Higher Dietary Intake of Advanced Glycation End Products Is Associated with Faster Cognitive Decline in Community-Dwelling Older Adults
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Cognition and Cognitive Status Assessment
2.3. Assessment of Dietary AGEs
2.4. Other Covariates
2.5. Statistical Analyses
3. Results
3.1. Description of the Analytic Cohort
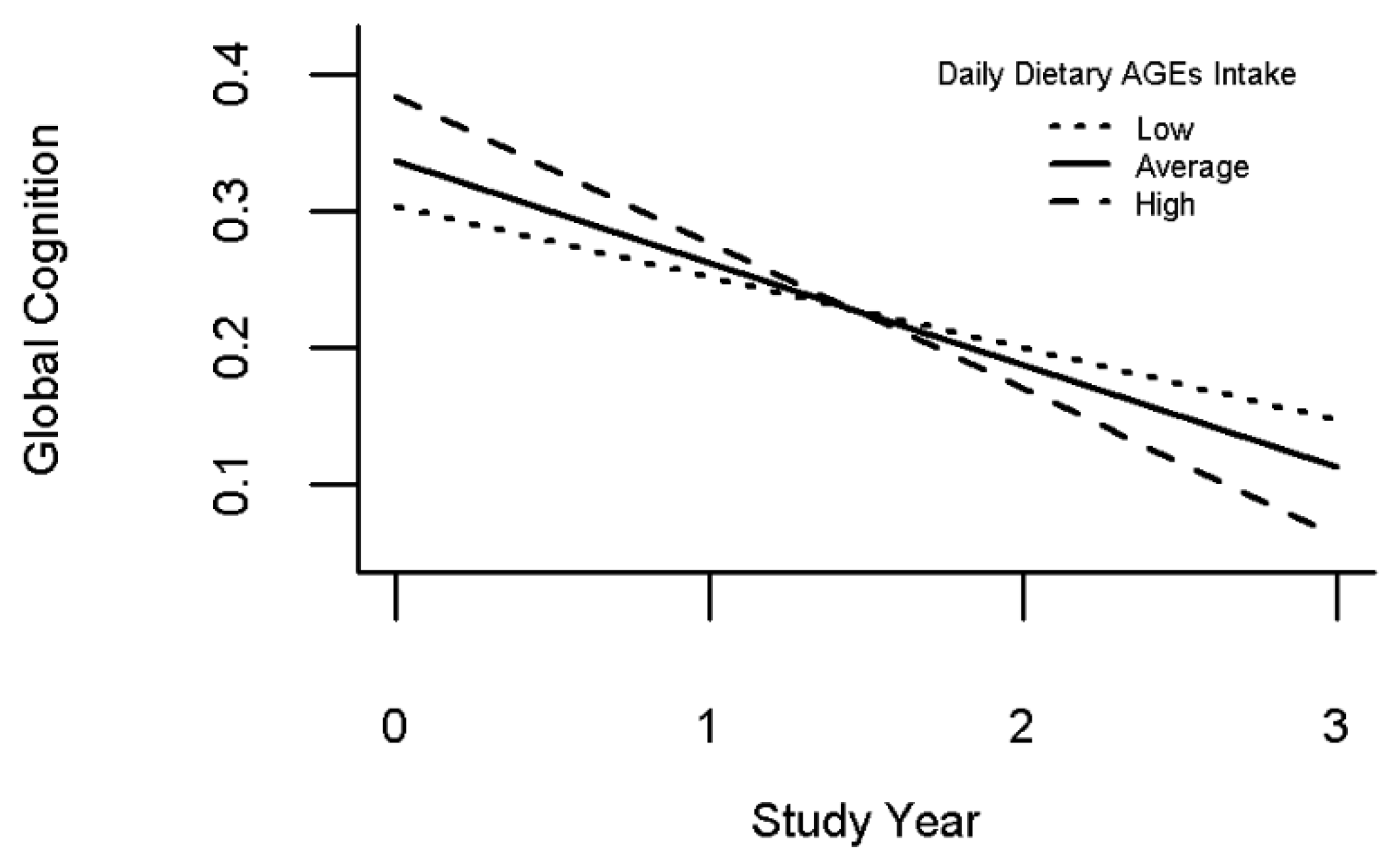
3.2. Associations of Dietary AGEs with Baseline Cognition and with Cognitive Decline
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimer’s Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Manly, J.J.; Schupf, N.; Luchsinger, J.A. Mediterranean diet and mild cognitive impairment. Arch. Neurol. 2009, 66, 216–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, G.D.; Chen, H.; Bertoni, A.G.; Rapp, S.R.; Fitzpatrick, A.L.; Luchsinger, J.A.; Wood, A.C.; Hughes, T.M.; Burke, G.L.; Hayden, K.M. DASH diet adherence and cognitive function: Multi-ethnic study of atherosclerosis. Clin. Nutr. ESPEN 2021, 46, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Garay-Sevilla, M.E.; Beeri, M.S.; de la Maza, M.P.; Rojas, A.; Salazar-Villanea, S.; Uribarri, J. The potential role of dietary advanced glycation endproducts in the development of chronic non-infectious diseases: A narrative review. Nutr. Res. Rev. 2020, 33, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Peppa, M.; Uribarri, J.; Vlassara, H. Aging and glycoxidant stress. Hormones 2008, 7, 123–132. [Google Scholar] [CrossRef]
- Lubitz, I.; Ricny, J.; Atrakchi-Baranes, D.; Shemesh, C.; Kravitz, E.; Liraz-Zaltsman, S.; Maksin-Matveev, A.; Cooper, I.; Leibowitz, A.; Uribarri, J.; et al. High dietary advanced glycation end products are associated with poorer spatial learning and accelerated Abeta deposition in an Alzheimer mouse model. Aging Cell 2016, 15, 309–316. [Google Scholar] [CrossRef]
- Cai, W.; Uribarri, J.; Zhu, L.; Chen, X.; Swamy, S.; Zhao, Z.; Grosjean, F.; Simonaro, C.; Kuchel, G.A.; Schnaider-Beeri, M.; et al. Oral glycotoxins are a modifiable cause of dementia and the metabolic syndrome in mice and humans. Proc. Natl. Acad. Sci. USA 2014, 111, 4940–4945. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, T.; Cai, W.; Peppa, M.; Dardaine, V.; Baliga, B.S.; Uribarri, J.; Vlassara, H. Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet. Assoc. 2004, 104, 1287–1291. [Google Scholar] [CrossRef]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916. [Google Scholar] [CrossRef] [Green Version]
