Antioxidant and Anti-Atherogenic Activities of Essential Oils from Myrtus communis L. and Laurus nobilis L. in Rat
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Materials
2.2. Extraction of the Essential Oil
2.3. Determination of the Composition of EOs
2.4. Animal Study
2.4.1. Experimental Animals and Ethics
2.4.2. Animals, Experimental Treatment and Organ Processing
2.5. Body Weight
2.6. Sample Collection, Preparation and Storage of Tissue Samples
2.7. Relative Mass of Individual Organs
2.8. Antioxidant Status of Tissues
2.9. Blood Sample Collection and Laboratory Analysis
2.9.1. Estimation of Serum Liver Enzyme Activity and Kidney Function
2.9.2. Lipid Parameters and Atherogenic Risk Predictor Indices (ARPI) Calculation
2.9.3. Measurement of Erythrocyte Osmotic Fragility
2.10. Intestinal Contents and Isolation of Gut Microorganisms
2.10.1. Intestinal Content Sampling and Analysis of Gut Probiotic Bacteria and Enterobacteriaceae
2.10.2. Fecal Enzyme Activity
2.11. Histopathological Analysis of the Liver and Gut
2.12. Statistics
3. Results
3.1. Chemical Composition of EOs
3.2. Body Weight Change
3.3. Fecal Microbial Count
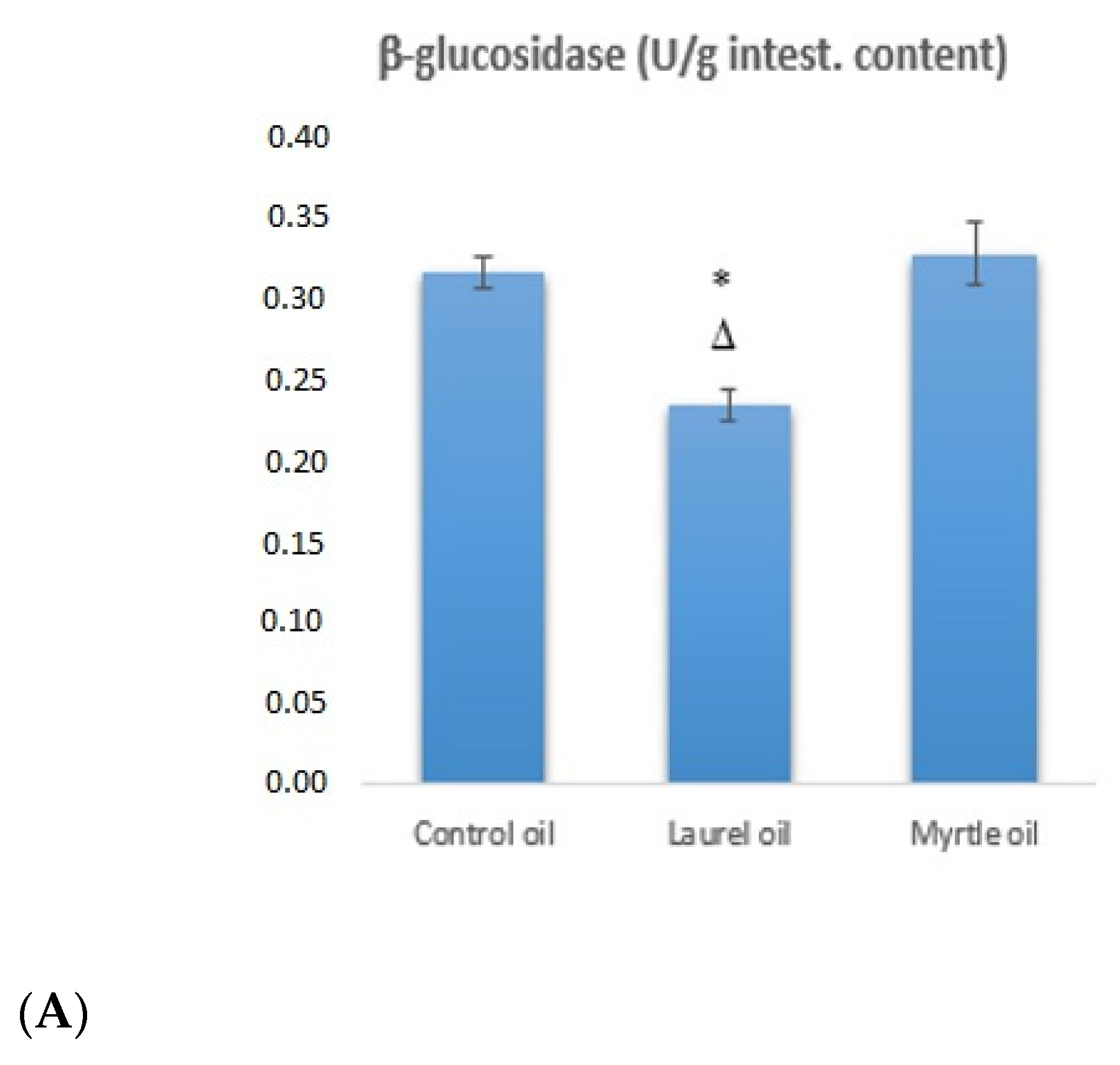
3.4. Fecal Bacterial Enzyme Activity
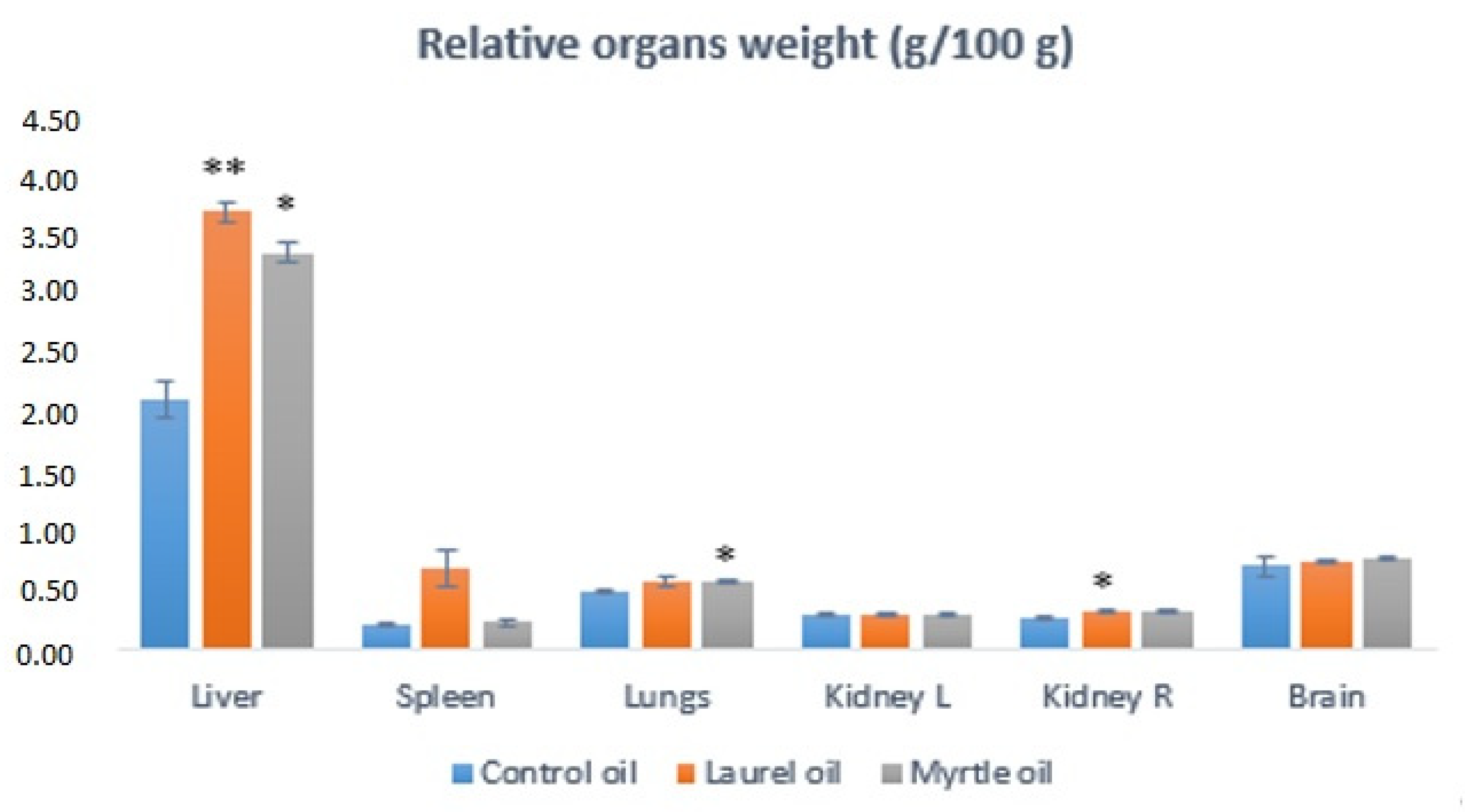
3.5. Relative Organs Weights
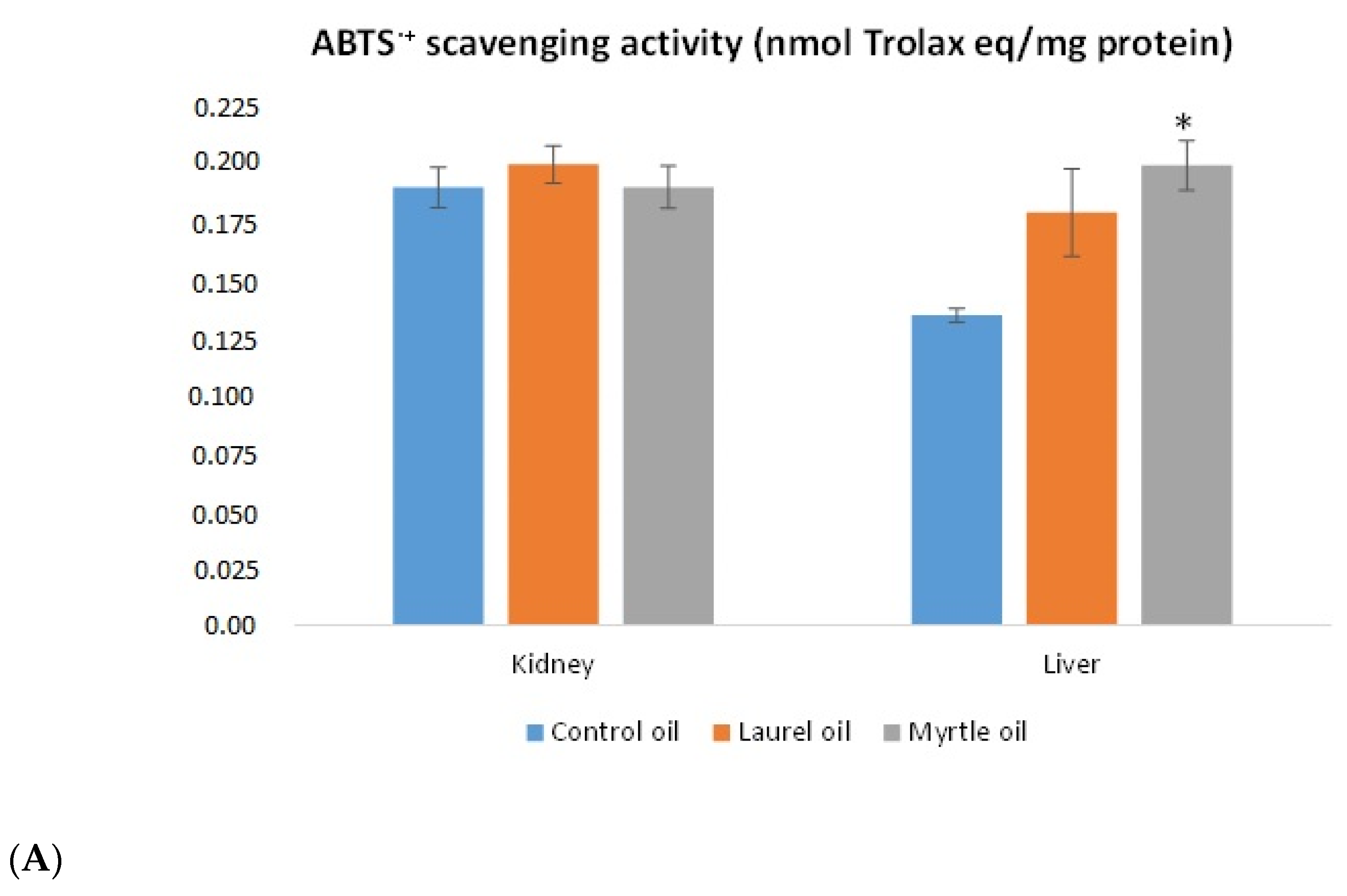
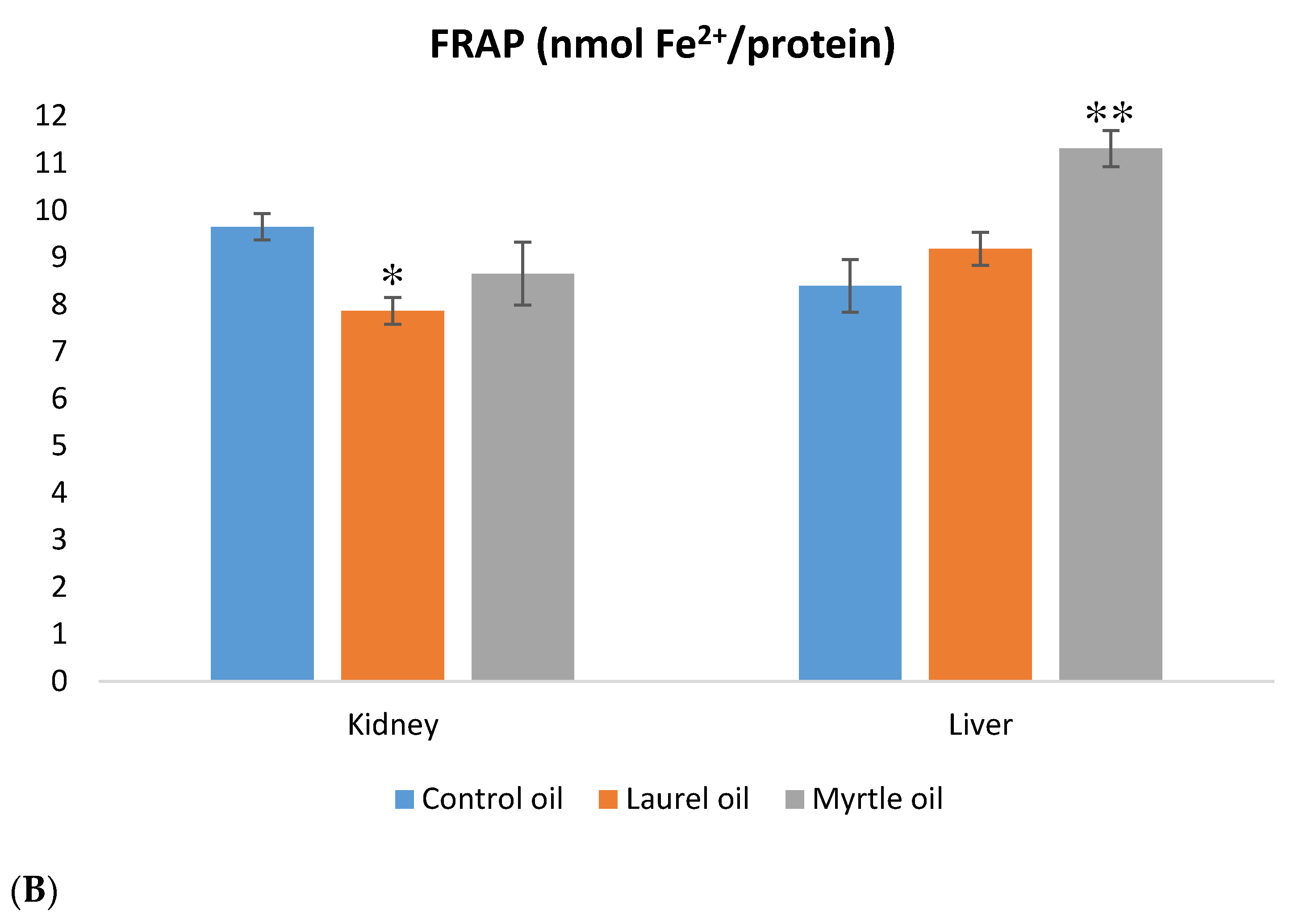
3.6. Antioxidative Capacity of the Liver and Kidney
3.7. Osmotic Fragility Curve
3.8. Biochemical Parameters as Indicators of Liver and Kidney Function
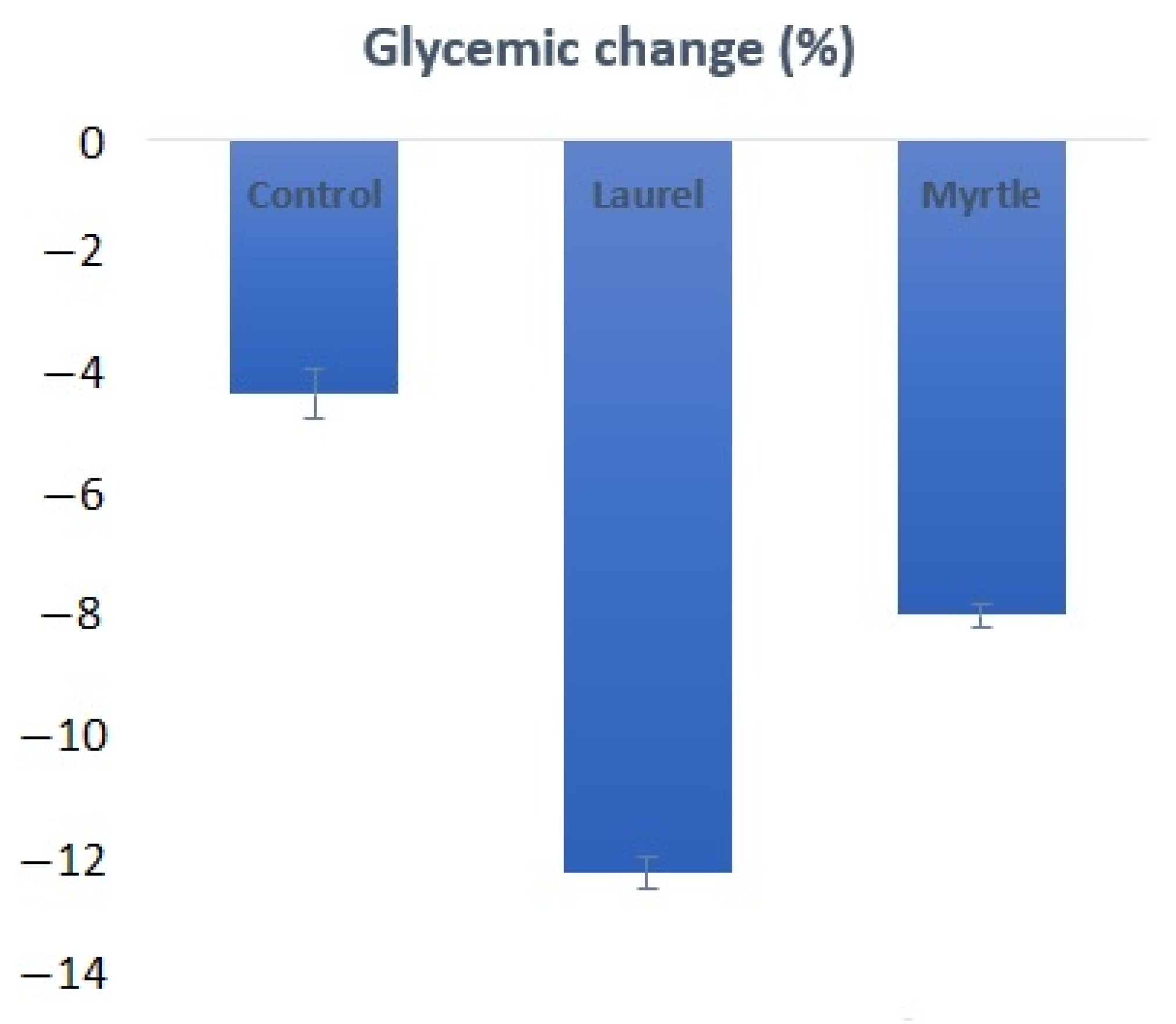
3.9. Glycemic Change
3.10. Lipid Profile Analysis and Atherogenic Indices
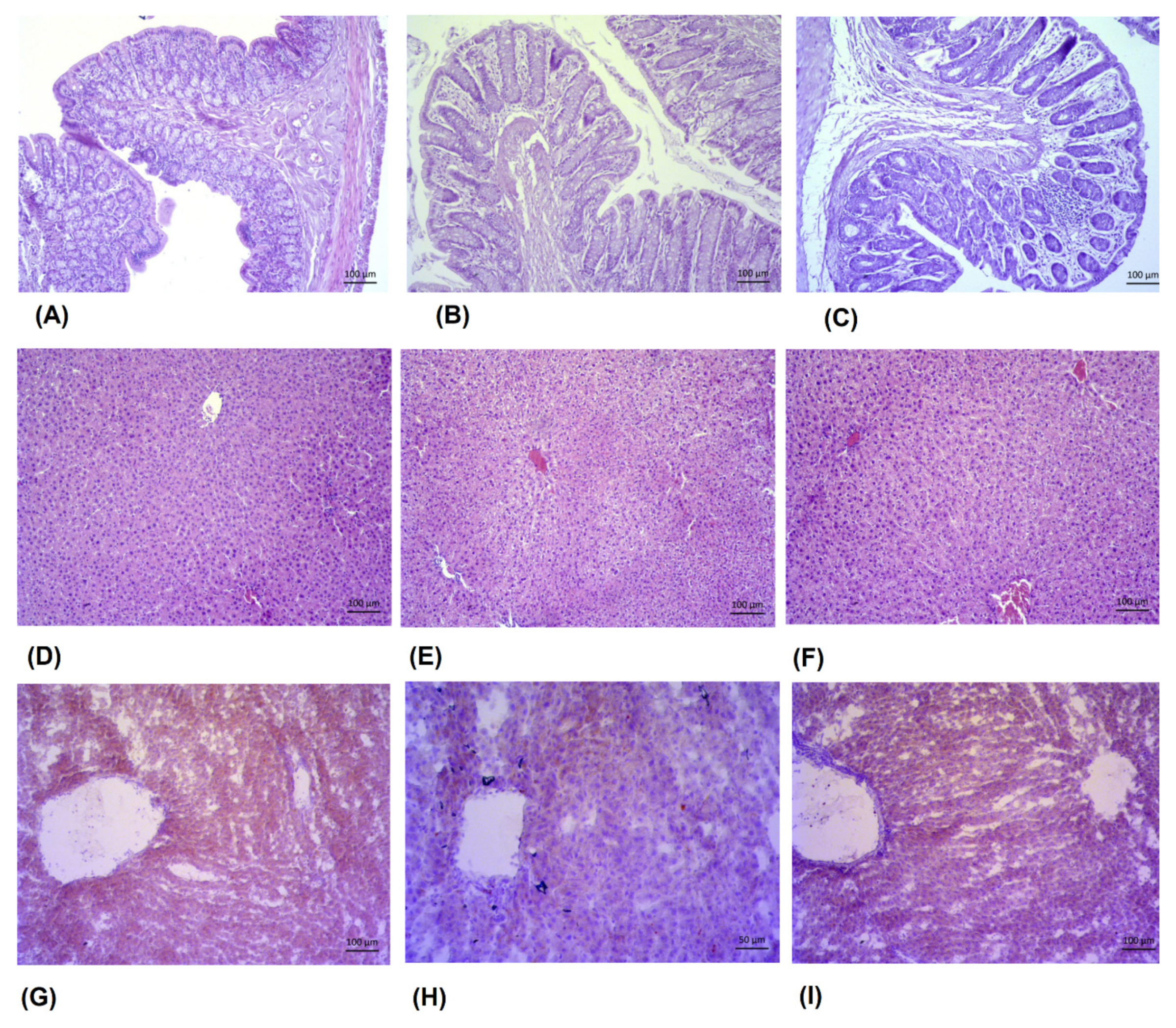
3.11. Morphometric Analyses of the Intestine and Reduction in Lipid Deposition in Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Correction to: Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotechnol. 2020, 11, 50. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Yiap, B.C.; Ping, H.C.; Lim, S.H.E. Essential Oils, A New Horizon in Combating Bacterial Antibiotic Resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Saljoughian, S.; Roohinejad, S.; Bekhit, A.E.-D.A.; Greiner, R.; Omidizadeh, A.; Nikmaram, N.; Mousavi, K.A. The effects of food essential oils on cardiovascular diseases: A review. Crit. Rev. Food Sci. Nutr. 2017, 58, 1688–1705. [Google Scholar] [CrossRef] [PubMed]
- Giannenas, I.; Tzora, A.; Sarakatsianos, I.; Karamoutsios, A.; Skoufos, S.; Papaioannou, N.; Anastasiou, I.; Skoufos, I. The Effectiveness of the Use of Oregano and Laurel Essential Oils in Chicken Feeding. Ann. Anim. Sci. 2016, 16, 779–796. [Google Scholar] [CrossRef]
