Changes in Kidney Fat upon Dietary-Induced Weight Loss
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Laboratory Methods
2.3. Imaging
2.4. Statistical Analyses
3. Results
3.1. Characteristics of the Study Population at Baseline
3.2. Correlations of Kidney Fat Content with Blood Biomarkers, Blood Pressure, Anthropometrics and Body Fat Depots
3.3. Effects of Weight Loss on Kidney Fat Content, Creatinine, and Glomerular Filtration Rate (GFR)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, G.A.; Heisel, W.E.; Afshin, A.; Jensen, M.D.; Dietz, W.H.; Long, M.; Kushner, R.F.; Daniels, S.R.; Wadden, T.A.; Tsai, A.G.; et al. The Science of Obesity Management: An Endocrine Society Scientific Statement. Endocr. Rev. 2018, 39, 79–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar]
- Alpert, M.A.; Omran, J.; Bostick, B.P. Effects of Obesity on Cardiovascular Hemodynamics, Cardiac Morphology, and Ventricular Function. Curr. Obes. Rep. 2016, 5, 424–434. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metab. Clin. Exp. 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Kim, D.D.; Basu, A. Estimating the Medical Care Costs of Obesity in the United States: Systematic Review, Meta-Analysis, and Empirical Analysis. Value Health 2016, 19, 602–613. [Google Scholar] [CrossRef] [Green Version]
- Tremmel, M.; Gerdtham, U.G.; Nilsson, P.M.; Saha, S. Economic Burden of Obesity: A Systematic Literature Review. Int. J. Environ. Res. Public Health 2017, 14, 435. [Google Scholar] [CrossRef]
- Hamilton, D.; Dee, A.; Perry, I.J. The lifetime costs of overweight and obesity in childhood and adolescence: A systematic review. Obes. Rev. 2018, 19, 452–463. [Google Scholar] [CrossRef]
- Lehnert, T.; Sonntag, D.; Konnopka, A.; Riedel-Heller, S.; König, H.H. Economic costs of overweight and obesity. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 105–115. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Romano, L.; Di Renzo, L.; Di Lorenzo, N.; Cenname, G.; Gualtieri, P. Obesity: A preventable, treatable, but relapsing disease. Nutrition 2020, 71, 110615. [Google Scholar] [CrossRef] [PubMed]
- Britton, K.A.; Fox, C.S. Ectopic fat depots and cardiovascular disease. Circulation 2011, 124, e837–e841. [Google Scholar] [CrossRef] [PubMed]
