Enhanced Walking-Induced Fat Oxidation by New Zealand Blackcurrant Extract Is Body Composition-Dependent in Recreationally Active Adult Females
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Preliminary Session
2.4. Supplementation for the Experimental Sessions
2.5. Experimental Sessions
2.6. Statistical Analysis
3. Results
3.1. Walking-Induced Physiological Responses
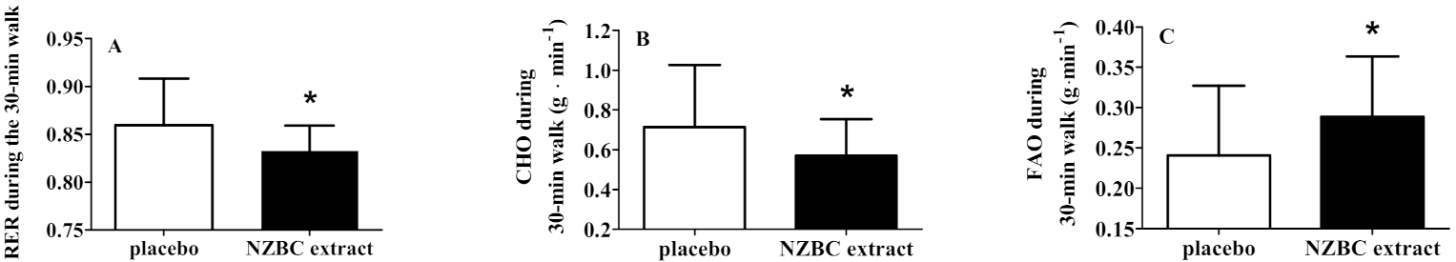
3.2. Walking-Induced Metabolic Responses
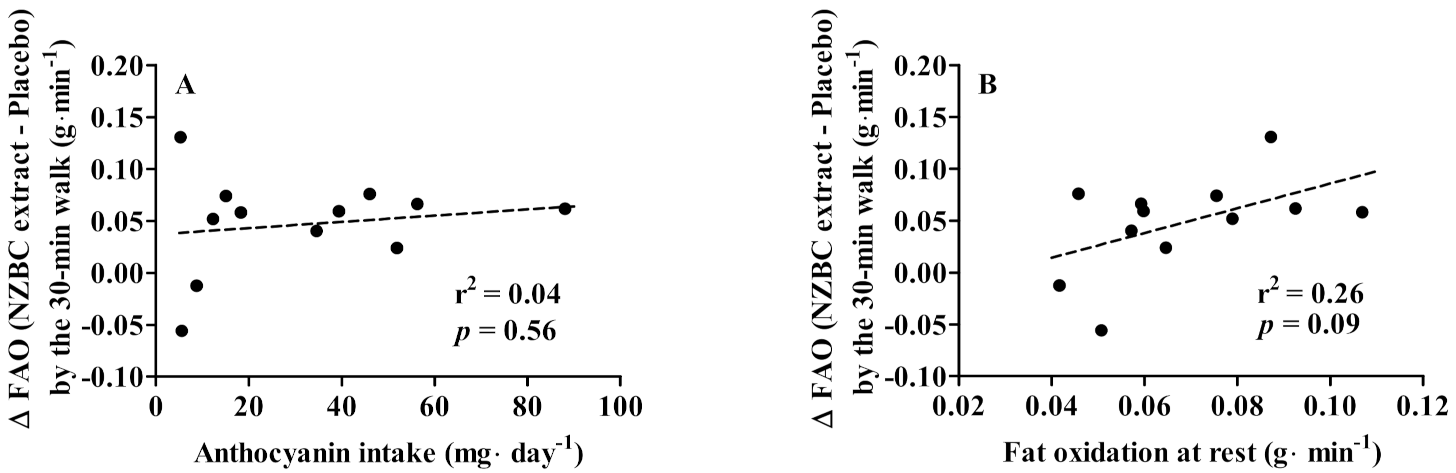
3.3. Habitual Anthcoyanin Intake, Fat Oxidation at Rest, and Walking-Induced Fat Oxidation
3.4. Body Mass, Body Composition, and Walking-Induced Fat Oxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Rosenblatt, J.; Wolfe, R.R. Substrate metabolism during different exercise intensities in endurance-trained women. J. Appl. Physiol. 2000, 88, 1704–1714. [Google Scholar] [CrossRef] [PubMed]
- Lambert, E.V.; Speechly, D.P.; Dennis, S.C.; Noakes, T.D. Enhanced endurance in trained cyclists during moderate intensity exercise following 2 weeks adaptation to a high fat diet. Eur. J. Appl. Physiol. 1994, 69, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Coggan, A.R.; Kohrt, W.M.; Spina, R.J.; Bier, D.M.; Holloszy, J.O. Endurance training decreases plasma glucose turnover and oxidation during moderate-intensity exercise in men. J. Appl. Physiol. 1990, 68, 990–996. [Google Scholar] [CrossRef]
- Devries, M.M.; Hamadeh, M.J.; Phillips, S.M.; Tarnapolsky, M.A. Menstrual cycle phase and sex influence muscle glycogen utilization and glucose turnover during moderate-intensity endurance exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1120–R1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chenevière, X.; Malatesta, D.; Gojanovi, B.; Borrani, F. Differences in whole-body fat oxidation kinetics between cycling and running. Eur. J. Appl. Physiol. 2010, 109, 1037–1045. [Google Scholar] [CrossRef]
- Maunder, E.; Plews, D.J.; Merien, F.; Kilding, A.E. Exercise intensity regulates the effect of heat stress on substrate oxidation rates during exercise. Eur. J. Sport Sci. 2020, 20, 935–943. [Google Scholar] [CrossRef]
- Ruiz-Moreno, C.; Gutiérrez-Hellín, J.; Amaro-Gahete, F.J.; González-García, J.; Giráldez-Costas, V.; Pérez- García, V.; Del Coso, J. Caffeine increases whole-body fat oxidation during 1 h of cycling at Fatmax. J. Nutr. 2021, 60, 2077–2085. [Google Scholar] [CrossRef]
- Venables, M.C.; Hulston, C.J.; Cox, H.R.; Jeukendrup, A.E. Green tea extract ingestion, fat oxidation, and glucose tolerance in healthy humans. Am. J. Clin. Nutr. 2008, 87, 778–784. [Google Scholar] [CrossRef] [Green Version]
- Willems, M.E.T.; Fry, H.L.; Belding, M.A.; Kaviani, M. Three Weeks Daily Intake of Match Green Tea Powder Affects Substrate Oxidation during Moderate-Intensity Exercise in Females. J. Diet. Suppl. 2021, 18, 566–576. [Google Scholar] [CrossRef]