- El Hartiti, H.; El Mostaphi, A.; Barrahi, M.; Ben Ali, A.; Chahboun, N.; Amiyare, R.; Zarrouk, A.; Bourkhiss, B.; Ouhssine, M. Chemical composition and antibacterial activity of the essential oil of Myrtus communis leaves. Karbala Int. J. Mod. Sci. 2020, 6, 251–258. [Google Scholar] [CrossRef]
- Li, Y.; Fu, X.; Ma, X.; Geng, S.; Jiang, X.; Huang, Q.; Hu, C.; Han, X. Intestinal Microbiome-Metabolome Responses to Essential Oils in Piglets. Front. Microbiol. 2018, 28, 9. [Google Scholar] [CrossRef]
- Du, E.; Gan, L.; Li, Z.; Wang, W.; Liu, D.; Guo, Y. In vitro antibacterial activity of thymol and carvacrol and their effects on broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2015, 6, 58. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, Y.; Zhu, P.; Nasser, M.I.; Zhang, X.; Zhao, M. Implication of Gut Microbiota in Cardiovascular Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 5394096. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Kustrin, E.; Morton, D.W. Essential oils and functional herbs for healthy aging. Neural Regen. Res. 2019, 14, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, H.A. Essential Oil as Antimicrobial Agents: Efficacy, Stability, and Safety Issues for Food Application. In Essential Oils-Bioactive Compounds, New Perspectives and Applications; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Oršolić, N.; Jazvinšćak Jembrek, M.; Terzić, S. Honey and Quercetin reduces ochratoxin A-induced DNA damage in the liver and the kidney through the modulation of intestinal microflora. Food Agric. Immunol. 2017, 28, 812–833. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. The Changing Face of the Family Enterobacteriaceae (Order: “Enterobacterales”): New Members, Taxonomic Issues, Geographic Expansion, and New Diseases and Disease Syndromes. Clin. Microbiol. Rev. 2021, 34, e00174-20. [Google Scholar] [CrossRef] [PubMed]
- Chamseddine, A.N.; Ducreux, M.; Armand, J.P.; Paoletti, X.; Satar, T.; Paci, A.; Mir, O. Intestinal bacterial β-glucuronidase as a possible predictive biomarker of irinotecan-induced diarrhea severity. Pharmacol. Ther. 2019, 199, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dabek, M.; McCrae, S.I.; Stevens, V.J.; Duncan, S.H.; Louis, P. Distribution of β-glucosidase and β-glucuronidase activity and of β-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol. Ecol. 2008, 66, 487–495. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis: Changes in Official Methods of Analysis Made at the Annual Meeting; Association of Official Analytical Chemists: Rockville, MD, USA, 1990. [Google Scholar]