- West, R.K.; Moshier, E.; Lubitz, I.; Schmeidler, J.; Godbold, J.; Cai, W.; Uribarri, J.; Vlassara, H.; Silverman, J.M.; Beeri, M.S. Dietary advanced glycation end products are associated with decline in memory in young elderly. Mech. Ageing Dev. 2014, 140, 10–12. [Google Scholar] [CrossRef] [Green Version]
- Perrone, L.; Grant, W.B. Observational and ecological studies of dietary advanced glycation end products in national diets and Alzheimer’s disease incidence and prevalence. J. Alzheimer’s Dis. 2015, 45, 965–979. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.A.; Buchman, A.S.; Boyle, P.A.; Barnes, L.L.; Wilson, R.S.; Schneider, J.A. Religious Orders Study and Rush Memory and Aging Project. J. Alzheimer’s Dis. 2018, 64, S161–S189. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.A.; Schneider, J.A.; Buchman, A.S.; Mendes de Leon, C.; Bienias, J.L.; Wilson, R.S. The Rush Memory and Aging Project: Study design and baseline characteristics of the study cohort. Neuroepidemiology 2005, 25, 163–175. [Google Scholar] [CrossRef]
- Wilson, R.S.; Barnes, L.L.; Krueger, K.R.; Hoganson, G.; Bienias, J.L.; Bennett, D.A. Early and late life cognitive activity and cognitive systems in old age. J. Int. Neuropsychol. Soc. 2005, 11, 400–407. [Google Scholar] [CrossRef]
- Crum, R.M.; Anthony, J.C.; Bassett, S.S.; Folstein, M.F. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993, 269, 2386–2391. [Google Scholar] [CrossRef]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, R.S.; Boyle, P.A.; James, B.D.; Leurgans, S.E.; Buchman, A.S.; Bennett, D.A. Negative social interactions and risk of mild cognitive impairment in old age. Neuropsychology 2015, 29, 561–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uribarri, J.; Peppa, M.; Cai, W.; Goldberg, T.; Lu, M.; Baliga, S.; Vassalotti, J.A.; Vlassara, H. Dietary glycotoxins correlate with circulating advanced glycation end product levels in renal failure patients. Am. J. Kidney Dis. 2003, 42, 532–538. [Google Scholar] [CrossRef]
- Fayemendy, P.; Mabiama, G.; Vernier, T.; Massoulard-Gainant, A.; Villemonteix, C.; Desport, J.C.; Jesus, P. Nutritional status, dementia, and mobility among nursing home’s residents: First exhaustive cross-sectional study in Limousin territory (France). PLoS ONE 2021, 16, e0250595. [Google Scholar] [CrossRef]
- Boyle, P.A.; Wilson, R.S.; Aggarwal, N.T.; Arvanitakis, Z.; Kelly, J.; Bienias, J.L.; Bennett, D.A. Parkinsonian signs in subjects with mild cognitive impairment. Neurology 2005, 65, 1901–1906. [Google Scholar] [CrossRef]
- Fitzmaurice, G.; Laird, N.; Ware, J. Applied Longitudinal Analysis; Wiley Interscience: New York, NY, USA, 2004. [Google Scholar]