- Cook, M.D.; Myers, S.D.; Blacker, S.D.; Willems, M.E.T. New Zealand blackcurrant extract improves cycling performance and fat oxidation in cyclists. Eur. J. Appl. Physiol. 2015, 115, 2357–2365. [Google Scholar] [CrossRef]
- Frandsen, J.; Hansen, I.M.D.; Wismann, J.F.; Olsen, M.H.; Brage-Andersen, M.R.; Sahl, R.E.; Hansen, M.; Ingersen, A.; Modvig, J.L.; Schmücker, M.; et al. Maximal Fat Oxidation Rate is Higher in Fit Women and Unfit Women with Obesity, Compared to Normal-weight Unfit Women. J. Clin. Endocrinol. Metabol. 2021, 106, e4389–e4399. [Google Scholar] [CrossRef] [PubMed]
- Kerhervé, H.A.; Harvey, L.M.; Eagles, A.N.; McLellan, C.; Lovell, D. Similar rates of fat oxidation during graded submaximal exercise in women of different body composition. PLoS ONE 2020, 15, e0242551. [Google Scholar] [CrossRef] [PubMed]
- Isacco, L.; Ennequin, G.; Boisseau, N. Effect of Fat Mass Localization on Fat Oxidation during Endurance Exercise in Women. Front. Physiol. 2020, 11, 585137. [Google Scholar] [CrossRef] [PubMed]
- De Lima, L.P.; de Paula Barbosa, A. A review of the lipolytic effects and the reduction of abdominal fat from bioactive compounds and moro orange extracts. Heliyon 2021, 7, e07695. [Google Scholar] [CrossRef]
- Nackers, L.M.; Middleton, K.R.; Dubyak, P.J.; Daniels, M.J.; Anton, S.D.; Perri, M.G. Effects of prescribing 1,000 versus 1,500 kilocalories per day in the behavioral treatment of obesity: A randomized trial. Obesity 2013, 21, 2481–2487. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Park, J.; Lim, K. Nutrition Supplements to Stimulate Lipolysis: A Review in Relation to Endurance Exercise Capacity. J. Nutr. Sci. Vitaminol. 2016, 62, 141–161. [Google Scholar] [CrossRef] [Green Version]
- Şahin, M.A.; Bilgiç, P.; Montanari, S.; Willems, M.E.T. Daily and not Every-Other-Day Intake of Anthocyanin-Rich New Zealand Blackcurrant Extract Alters Substrate Oxidation during Moderate-Intensity Walking in Adult Males. J. Diet. Suppl. 2022; in press. [Google Scholar] [CrossRef]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [Green Version]
- Bonen, A.; Haynes, F.J.; Watson-Wright, W.; Sopper, M.M.; Pierce, G.N.; Low, M.P.; Graham, T.E. Effects of menstrual cycle on metabolic responses to exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 1506–1513. [Google Scholar] [CrossRef]