- Oršolić, N.; Landeka Jurčević, I.; Đikić, D.; Rogić, D.; Odeh, D.; Balta, V.; Perak Junaković, E.; Terzić, S.; Jutrić, D. Effect of Propolis on Diet-Induced Hyperlipidemia and Atherogenic Indices in Mice. Antioxidants 2019, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Sirovina, D.; Odeh, D.; Gajski, G.; Balta, V.; Šver, L.; Jazvinšćak Jembrek, M. Efficacy of Caffeic Acid on Diabetes and Its Complications in the Mouse. Molecules 2021, 26, 3262. [Google Scholar] [CrossRef]
- Ilić, I.; Oršolić, N.; Rođak, E.; Odeh, D.; Lovrić, M.; Mujkić, R.; Delaš Aždajić, M.; Grgić, A.; Tolušić Levak, M.; Vargek, M.; et al. The effect of high-fat diet and 13-cis retinoic acid application on lipid profile, glycemic response and oxidative stress in female Lewis rats. PLoS ONE 2020, 15, e0238600. [Google Scholar] [CrossRef]
- Clinical Laboratory Standards Institute. Procedures for the Collection of Diagnostic Blood Specimens by Venipuncture—CLSI: H3-A6, 6th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2007. [Google Scholar]
- World Health Organization (WHO). Guidelines on Drawing Blood: Best Practices in Phlebotomy; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Balta, V.; Đikić, D.; Crnić, I.; Odeh, D.; Oršolić, N.; Kmetič, I.; Murati, T.; Dragović-Uzelac, V.; Landeka Jurčrvić, I. The effects of four-week intake of blackthorn flower extract on mice tissue antioxidant status and phenolic content. Pol. J. Food Nutr. Sci. 2020, 70, 361–375. [Google Scholar] [CrossRef]
- Oršolić, N.; Nemrava, J.; Jeleč, Ž.; Kukolj, M.; Odeh, D.; Terzić, S.; Fureš, R.; Bagatin, T.; Bagatin, D. The Beneficial Effect of Proanthocyanidins and Icariin on Biochemical Markers of Bone Turnover in Rats. Int. J. Mol. Sci. 2018, 19, 2746. [Google Scholar] [CrossRef]
- Reznick, A.Z.; Packer, L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol. 1994, 233, 357–363. [Google Scholar] [CrossRef]
- Schumann, G.; Klauke, R.; Canalias, F.; Bossert-Reuther, S.; Franck, P.F.; Gella, F.J.; Jørgensen, P.J.; Kang, D.; Lessinger, J.M.; Panteghini, M.; et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 °C. Part 9: Reference procedure for the measurement of catalytic concentration of alkaline phosphatase; International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Scientific Division, Committee on Reference Systems of Enzymes (C-RSE). Clin. Chem. Lab. Med. 2011, 49, 1439–1446. [Google Scholar] [PubMed]