- Montani, J.P.; Carroll, J.F.; Dwyer, T.M.; Antic, V.; Yang, Z.; Dulloo, A.G. Ectopic fat storage in heart, blood vessels and kidneys in the pathogenesis of cardiovascular diseases. Int. J. Obes. Relat. Metab. Disord. 2004, 28 (Suppl. S4), S58–S65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelicha, H.; Schwarzfuchs, D.; Shelef, I.; Gepner, Y.; Tsaban, G.; Tene, L.; Yaskolka Meir, A.; Bilitzky, A.; Komy, O.; Cohen, N.; et al. Changes of renal sinus fat and renal parenchymal fat during an 18-month randomized weight loss trial. Clin. Nutr. 2018, 37, 1145–1153. [Google Scholar] [CrossRef]
- Bobulescu, I.A.; Lotan, Y.; Zhang, J.; Rosenthal, T.R.; Rogers, J.T.; Adams-Huet, B.; Sakhaee, K.; Moe, O.W. Triglycerides in the human kidney cortex: Relationship with body size. PLoS ONE 2014, 9, e101285. [Google Scholar] [CrossRef] [Green Version]
- Chughtai, H.L.; Morgan, T.M.; Rocco, M.; Stacey, B.; Brinkley, T.E.; Ding, J.; Nicklas, B.; Hamilton, C.; Hundley, W.G. Renal sinus fat and poor blood pressure control in middle-aged and elderly individuals at risk for cardiovascular events. Hypertension 2010, 56, 901–906. [Google Scholar] [CrossRef] [Green Version]
- Foster, M.C.; Hwang, S.J.; Porter, S.A.; Massaro, J.M.; Hoffmann, U.; Fox, C.S. Fatty kidney, hypertension, and chronic kidney disease: The Framingham Heart Study. Hypertension 2011, 58, 784–790. [Google Scholar] [CrossRef] [Green Version]
- Murakami, Y.; Nagatani, Y.; Takahashi, M.; Ikeda, M.; Miyazawa, I.; Morino, K.; Ohkubo, T.; Maegawa, H.; Nitta, N.; Sakai, H.; et al. Renal sinus fat volume on computed tomography in middle-aged patients at risk for cardiovascular disease and its association with coronary artery calcification. Atherosclerosis 2016, 246, 374–381. [Google Scholar] [CrossRef]
- Sijens, P.E.; Edens, M.A.; Bakker, S.J.; Stolk, R.P. MRI-determined fat content of human liver, pancreas and kidney. World J. Gastroenterol. 2010, 16, 1993–1998. [Google Scholar] [CrossRef]
- Hall, J.E.; Brands, M.W.; Henegar, J.R.; Shek, E.W. Abnormal kidney function as a cause and a consequence of obesity hypertension. Clin. Exp. Pharmacol. Physiol. 1998, 25, 58–64. [Google Scholar] [CrossRef]
- Jiang, Y.; Spurny, M.; Schubel, R.; Nonnenmacher, T.; Schlett, C.L.; von Stackelberg, O.; Ulrich, C.M.; Kaaks, R.; Kauczor, H.U.; Kuhn, T.; et al. Changes in Pancreatic Fat Content Following Diet-Induced Weight Loss. Nutrients 2019, 11, 912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schubel, R.; Nattenmuller, J.; Sookthai, D.; Nonnenmacher, T.; Graf, M.E.; Riedl, L.; Schlett, C.L.; von Stackelberg, O.; Johnson, T.; Nabers, D.; et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Schubel, R.; Graf, M.E.; Nattenmuller, J.; Nabers, D.; Sookthai, D.; Gruner, L.F.; Johnson, T.; Schlett, C.L.; von