- Morris, M.C.; Wang, Y.; Barnes, L.L.; Bennett, D.A.; Dawson-Hughes, B.; Booth, S.L. Nutrients and bioactives in green leafy vegetables and cognitive decline: Prospective study. Neurology 2017, 90, e214–e222. [Google Scholar] [CrossRef] [PubMed]
- Samieri, C.; Morris, M.C.; Bennett, D.A.; Berr, C.; Amouyel, P.; Dartigues, J.F.; Tzourio, C.; Chasman, D.I.; Grodstein, F. Fish intake, genetic predisposition to alzheimer’s disease and decline in global cognition and memory in five cohorts of older persons. Am. J. Epidemiol. 2017, 187, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C. Dietary fat composition and dementia risk. Neurobiol. Aging 2014, 35 (Suppl. 2), S59–S64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlassara, H.; Uribarri, J.; Cai, W.; Striker, G. Advanced glycation end product homeostasis: Exogenous oxidants and innate defenses. Ann. N. Y. Acad. Sci. 2008, 1126, 46–52. [Google Scholar] [CrossRef]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid. Med. Cell. Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef]
- Sun, Y.; Rawish, E.; Nording, H.M.; Langer, H.F. Inflammation in Metabolic and Cardiovascular Disorders-Role of Oxidative Stress. Life 2021, 11, 672. [Google Scholar] [CrossRef]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef]
- Bar, K.J.; Franke, S.; Wenda, B.; Muller, S.; Kientsch-Engel, R.; Stein, G.; Sauer, H. Pentosidine and N(epsilon)-(carboxymethyl)-lysine in Alzheimer′s disease and vascular dementia. Neurobiol. Aging 2003, 24, 333–338. [Google Scholar] [CrossRef]
- Girones, X.; Guimera, A.; Cruz-Sanchez, C.Z.; Ortega, A.; Sasaki, N.; Makita, Z.; Lafuente, J.V.; Kalaria, R.; Cruz-Sanchez, F.F. N epsilon-carboxymethyllysine in brain aging, diabetes mellitus, and Alzheimer’s disease. Free Radic. Biol. Med. 2004, 36, 1241–1247. [Google Scholar] [CrossRef]
- Kuhla, B.; Luth, H.J.; Haferburg, D.; Boeck, K.; Arendt, T.; Munch, G. Methylglyoxal, glyoxal, and their detoxification in Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2005, 1043, 211–216. [Google Scholar] [CrossRef]
- Beeri, M.S.; Moshier, E.; Schmeidler, J.; Godbold, J.; Uribarri, J.; Reddy, S.; Sano, M.; Grossman, H.T.; Cai, W.; Vlassara, H.; et al. Serum concentration of an inflammatory glycotoxin, methylglyoxal, is associated with increased cognitive decline in elderly individuals. Mech. Ageing Dev. 2011, 132, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Lindquist, K.; Schwartz, A.V.; Vitartas, C.; Vittinghoff, E.; Satterfield, S.; Simonsick, E.M.; Launer, L.; Rosano, C.; Cauley, J.A.; et al. Advanced glycation end product level, diabetes, and accelerated cognitive aging. Neurology 2011, 77, 1351–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabara, Y.; Ikezoe, T.; Yamanaka, M.; Setoh, K.; Segawa, H.; Kawaguchi, T.; Kosugi, S.; Nakayama, T.; Ichihashi, N.; Tsuboyama, T.; et al. Advanced Glycation End Product Accumulation Is Associated With Low Skeletal Muscle Mass, Weak Muscle Strength, and Reduced Bone Density: The Nagahama Study. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1446–1453. [Google Scholar] [CrossRef]