- International Organization for Standardization (ISO). Milk Products—Enumeration of Presumptive Bifidobacteria—Colony Count Technique at 37 Degrees C; ISO 29981:2010; International Organization for Standardization: Geneva, Switzerland, 2010. [Google Scholar]
- International Organization for Standardization (ISO). Milk products —Enumeration of Presumptive Lactobacillus Acidophilus on a Selective Medium —Colony-Count Technique at 37 Degrees C; ISO 20128:2006; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- International Organization for Standardization (ISO). Microbiology of the Food Chain–Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part. 2: Colony-Count Technique; ISO 21528-2:2017; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Juśkiewicz, J.; Zduńczyk, Z.; Wróblewska, M.; Gulewicz, K. Influence of oligosaccharide extracts from pea and lupin seeds on caecal fermentation in rats. J. Anim. Feed Sci. 2003, 12, 289–298. [Google Scholar] [CrossRef][Green Version]
- Ajiboye, J.A.; Erukainure, O.L.; Lawal, B.A.; Nwachukwu, V.A.; Tugbobo-Amisu, A.O.; Okafor, E.N. Comparative alteration in atherogenic indices and hypocholesteremic effect of palm oil and palm oil mill effluent in normal albino rats. Heliyon 2015, 1, e00010. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Wang, S.; Han, R.; Cao, Y.; Hua, W.; Mao, Y.; Zhang, X.; Pang, X.; Wei, C.; et al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010, 4, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Little, M.S.; Pellock, S.J.; Walton, W.G.; Tripathy, A.; Redinbo, M.R. Structural basis for the regulation of β-glucuronidase expression by human gut Enterobacteriaceae. Proc. Nat. Acad. Sci. USA 2017, 115, 152–161. [Google Scholar] [CrossRef]
- Aleksic, V.; Knezevic, P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol. Res. 2014, 169, 240–254. [Google Scholar] [CrossRef]
- O’Connor, K.; Morrissette, M.; Strandwitz, P.; Ghiglieri, M.; Caboni, M.; Liu, H.; Khoo, C.; O’Onofrio, A.; Lewis, K. Cranberry extracts promote growth of Bacteroidaceae and decrease abundance of Enterobacteriaceae in a human gut simulator model. PLoS ONE 2019, 14, e0224836. [Google Scholar] [CrossRef]
- Bennadja, S.; Tlili Ait Kaki, Y.; Djahoudi, A.; Hadef, Y.; Chefrour, A. Antibiotic Activity of the Essential Oil of Laurel (Laurus nobilis L.) on Eight Bacterial Strains. J. Life Sci. 2013, 7, 814–819. [Google Scholar] [CrossRef]
- Mallappa Kumara, S.; Mohd Sayeed, A.; Uma Rani, S. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based Complement. Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef]