Stackelberg, O.; Kirsten, R.; et al. The effects of intermittent calorie restriction on metabolic health: Rationale and study design of the HELENA Trial. Contemp. Clin. Trials 2016, 51, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I., 3rd; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Friedman, A.N.; Moe, S.; Fadel, W.F.; Inman, M.; Mattar, S.G.; Shihabi, Z.; Quinney, S.K. Predicting the glomerular filtration rate in bariatric surgery patients. Am. J. Nephrol. 2014, 39, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Zhong, X.; Nickel, M.D.; Kannengiesser, S.A.; Dale, B.M.; Kiefer, B.; Bashir, M.R. Liver fat quantification using a multi-step adaptive fitting approach with multi-echo GRE imaging. Magn. Reson. Med. 2014, 72, 1353–1365. [Google Scholar] [CrossRef]
- Idilman, I.S.; Tuzun, A.; Savas, B.; Elhan, A.H.; Celik, A.; Idilman, R.; Karcaaltincaba, M. Quantification of liver, pancreas, kidney, and vertebral body MRI-PDFF in non-alcoholic fatty liver disease. Abdom. Imaging 2015, 40, 1512–1519. [Google Scholar] [CrossRef]
- Kuhn, T.; Nonnenmacher, T.; Sookthai, D.; Schubel, R.; Quintana Pacheco, D.A.; von Stackelberg, O.; Graf, M.E.; Johnson, T.; Schlett, C.L.; Kirsten, R.; et al. Anthropometric and blood parameters for the prediction of NAFLD among overweight and obese adults. BMC Gastroenterol. 2018, 18, 113. [Google Scholar] [CrossRef] [Green Version]
- Schubel, R.; Nonnenmacher, T.; Sookthai, D.; Gonzalez Maldonado, S.; Sowah, S.A.; von Stackelberg, O.; Schlett, C.L.; Grafetstatter, M.; Nabers, D.; Johnson, T.; et al. Similar Weight Loss Induces Greater Improvements in Insulin Sensitivity and Liver Function among Individuals with NAFLD Compared to Individuals without NAFLD. Nutrients 2019, 11, 544. [Google Scholar] [CrossRef] [Green Version]
- Wald, D.; Teucher, B.; Dinkel, J.; Kaaks, R.; Delorme, S.; Boeing, H.; Seidensaal, K.; Meinzer, H.P.; Heimann, T. Automatic quantification of subcutaneous and visceral adipose tissue from whole-body magnetic resonance images suitable for large cohort studies. J. Magn. Reson. Imaging 2012, 36, 1421–1434. [Google Scholar] [CrossRef]
- Pannen, S.T.; Maldonado, S.G.; Nonnenmacher, T.; Sowah, S.A.; Gruner, L.F.; Watzinger, C.; Nischwitz, K.; Ulrich, C.M.; Kaaks, R.; Schubel, R.; et al. Adherence and Dietary Composition during Intermittent vs. Continuous Calorie Restriction: Follow-Up Data from a Randomized Controlled Trial in Adults with Overweight or Obesity. Nutrients 2021, 13, 1195. [Google Scholar] [CrossRef] [PubMed]