- Albers, M.W.; Gilmore, G.C.; Kaye, J.; Murphy, C.; Wingfield, A.; Bennett, D.A.; Boxer, A.L.; Buchman, A.S.; Cruickshanks, K.J.; Devanand, D.P.; et al. At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 70–98. [Google Scholar] [CrossRef] [Green Version]
- Boyle, P.A.; Buchman, A.S.; Wilson, R.S.; Leurgans, S.E.; Bennett, D.A. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch. Neurol. 2009, 66, 1339–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, W.; Chen, H.; Li, Y. The potential mechanisms of Abeta-receptor for advanced glycation end-products interaction disrupting tight junctions of the blood-brain barrier in Alzheimer’s disease. Int. J. Neurosci. 2014, 124, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Dobi, A.; Rosanaly, S.; Devin, A.; Baret, P.; Meilhac, O.; Harry, G.J.; d’Hellencourt, C.L.; Rondeau, P. Advanced glycation end-products disrupt brain microvascular endothelial cell barrier: The role of mitochondria and oxidative stress. Microvasc. Res. 2021, 133, 104098. [Google Scholar] [CrossRef] [PubMed]
- Akhter, F.; Chen, D.; Akhter, A.; Sosunov, A.A.; Chen, A.; McKhann, G.M.; Yan, S.F.; Yan, S.S. High Dietary Advanced Glycation End Products Impair Mitochondrial and Cognitive Function. J. Alzheimer’s Dis. 2020, 76, 165–178. [Google Scholar] [CrossRef]
- Moran, C.; Munch, G.; Forbes, J.M.; Beare, R.; Blizzard, L.; Venn, A.J.; Phan, T.G.; Chen, J.; Srikanth, V. Type 2 diabetes, skin autofluorescence, and brain atrophy. Diabetes 2015, 64, 279–283. [Google Scholar] [CrossRef] [Green Version]
- Ying, L.; Shen, Y.; Zhang, Y.; Wang, Y.; Liu, Y.; Yin, J.; Wang, Y.; Yin, J.; Zhu, W.; Bao, Y.; et al. Advanced glycation end products via skin autofluorescence as potential marker of carotid atherosclerosis in patients with type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3449–3456. [Google Scholar] [CrossRef]
| Clinical Characteristics at Study Baseline (n = 684) | |
|---|---|
| Age, years | 82.46 (7.24) |
| Women, % | 77.05 |
| Education, years | 15.93 (3.02) |
| Race, % White | 93.9% |
| BMI, kg/m2 | 27.61 (5.41) |
| Vascular risk factors, % | |
| Hypertension Diabetes Smoking (ever) | 57.31 16.81 43.71 |
| Vascular disease, % | |
| Claudication Stroke Heart attack Congestive heart failure | 13.01 9.36 5.70 5.99 |