- McIntosh, F.M.; Maison, N.; Holtrop, G.; Young, P.; Stevens, V.J.; Ince, J.; Johnstone, A.M.; Lobley, G.E.; Flint, H.J.; Louis, P. Phylogenetic distribution of genes encoding beta-glucuronidase activity inhuman colonic bacteria and the impact of diet on faecal glycosidase activities. Environ. Microbiol. 2012, 14, 1876–1887. [Google Scholar] [CrossRef]
- Flores, R.; Shi, J.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Association of Fecal Microbial Diversity and Taxonomy with Selected Enzymatic Functions. PLoS ONE 2012, 7, e39745. [Google Scholar] [CrossRef]
- Maheux, A.F.; Dion-Dupont, V.; Bouchard, S.; Bisson, M.-A.; Bergeron, M.G.; Rodriguez, M.J. Comparison of four β-glucuronidase and β-galactosidase-based commercial culture methods used to detect Escherichia coli and total coliforms in water. J. Water Health 2015, 13, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Zhu, X.; Liu, G.; Kan, Q.; Chen, T.; Chen, Y.; Cao, Y. Dietary citrus peel essential oil ameliorates hypercholesterolemia and hepatic steatosis by modulating lipid and cholesterol homeostasis. Food Funct. 2020, 11, 7217–7230. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Losa, R.; Zweifel, B.; Wallace, R.J. Sensitivity of pathogenic and commensal bacteria from the human colon to essential oils. Microbiology 2012, 158, 2870–2877. [Google Scholar] [CrossRef]
- Bozkurt, M.; Küçükyilmaz, K.; Uğur Çatli, A.; Özyildiz, Z.; Çinar, M.; Çabuk, M.; Çoven, F. Influences of an essential oil mixture supplementation to cornversuswheat-based practical diets on growth, organ size, intestinal morphology and immune response of male and female broilers. Ital. J. Anim. Sci. 2012, 11, e54. [Google Scholar] [CrossRef]
- Horošová, K.; Bujňáková, D.; Kmet, V. Effect of oregano essential oil on chicken lactobacilli and E. coli. Folia Microbiol. 2006, 51, 278–280. [Google Scholar] [CrossRef]
- Muhl, A.; Liebert, F. Growth and parameters of microflora in intestinal and faecal samples of piglets due to application of a phytogenic feed additive. J. Anim. Physiol. Anim. Nutr. 2007, 91, 411–418. [Google Scholar] [CrossRef]
- Cross, D.E.; McDevitt, R.M.; Hillman, K.; Acamovic, T. The effect of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. Br. Poult. Sci. 2007, 48, 496–506. [Google Scholar] [CrossRef]
- Barone, M.; Lofano, K.; De Tullio, N.; Licino, R.; Albano, F.; Di Leo, A. Dietary, Endocrine, and Metabolic Factors in the Development of Colorectal Cancer. J. Gastrointest. Cancer 2011, 43, 13–19. [Google Scholar] [CrossRef]
- Dashnyam, P.; Mudududdla, R.; Hsieh, T.-J.; Lin, T.-C.; Lin, H.-Y.; Chen, P.-Y.; Hsu, C.-Y.; Lin, C.-H. β-Glucuronidases of opportunistic bacteria are the major contributors to xenobiotic-induced toxicity in the gut. Sci. Rep. 2018, 8, 16372. [Google Scholar] [CrossRef]