- Bakdash, J.Z.; Marusich, L.R. Repeated Measures Correlation. Front. Psychol. 2017, 8, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, O.J. Multiple comparisons among means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]
- Spurny, M.; Jiang, Y.; Sowah, S.A.; Schubel, R.; Nonnenmacher, T.; Bertheau, R.; Kirsten, R.; Johnson, T.; Hillengass, J.; Schlett, C.L.; et al. Changes in Bone Marrow Fat upon Dietary-Induced Weight Loss. Nutrients 2020, 12, 1509. [Google Scholar] [CrossRef] [PubMed]
- Navarro Diaz, M. Consequences of morbid obesity on the kidney. Where are we going? Clin. Kidney J. 2016, 9, 782–787. [Google Scholar] [CrossRef]
- Mende, C.W.; Einhorn, D. Fatty Kidney Disease: A New Renal and Endocrine Clinical Entity? Describing the Role of the Kidney in Obesity, Metabolic Syndrome, and Type 2 Diabetes. Endocr. Pract. 2019, 25, 854–858. [Google Scholar] [CrossRef]
- Giordani, I.; Malandrucco, I.; Donno, S.; Picconi, F.; Di Giacinto, P.; Di Flaviani, A.; Chioma, L.; Frontoni, S. Acute caloric restriction improves glomerular filtration rate in patients with morbid obesity and type 2 diabetes. Diabetes Metab. 2014, 40, 158–160. [Google Scholar] [CrossRef]
- Motie, M.; Evangelista, L.S.; Lombardo, D.; Hoi, J.; Horwich, T.B.; Hamilton, M.; Fonarow, G.C. Effect of weight loss on renal function in overweight and obese patients with heart failure. Diabetes Metab. Syndr. 2017, 11, 95–98. [Google Scholar] [CrossRef] [Green Version]
- von Scholten, B.J.; Davies, M.J.; Persson, F.; Hansen, T.W.; Madsbad, S.; Endahl, L.; Jepsen, C.H.; Rossing, P. Effect of weight reductions on estimated kidney function: Post-hoc analysis of two randomized trials. J. Diabetes Its Complicat. 2017, 31, 1164–1168. [Google Scholar] [CrossRef]
- Friedman, A.N.; Wang, J.; Wahed, A.S.; Docherty, N.G.; Fennern, E.; Pomp, A.; Purnell, J.Q.; le Roux, C.W.; Wolfe, B. The Association between Kidney Disease and Diabetes Remission in Bariatric Surgery Patients with Type 2 Diabetes. Am. J. Kidney Dis. 2019, 74, 761–770. [Google Scholar] [CrossRef]
- Favre, G.; Schiavo, L.; Lemoine, S.; Esnault, V.L.M.; Iannelli, A. Longitudinal assessment of renal function in native kidney after bariatric surgery. Surg. Obes. Relat. Dis. 2018, 14, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Lieske, J.C.; Collazo-Clavell, M.L.; Sarr, M.G.; Rule, A.D.; Bergstralh, E.J.; Kumar, R. Gastric bypass surgery and measured and estimated GFR in women. Am. J. Kidney