| Dietary AGEs | |
| Daily total Meat Poultry Fish Cheese Spreads Processed foods | 16.00 (8.04) 27.44 (25.77) 27.00 (22.04) 12.91 (15.37) 12.42 (11.17) 11.30 (7.85) 21.02 (23.56) |
| Dietary AGEs Intake is Associated with Decline in Global Cognition | |
|---|---|
| Model Term | Estimate (S.E, p-Value) |
| Time | −0.077 (0.011, <0.001) |
| Age | −0.027 (0.003, <0.001) |
| Sex | −0.140 (0.045, 0.002) |
| Years of Education | 0.040 (0.006, <0.001) |
| Race | −0.472 (0.087, <0.001) |
| BMI | 0.004 (0.003, 0.254) |
| Daily dAGE | 0.004 (0.002, 0.100) |
| Time*daily dAGE | −0.003 (0.001, 0.015) |
| Dietary AGEs Intake and Declining Cognitive Domains | ||
|---|---|---|
| Cognitive Ability Outcome | Model Term | Estimate (S.E, p-Value) |
| Global Cognition | Time | −0.078 (0.011, <0.001) |
| Time × dietary AGEs intake | −0.003 (0.001, 0.015) | |
| Semantic Memory | Time | −0.072 (0.013, <0.001) |
| Time × dietary AGEs intake | <0.001 (0.001, 0.923) | |
| Episodic Memory | Time | −0.080 (0.015, <0.001) |
| Time × dietary AGEs intake | −0.004 (0.002, 0.015) | |
| Working Memory | Time | 0.021 (0.016, 0.193) |
| Time × dietary AGEs intake | −0.001 (0.002, 0.380) | |
| Perceptual Speed | Time | −0.117 (0.013, <0.001) |
| Time × dietary AGEs intake | −0.003 (0.001, 0.049) | |
| Visuospatial Abilities | Time | −0.041 (0.018, 0.027) |
| Time × dietary AGEs intake | −0.001 (0.002, 0.449) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnaider Beeri, M.; Lotan, R.; Uribarri, J.; Leurgans, S.; Bennett, D.A.; Buchman, A.S. Higher Dietary Intake of Advanced Glycation End Products Is Associated with Faster Cognitive Decline in Community-Dwelling Older Adults. Nutrients 2022, 14, 1468. https://doi.org/10.3390/nu14071468
Schnaider Beeri M, Lotan R, Uribarri J, Leurgans S, Bennett DA, Buchman AS. Higher Dietary Intake of Advanced Glycation End Products Is Associated with Faster Cognitive Decline in Community-Dwelling Older Adults. Nutrients. 2022; 14(7):1468. https://doi.org/10.3390/nu14071468
Chicago/Turabian StyleSchnaider Beeri, Michal, Roni Lotan, Jaime Uribarri, Sue Leurgans, David A. Bennett, and Aron S. Buchman. 2022. "Higher Dietary Intake of Advanced Glycation End Products Is Associated with Faster Cognitive Decline in Community-Dwelling Older Adults" Nutrients 14, no. 7: 1468. https://doi.org/10.3390/nu14071468
APA StyleSchnaider Beeri, M., Lotan, R., Uribarri, J., Leurgans, S., Bennett, D. A., & Buchman, A. S. (2022). Higher Dietary Intake of Advanced Glycation End Products Is Associated with Faster Cognitive Decline in Community-Dwelling Older Adults. Nutrients, 14(7), 1468. https://doi.org/10.3390/nu14071468