- Gloux, K.; Berteau, O.; El Oumami, H.; Beguet, F.; Leclerc, M.; Dore, J. A metagenomic-glucuronidase uncovers a core adaptive function of the human intestinal microbiome. Proc. Nat. Acad. Sci. USA 2010, 108, 4539–4546. [Google Scholar] [CrossRef]
- Pellock, S.J.; Redinbo, M.R. Glucuronides in the gut: Sugar-driven symbioses between microbe and host. J. Biol. Chem. 2017, 292, 8569–8576. [Google Scholar] [CrossRef] [PubMed]
- Awolade, P.; Cele, N.; Kerru, N.; Gummidi, L.; Oluwakemi, E.; Singh, P. Therapeutic significance of β-glucuronidase activity and its inhibitors: A review. Eur. J. Med. Chem. 2019, 187, 111921. [Google Scholar] [CrossRef] [PubMed]
- De Preter, V.; Raemen, H.; Cloetens, L.; Houben, E.; Rutgeerts, P.; Verbeke, K. Effect of dietary intervention with different pre- and probiotics on intestinal bacterial enzyme activities. Eur. J. Clin. Nutr. 2007, 62, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Pool-Zobel, B.; van Loo, J.; Rowland, I.; Roberfroid, M.B. Experimental evidences on the potential of prebiotic fructans to reduce the risk of colon cancer. Br. J. Nutr. 2002, 87, 273–281. [Google Scholar] [CrossRef]
- Nemeth, K.; Plumb, G.W.; Berrin, J.-G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell?—Glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Michlmayr, H.; Kneifel, W. β-Glucosidase activities of lactic acid bacteria: Mechanisms, impact on fermented food and human health. FEMS Microbiol. Lett. 2013, 352, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hijová, E.; Kuzma, J.; Strojný, L.; Bomba, A.; Bertková, I.; Chmelárová, A.; Hertelyová, Z.; Benetinová, V.; Štofilová, J.; Ambro, Ľ. Ability of Lactobacillus plantarum LS/07 to modify intestinal enzymes activity in chronic diseases prevention. Acta Biochim. Pol. 2016, 64, 113–116. [Google Scholar] [CrossRef]
- Kondratyuk, T.P.; Pezzuto, J.M. Natural Product Polyphenols of Relevance to Human Health. Arch. Physiol. Biochem. 2004, 42, 46–63. [Google Scholar] [CrossRef]
- Harassi, Y.; Tilaoui, M.; Idir, A.; Frédéric, J.; Baudino, S.; Ajouaoi, S.; Mouse, H.A.; Zyad, A. Phytochemical analysis, cytotoxic and antioxidant activities of Myrtus communis essential oil from Morocco. J. Complement. Integr. Med. 2019, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bouzabata, A.; Cabral, C.; Gonçalves, M.J.; Cruz, M.T.; Bighelli, A.; Cavaleiro, C.; Casanova, J.; Tomi, F.; Salgueiro, L. Myrtus communis L. as source of a bioactive and safe essential oil. Food Chem. Toxicol. 