Dis. 2014, 64, 663–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coupaye, M.; Flamant, M.; Sami, O.; Calabrese, D.; Msika, S.; Bogard, C.; Vidal-Petiot, E.; Ledoux, S. Determinants of Evolution of Glomerular Filtration Rate After Bariatric Surgery: A 1-Year Observational Study. Obes. Surg. 2017, 27, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Ngoh, C.L.Y.; So, J.B.Y.; Tiong, H.Y.; Shabbir, A.; Teo, B.W. Effect of weight loss after bariatric surgery on kidney function in a multiethnic Asian population. Surg. Obes. Relat. Dis. 2016, 12, 600–605. [Google Scholar] [CrossRef]
- Notohamiprodjo, M.; Goepfert, M.; Will, S.; Lorbeer, R.; Schick, F.; Rathmann, W.; Martirosian, P.; Peters, A.; Muller-Peltzer, K.; Helck, A.; et al. Renal and renal sinus fat volumes as quantified by magnetic resonance imaging in subjects with prediabetes, diabetes, and normal glucose tolerance. PLoS ONE 2020, 15, e0216635. [Google Scholar] [CrossRef] [Green Version]
- Mann, J.F.; Yi, Q.L.; Gerstein, H.C. Albuminuria as a predictor of cardiovascular and renal outcomes in people with known atherosclerotic cardiovascular disease. Kidney Int. Suppl. 2004, 66, S59–S62. [Google Scholar] [CrossRef] [Green Version]
- Moisi, M.I.; Bungau, S.G.; Vesa, C.M.; Diaconu, C.C.; Behl, T.; Stoicescu, M.; Toma, M.M.; Bustea, C.; Sava, C.; Popescu, M.I. Framing Cause-Effect Relationship of Acute Coronary Syndrome in Patients with Chronic Kidney Disease. Diagnostics 2021, 11, 1518. [Google Scholar] [CrossRef]
- Hillege, H.L.; Fidler, V.; Diercks, G.F.; van Gilst, W.H.; de Zeeuw, D.; van Veldhuisen, D.J.; Gans, R.O.; Janssen, W.M.; Grobbee, D.E.; de Jong, P.E.; et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002, 106, 1777–1782. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Mann, J.F.; Yi, Q.; Zinman, B.; Dinneen, S.F.; Hoogwerf, B.; Halle, J.P.; Young, J.; Rashkow, A.; Joyce, C.; et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001, 286, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Stefan, N.; Artunc, F.; Heyne, N.; Machann, J.; Schleicher, E.D.; Haring, H.U. Obesity and renal disease: Not all fat is created equal and not all obesity is harmful to the kidneys. Nephrol. Dial. Transpl. 2016, 31, 726–730. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Dekkers, I.A.; Huang, L.; Tao, Q.; Paiman, E.H.M.; Bizino, M.B.; Jazet, I.M.; Lamb, H.J. Renal sinus fat volume in type 2 diabetes mellitus is associated with glycated hemoglobin and metabolic risk factors. J. Diabetes Complicat. 