Nutrients, 14(7), 1487. https://doi.org/10.3390/nu14071487