2015, 75, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.J.; Costa, R.J.O.; Cesário, F.R.A.S.; Rodrigues, L.B.; da Costa, J.G.M.; Coutinho, H.D.M.; Galvao, H.B.F.; de Menezes, I.R.A. Activity of essential oils of Piper aduncum anf and Cinnamomum zeylanicum by evaluating osmotic and morphologic fragility of erythrocytes. Eur. J. Integr. Med. 2016, 8, 505–512. [Google Scholar] [CrossRef]





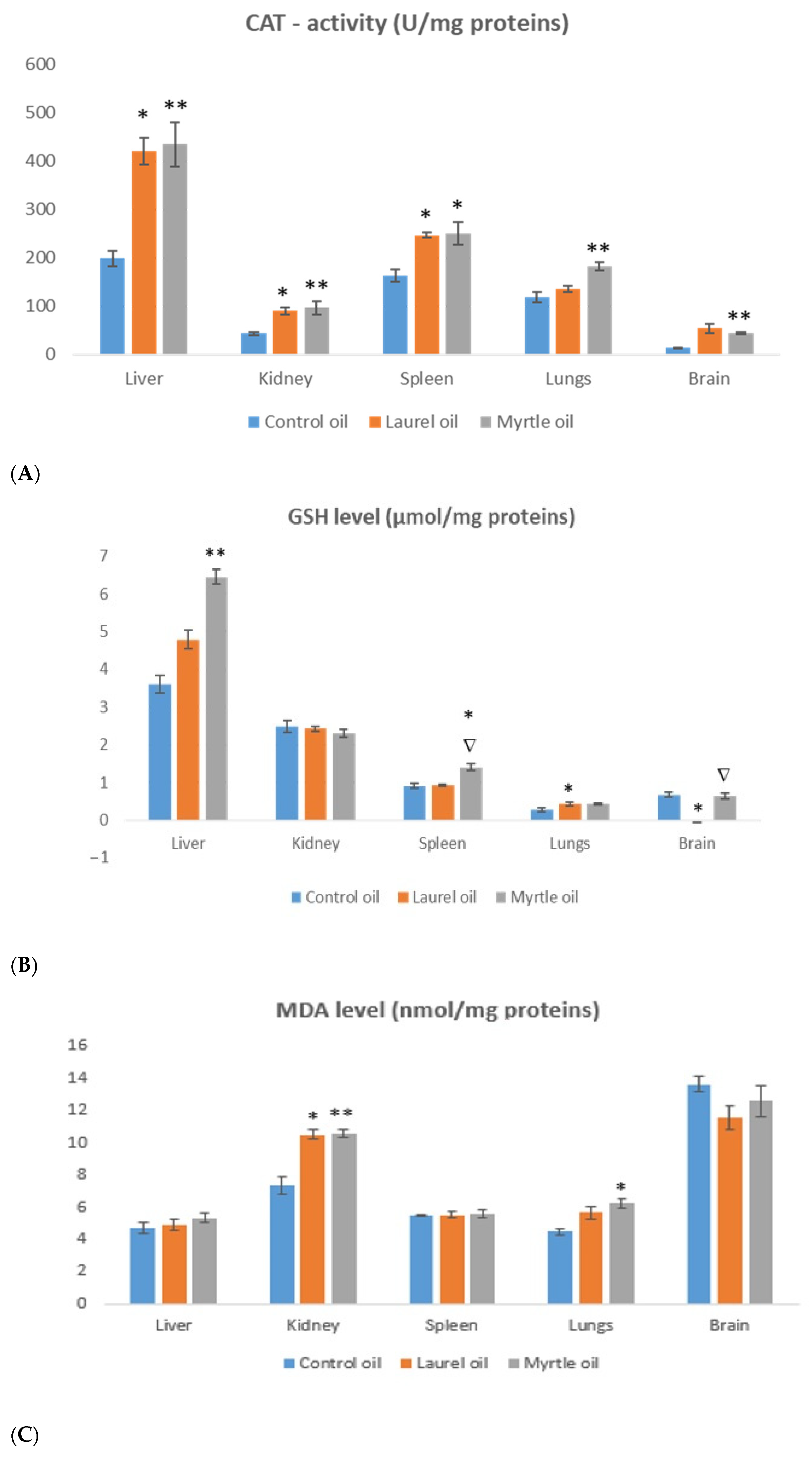
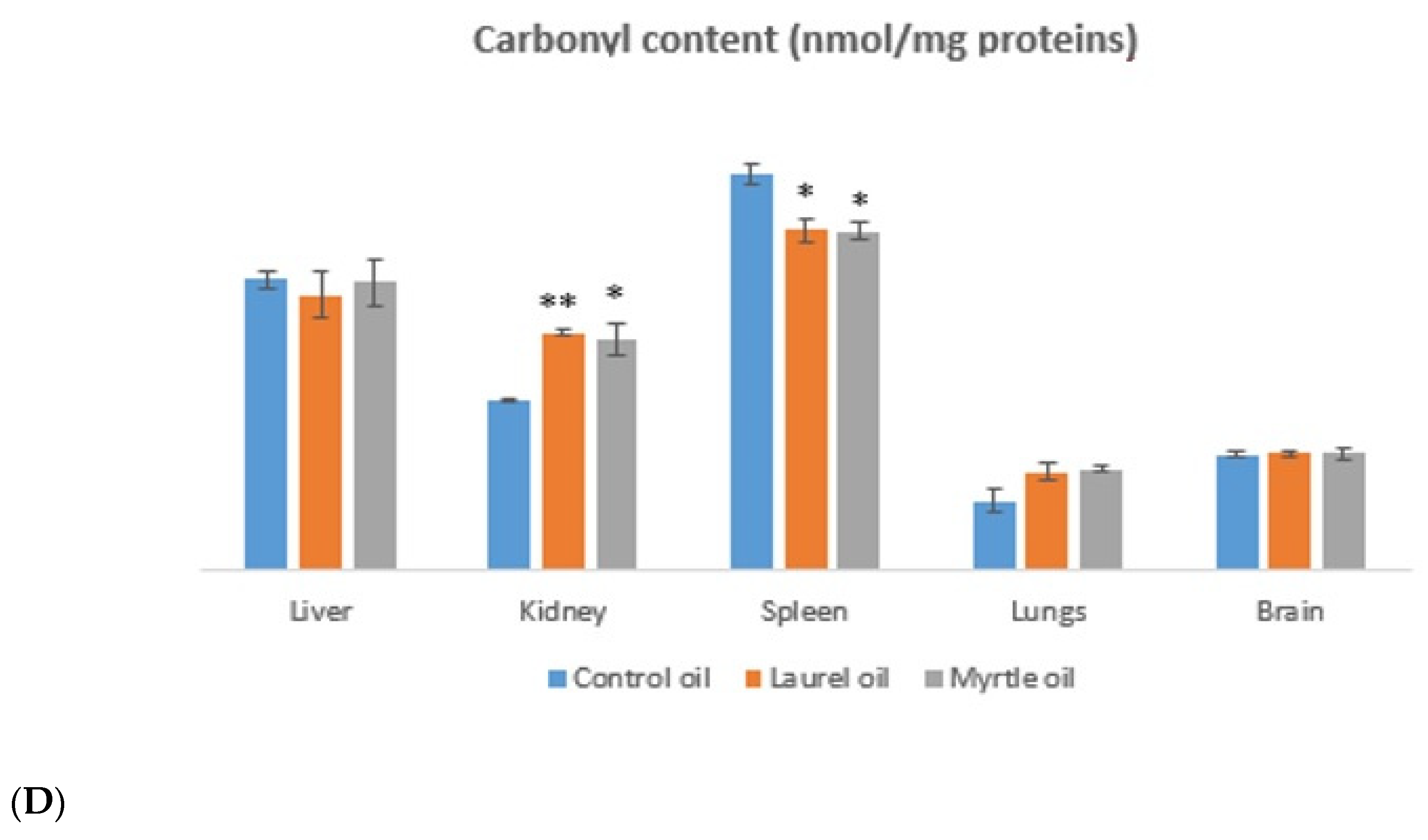

| Essential Oil | Latin Name | Plant Family | Plant Tissues Used |
|---|---|---|---|
| Laurel oil | Laurus nobilis L. | Lauraceae | Leaves |
| Myrtle oil | Myrtus communis L. | Myrtaceae | Leaves |
| Compound | Content in Laurel Essential Oil (mg/mL Oil) | Content in Myrtle Essential Oil (mg/mL Oil) |
|---|---|---|
| α-thujene a | 4.46 ± 0.07 | 0.013 ± 0.002 |
| α-pinene | 61.60 ± 0.90 | 193.75 ± 1.53 |
| Camphene | 11.08 ± 0.27 | 1.08 ± 0.08 |
| Sabinene b | 62.85 ± 1.00 | n.d. |
| β-pinene | 28.18 ± 0.23 | 2.35 ± 0.03 |
| Myrcene | 16.60 ± 0.12 | 2.68 ± 0.01 |
| α-phellandrene | 3.12 ± 0.03 | 1.66 ± 0.01 |
| 3-carene | 1.37 ± 0.01 | 0.48 ± 0.01 |
| α-terpinene | 14.04 ± 0.18 | n.d. |
| p-cymene | 7.03 ± 0.01 | 3.45 ± 0.01 |
| d-limonene | 10.61 ± 0.21 | 69.25 ± 0.71 |
| Eucalyptol | 347.45 ± 6.66 | 244.60 ± 1.63 |
| γ-terpinene | 5.25 ± 0.02 | n.d. |
| cis-sabinene hydrate b | 0.87 ± 0.01 | n.d. |
| α-terpinolene c | 4.17 ± 0.32 | n.d. |
| Linalool | 55.44 ± 0.16 | 19.36 ± 0.08 |
| δ-terpineol d | 6.95 ± 0.05 | n.d. |
| Terpinen-4-ol d | 32.85 ± 0.22 | 31.62 ± 0.20 |
| α-terpineol | 23.08 ± 0.08 | 26.26 ± 0.34 |
| Linalyl acetate e | 4.42 ± 0.08 | n.d. |
| Bornyl acetate d | 8.90 ± 0.03 | n.d. |
| δ-terpinyl acetate d | 8.31 ± 0.11 | n.d. |
| α-terpinyl acetate d | 148.48 ± 0.63 | 4.53 ± 0.05 |
| Eugenol | 14.34 ± 0.17 | n.d. |
| Methyleugenol f | 30.67 ± 0.23 | 9.88 ± 0.07 |
| Camphor | n.d. | 0.56 ± 0.08 |
| Carvone | n.d. | 2.14 ± 0.07 |
| Geraniol | n.d. | 6.21 ± 0.04 |
| Myrtenyl acetate | n.d. | 146.10 ± 0.84 |
| Estragole | n.d. | 0.013 ± 0.003 |
| Geranyl acetate g | n.d. | 20.71 ± 0.08 |
| Myrtenol | n.d. | 3.92 ± 0.04 |
| Treatments a | pH Value (Mean ± SE) | Minimum | Maximum |
|---|---|---|---|
| Control | 7.37 ± 0.17 | 6.84 | 7.7 |
| Laurel | 7.35 ± 0.21 | 6.82 | 7.96 |
| Myrtle | 7.44 ± 0.13 | 6.95 | 7.71 |
| Parameters | Treatments a (X + SEM) | ||
|---|---|---|---|
| Control | Laurel | Myrtle | |
| ALP (U/L) | 44.33 ± 3.60 | 68.00 ± 4.94 *∆ | 45.000 ± 3.596 |
| ALT (U/L) | 44.00 ± 3.48 | 27.33 ± 1.87 * | 28.000 ± 1.317 * |