2021, 35, 107973. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Cherney, D.Z.I. The actions of SGLT2 inhibitors on metabolism, renal function and blood pressure. Diabetologia 2018, 61, 2098–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nattenmueller, J.; Hoegenauer, H.; Boehm, J.; Scherer, D.; Paskow, M.; Gigic, B.; Schrotz-King, P.; Grenacher, L.; Ulrich, C.; Kauczor, H.U. CT-based compartmental quantification of adipose tissue versus body metrics in colorectal cancer patients. Eur. Radiol. 2016, 26, 4131–4140. [Google Scholar] [CrossRef] [PubMed]

| Q1 (n = 35) | Q2 (n = 34) | Q3 (n = 35) | Q4 (n = 33) | |
|---|---|---|---|---|
| ≤2% | >2% to ≤4.5% | >4.5% to ≤7.5% | >7.5% | |
| Demographics | ||||
| Women (n (%)) | 19 (54.3) | 14 (41.2) | 18 (51.4) | 17 (51.5) |
| Age (years) | 50.9 ± 6.4 | 50.8 ± 8.4 | 51.2 ± 7.9 | 47.3 ± 8.5 |
| Anthropometrics | ||||
| Waist circumference (cm) | 106.1 ± 12.0 | 104.9 ± 10.7 | 103.1 ± 10.8 | 103.5 ± 12.2 |
| BMI (kg/m2) | 32.2 ± 4.1 | 30.8 ± 3.6 | 31.0 ± 3.4 | 31.6 ± 3.6 |
| Blood pressure | ||||
| Systolic BP (mmHg) | 139.5 ± 11.2 | 132.7 ± 14.2 | 136.5 ± 14.6 | 140.4 ± 22.8 |
| Diastolic BP (mmHg) | 90.0 ± 8.2 | 85.6 ± 8.2 | 87.2 ± 7.8 | 87.0 ± 10.2 |
| Fat depots | ||||
| VAT (L) | 5.3 ± 2.2 | 4.8 ± 2.1 | 4.8 ± 2.0 | 4.7 ± 2.1 |
| SAT (L) | 13.1 ± 4.6 | 11.2 ± 2.9 | 12.1 ± 3.9 | 12.9 ± 4.1 |
| Total kidney fat content (%) | 58.9 ± 18.5 | 52.3 ± 18.4 | 52.0 ± 16.8 | 52.8 ± 13.3 |
| Kidney cortex fat content (%) | 3.6 ± 1.8 | 3.2 ± 1.2 | 2.9 ± 1.2 | 3.4 ± 1.8 |
| Kidney sinus fat content (%) | 55.3 ± 18.3 | 49.2 ± 18.4 | 49.1 ± 16.8 | 49.4 ± 13.2 |
| Liver fat content (%) | 7.1 ± 4.4 | 8.8 ± 7.8 | 7.9 ± 6.5 | 7.4 ± 4.9 |
| Liver function | ||||
| ALT (U/L) | 25.0 ± 7.3 | 30.8 ± 13.9 | 26.6 ± 12.2 | 24.8 ± 9.9 |
| AST (U/L) | 21.7 ± 4.0 | 25.4 ± 6.8 | 22.3 ± 3.9 | 22.4 ± 5.2 |
| GGT (U/L) | 29.4 ± 14.0 | 25.5 ± 16.2 | 29.8 ± 19.7 | 24.3 ± 12.6 |
| Lipid metabolism | ||||
| Triglycerides (mg/dL) | 138.9 ± 65.7 | 135.0 ± 91.8 | 145.3 ± 94.2 | 109.4 ± 55.0 |
| Cholesterol (mg/dL) | 210.3 ± 33.9 | 201.9 ± 36.9 | 214.5 ± 36.5 | 203.3 ± 32.0 |
| HDL (mg/dL) | 53.5 ± 14.8 | 53.1 ± 14.5 | 56.3 ± 13.5 | 53.4 ± 15.3 |
| LDL (mg/dL) | 129.1 ± 26.2 | 120.2 ± 25.7 | 129.2 ± 26.8 | 128.0 ± 27.9 |
| Glucose metabolism | ||||
| Glucose (mg/dL) | 93.5 ± 8.0 | 93.2 ± 7.0 | 94.9 ± 6.9 | 91.9 ± 8.3 |
| Insulin (mU/L) | 14.9 ± 7.8 | 12.0 ± 6.9 | 10.9 ± 5.1 | 11.4 ± 5.6 |
| HbA1c (%) | 5.4 ± 0.4 | 5.5 ± 0.3 | 5.5 ± 0.3 | 5.5 ± 0.3 |