- Şahin, M.A.; Bilgiç, P.; Montanari, S.; Willems, M.E.T. Intake Duration of Anthocyanin-Rich New Zealand Blackcurrant Extract Affects Metabolic Responses during Moderate Intensity Walking Exercise in Adult Males. J. Diet. Suppl. 2021, 18, 406–417. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- Willems, M.E.T.; Şahin, M.A.; Cook, M.D. Matcha Green Tea Drinks Enhance Fat Oxidation during Brisk Walking in Females. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 536–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeukendrup, A.E.; Wallis, G.A. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int. J. Sports Med. 2005, 26 (Suppl. 1), S28–S37. [Google Scholar] [CrossRef] [PubMed]
- Curran-Everett, D.; Benos, D.J. Guidelines for reporting statistics in journals by the American Physiological Society. Adv. Physiol. Educ. 2004, 28, 85–87. [Google Scholar] [CrossRef]
- Cook, M.D.; Myers, S.D.; Gault, M.L.; Edwards, V.C.; Willems, M.E.T. Dose effects of New Zealand blackcurrant on substrate oxidation and physiological responses during prolonged cycling. Eur. J. Appl. Physiol. 2017, 117, 1207–1216. [Google Scholar] [CrossRef]
- Strauss, J.A.; Willems, M.E.T.; Shepherd, S.O. New Zealand blackcurrant extract enhances fat oxidation during prolonged cycling in endurance-trained females. Eur. J. Appl. Physiol. 2018, 118, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
- Elliott-Sale, K.J.; Minahan, C.L.; de Jonge, X.A.K.J.; Ackerman, K.E.; Sipilä, S.; Constantini, N.W.; Lebrun, C.M.; Hackney, A.C. Methodological Considerations for Studies in Sport and Exercise Science with Women as Participants: A Working Guide for Standards of Practice for Research on Women. Sports Med. 2021, 51, 843–861. [Google Scholar] [CrossRef]
- Robinson, S.L.; Chambers, E.S.; Fletcher, G.; Wallis, G.A. Lipolytic Markers, Insulin and Resting Fat Oxidation are Associated with Maximal Fat Oxidation. Int. J. Sports Med. 2016, 37, 607–613. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willems, M.E.T.; Banic, M.; Cadden, R.; Barnett, L. Enhanced Walking-Induced Fat Oxidation by New Zealand Blackcurrant Extract Is Body Composition-Dependent in Recreationally Active Adult Females. Nutrients 2022, 14, 1475. https://doi.org/10.3390/nu14071475
Willems MET, Banic M, Cadden R, Barnett L. Enhanced Walking-Induced Fat Oxidation by New Zealand Blackcurrant Extract Is Body Composition-Dependent in Recreationally Active Adult Females. Nutrients. 2022; 14(7):1475. https://doi.org/10.3390/nu14071475
Chicago/Turabian StyleWillems, Mark E. T., Milena Banic, Roseanna Cadden, and Lara Barnett. 2022. "Enhanced Walking-Induced Fat Oxidation by New Zealand Blackcurrant Extract Is Body Composition-Dependent in Recreationally Active Adult Females" Nutrients 14, no. 7: 1475. https://doi.org/10.3390/nu14071475
APA StyleWillems, M. E. T., Banic, M., Cadden, R., & Barnett, L. (2022). Enhanced Walking-Induced Fat Oxidation by New Zealand Blackcurrant Extract Is Body Composition-Dependent in Recreationally Active Adult Females. Nutrients, 14(7), 1475. https://doi.org/10.3390/nu14071475