| AST (U/L) | 92.67 ± 4.90 | 63.00 ± 4.31 * | 61.333 ± 4.638 * |
| Amylase (U/L) | 883.67 ± 43.66 | 1106.33 ± 63.99 | 993.667 ± 33.107 |
| TP (g/L) | 66.33 ± 0.56 | 61.67 ± 1.11 * | 60.333 ± 0.211 ** |
| GLU (mmol/L) | 5.93 ± 0.40 | 5.43 ± 0.27 | 5.700 ± 0.179 |
| UREA (mmol/L) | 4.93 ± 0.24 | 5.67 ± 0.21 | 4.433 ± 0.220 ∇∇ |
| Creatinine (µmol/L) | 39.67 ± 4.89 | 30.67 ± 0.92 | 31.000 ± 0.365 |
| Parameters | Treatments a (X + SEM) | ||
|---|---|---|---|
| Control | Laurel | Myrtle | |
| TC (mmol/L) | 2.33 ± 0.15 | 1.40 ± 0.04 * | 1.47 ± 0.13 * |
| TG (mmol/L) | 1.17 ± 0.20 | 0.93 ± 0.09 | 1.03 ± 0.10 |
| HDL-C (mmol/L) | 0.68 ± 0.04 | 0.57 ± 0.02 | 0.55 ± 0.06 |
| LDL-C (mmol/L) | 0.11 ± 0.01 | 0.07 ± 0.02 * | 0.06 ± 0.00 * |
| VLDL-C (mmol/L) | 0.23 ± 0.04 | 0.18 ± 0.02 | 0.21 ± 0.02 |
| ARI (AC) = ((TC-HDL-C)/HDL-C) | 2.43 ± 0.13 | 0.19 ± 0.02 ***∆∆ | 1.67 ± 0.04 * |
| ARPI-1 (AIP) = (log (TG/HDL-C)) | 0.23 ± 0.04 | 0.212 ± 0.04 | 0.27 ± 0.04 |
| ARPI-2 = (LDL-C/HDL-C) | 0.16 ± 0.03 | 0.122 ± 0.00 | 0.10 ± 0.00 |
| ARPI-3 (CRR) = (TC/HDL-C) | 3.43 ± 0.13 | 2.441 ± 0.09 * | 2.67 ± 0.16 * |
| CPI = HDL-C/LDL-C | 6.18 ± 0.50 | 8.190 ± 0.34 * | 9.71 ± 0.43 * |
| IR = TG/HDL-C | 1.72 ± 0.15 | 1.63 ± 0.07 | 1.88 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odeh, D.; Oršolić, N.; Berendika, M.; Đikić, D.; Domjanić Drozdek, S.; Balbino, S.; Repajić, M.; Dragović-Uzelac, V.; Jurčević, I.L. Antioxidant and Anti-Atherogenic Activities of Essential Oils from Myrtus communis L. and Laurus nobilis L. in Rat. Nutrients 2022, 14, 1465. https://doi.org/10.3390/nu14071465
Odeh D, Oršolić N, Berendika M, Đikić D, Domjanić Drozdek S, Balbino S, Repajić M, Dragović-Uzelac V, Jurčević IL. Antioxidant and Anti-Atherogenic Activities of Essential Oils from Myrtus communis L. and Laurus nobilis L. in Rat. Nutrients. 2022; 14(7):1465. https://doi.org/10.3390/nu14071465
Chicago/Turabian StyleOdeh, Dyana, Nada Oršolić, Marija Berendika, Domagoj Đikić, Sandra Domjanić Drozdek, Sandra Balbino, Maja Repajić, Verica Dragović-Uzelac, and Irena Landeka Jurčević. 2022. "Antioxidant and Anti-Atherogenic Activities of Essential Oils from Myrtus communis L. and Laurus nobilis L. in Rat" Nutrients 14, no. 7: 1465. https://doi.org/10.3390/nu14071465
APA StyleOdeh, D., Oršolić, N., Berendika, M., Đikić, D., Domjanić Drozdek, S., Balbino, S., Repajić, M., Dragović-Uzelac, V., & Jurčević, I. L. (2022). Antioxidant and Anti-Atherogenic Activities of Essential Oils from Myrtus communis L. and Laurus nobilis L. in Rat. Nutrients, 14(7), 1465. https://doi.org/10.3390/nu14071465