| HOMA-IR | 3.5 ± 1.9 | 2.8 ± 1.8 | 2.6 ± 1.2 | 2.6 ± 1.4 |
| Kidney function | ||||
| Creatinine (mg/dL) | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| GFR (ml/min) | 100.6 ± 7.0 | 99.4 ± 6.4 | 100.2 ± 12.6 | 103.1 ± 8.2 |
| Albumin (g/L) | 44.0 ± 2.0 | 43.7 ± 2.2 | 43.2 ± 2.2 | 43.7 ± 2.4 |
| Inflammation | ||||
| CRP (ng/pL) | 7.0 ± 8.7 | 4.1 ± 5.5 | 3.8 ± 2.8 | 3.9 ± 3.8 |
| IFN-γ (ng/µL) | 16.6 ± 16.1 | 12.9 ± 12.9 | 17.5 ± 16.7 | 11.1 ± 7.8 |
| TNF-α (ng/µL) | 4.3 ± 2.7 | 4.0 ± 2.5 | 5.0 ± 2.6 | 4.2 ± 2.5 |
| IL-6 (ng/µL) | 2.0 ± 1.7 | 1.8 ± 3.5 | 1.3 ± 0.8 | 1.3 ± 1.1 |
| IL-8 (ng/µL) | 10.6 ± 4.4 | 14.2 ± 23.7 | 9.8 ± 4.8 | 10.6 ± 5.3 |
| LDH (U/L) | 197.3 ± 30.1 | 197.4 ± 26.8 | 192.7 ± 31.8 | 200.2 ± 28.3 |
| Adipokines | ||||
| Adiponectin (ng/mL) | 15.6 ± 8.4 | 18.7 ± 11.4 | 16.9 ± 11.4 | 19.9 ± 13.7 |
| Leptin (ng/mL) | 29.2 ± 25.3 | 19.8 ± 20.3 | 21.5 ± 15.2 | 29.7 ± 29.4 |
| Resistin (ng/mL) | 5.7 ± 2.5 | 5.4 ± 2.1 | 5.3 ± 1.5 | 6.2 ± 3.3 |
| Kidney Cortex | Kidney Sinus Fat | Total Kidney Fat | |||||||
|---|---|---|---|---|---|---|---|---|---|
| rrm | p | Adjusted p | rrm | p | Adjusted p | rrm | p | Adjusted p | |
| Anthropometrics | |||||||||
| BMI | 0.03 | 0.66 | 1.00 | 0.35 | <0.0001 * | <0.0001 * | 0.36 | <0.0001 * | <0.0001 * |
| Waist | −0.02 | 0.73 | 1.00 | 0.26 | <0.0001 * | <0.0001 * | 0.26 | <0.0001 * | <0.0001 * |
| Fat depots | |||||||||
| VAT | 0.02 | 0.70 | 1.00 | 0.38 | <0.0001 * | <0.0001 * | 0.39 | <0.0001 * | <0.0001 * |
| SAT | 0.01 | 0.89 | 1.00 | 0.31 | <0.0001 * | <0.0001 * | 0.33 | <0.0001 * | <0.0001 * |
| Liver fat content | 0.00 | 0.96 | 1.00 | 0.32 | <0.0001 * | <0.0001 * | 0.32 | <0.0001 * | <0.0001 * |
| Pancreas fat content | 0.02 | 0.76 | 1.00 | 0.07 | 0.25 | 0.74 | 0.08 | 0.19 | 0.58 |
| Blood pressure | |||||||||
| Systolic BP | 0.05 | 0.41 | 1.00 | 0.07 | 0.26 | 0.78 | 0.08 | 0.20 | 0.60 |
| Diastolic BP | 0.01 | 0.93 | 1.00 | 0.15 | 0.01 * | 0.04 * | 0.16 | 0.01 * | 0.03 * |
| Adipokine | |||||||||
| Leptin | 0.02 | 0.75 | 1.00 | 0.23 | <0.001 * | <0.001 * | 0.24 | <0.0001 * | <0.001 * |
| Adiponectin | −0.01 | 0.88 | 1.00 | 0.02 | 0.79 | 1.00 | 0.03 | 0.76 | 1.00 |
| Resistin | −0.16 | 0.01 * | 0.03 * | 0.01 | 0.84 | 1.00 | −0.02 | 0.80 | 1.00 |
| Glucose metabolism | |||||||||
| HOMA-IR | 0.05 | 0.38 | 1.00 | 0.12 | 0.06 | 0.17 | 0.13 | 0.04 * | 0.11 |
| Insulin | 0.06 | 0.37 | 1.00 | 0.10 | 0.12 | 0.36 | 0.11 | 0.08 | 0.25 |
| Glucose | 0.01 | 0.87 | 1.00 | 0.19 | 0.002 * | 0.01 * | 0.20 | <0.002 * | <0.004 * |
| IGF-1 | 0.02 | 0.70 | 1.00 | −0.11 | 0.07 | 0.21 | −0.12 | 0.06 | 0.18 |
| HbA1c | −0.07 | 0.26 | 0.77 | 0.11 | 0.08 | 0.24 | 0.11 | 0.09 | 0.27 |
| Lipid metabolism | |||||||||
| Triglycerides | 0.00 | 0.97 | 1.00 | 0.13 | 0.04 * | 0.12 | 0.14 | 0.03 * | 0.08 |
| Cholesterol | −0.05 | 0.42 | 1.00 | 0.23 | <0.001 * | <0.001 * | 0.23 | <0.001 * | <0.001 * |
| LDL | −0.08 | 0.21 | 0.64 | 0.16 | 0.01 * | 0.03 * | 0.15 | 0.02 * | 0.06 |
| HDL | −0.01 | 0.85 | 1.00 | 0.18 | <0.003 * | 0.01 * | 0.18 | <0.003 * | 0.01 * |
| Liver function tests | |||||||||
| ALT | 0.04 | 0.55 | 1.00 | 0.05 | 0.42 | 1.00 | 0.07 | 0.24 | 0.72 |
| AST | 0.00 | 0.97 | 1.00 | 0.01 | 0.89 | 1.00 | 0.02 | 0.73 | 1.00 |
| GGT | −0.01 | 0.92 | 1.00 | 0.31 | <0.0001 * | <0.0001 * | 0.32 | <0.0001 * | <0.0001 * |
| Inflammation | |||||||||
| CRP | 0.01 | 0.90 | 1.00 | 0.09 | 0.16 | 0.49 | 0.09 | 0.13 | 0.39 |
| TNF α | −0.02 | 0.77 | 1.00 | −0.03 | 0.68 | 1.00 | −0.03 | 0.66 | 1.00 |
| IFN | −0.04 | 0.50 | 1.00 | −0.02 | 0.76 | 1.00 | −0.02 | 0.69 | 1.00 |
| IL6 | −0.05 | 0.45 | 1.00 | −0.01 | 0.88 | 1.00 | −0.02 | 0.79 | 1.00 |
| IL8 | −0.13 | 0.04 * | 0.11 | 0.00 | 1.00 | 1.00 | −0.01 | 0.88 | 1.00 |
| LDH | −0.01 | 0.92 | 1.00 | 0.21 | 0.02 * | 0.05 * | 0.23 | 0.01 * | 0.03 * |
| Kidney function tests | |||||||||
| Creatinine | −0.07 | 0.25 | 0.76 | −0.05 | 0.38 | 1.00 | −0.08 | 0.22 | 0.66 |
| GFR | 0.06 | 0.33 | 0.99 | 0.03 | 0.58 | 1.00 | 0.05 | 0.42 | 1.00 |
| Albumin | 0.01 | 0.90 | 1.00 | 0.19 | <0.002 * | 0.01 * | 0.20 | <0.001 * | <0.003 * |
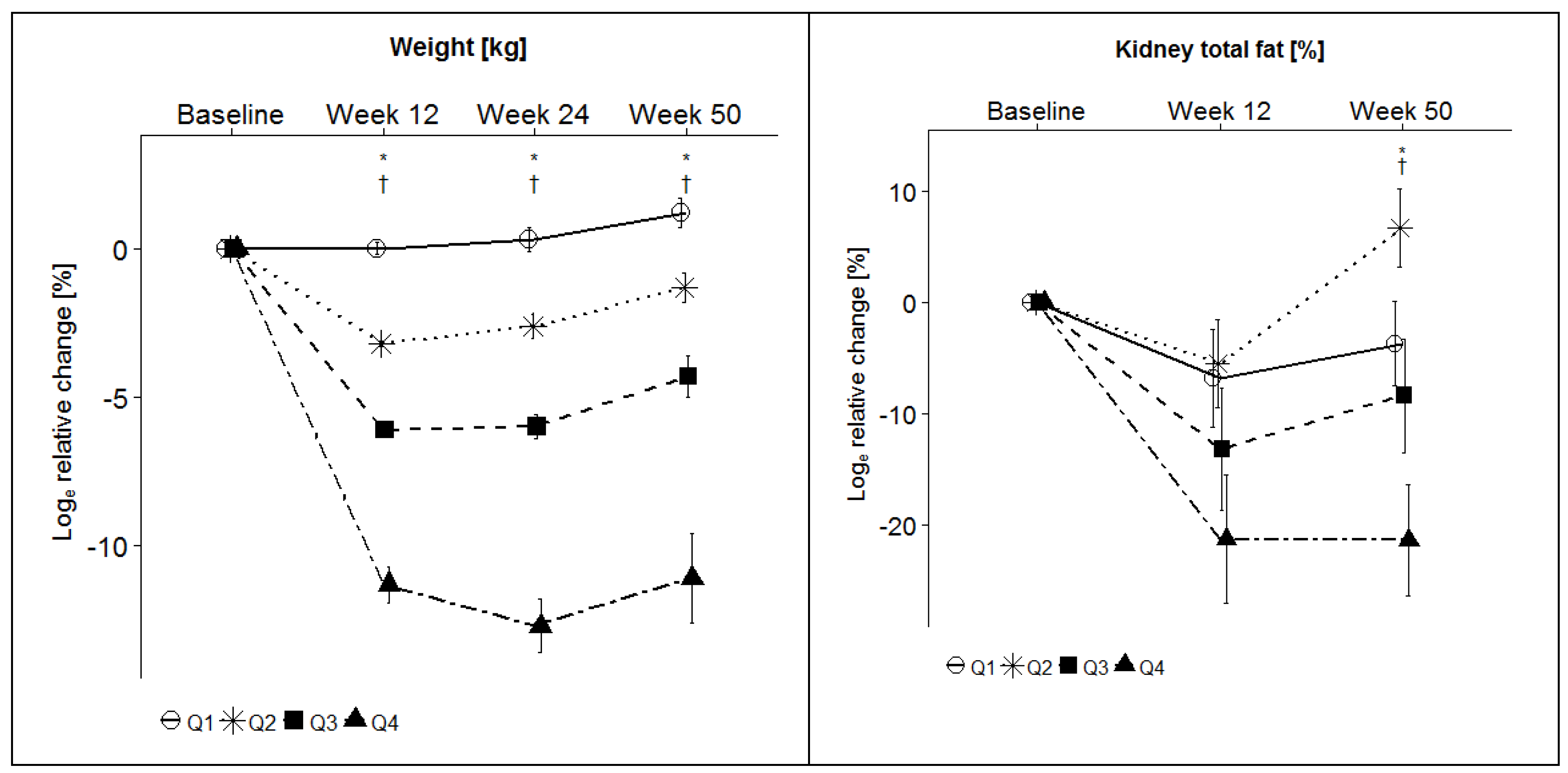
| Baseline | Week 12 | Loge Relative Change | p | Week 50 | Loge Relative Change | p | ||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | (Week 12) | Mean ± SD | (Week 50) | ||||
| Weight (kg) | Q1 | 94.8 ± 15.7 | 94.8 ± 15.6 | 0.0 ± 0.2 | <0.0001 * | 96.1 ± 16.1 | 1.3 ± 0.6 | <0.0001 * |
| Q2 | 93.7 ± 14.4 | 90.7 ± 14.0 | −3.3 ± 0.1 | 93.3 ± 13.9 | −1.3 ± 0.5 | |||
| Q3 | 93.9 ± 15.3 | 88.4 ± 14.4 | −6.1 ± 0.2 | 89.8 ± 15.8 | −4.4 ± 0.8 | |||
| Q4 | 94.7 ± 14.4 | 84.4 ± 12.8 | −11.5 ± 0.6 | 84.8 ± 14.2 | −11.2 ± 1.6 | |||
| Total kidney fat content (%) | Q1 | 58.9 ± 18.5 | 54.9 ± 18.6 | −6.8 ± 4.4 | 0.009 * | 57.4 ± 19.5 | −3.7 ± 3.8 | 0.002 * |
| Q2 | 52.3 ± 18.4 | 49.8 ± 17.1 | −5.5 ± 4.0 | 55.2 ± 17.6 | 6.7 ± 3.5 | |||
| Q3 | 52.0 ± 16.8 | 46.8 ± 16.0 | −13.2 ± 5.5 | 49.2 ± 18.4 | −8.4 ± 5.1 | |||
| Q4 | 52.8 ± 13.3 | 43.7 ± 13.4 | −21.3 ± 5.8 | 45.5 ± 16.5 | −21.4 ± 5.0 | |||
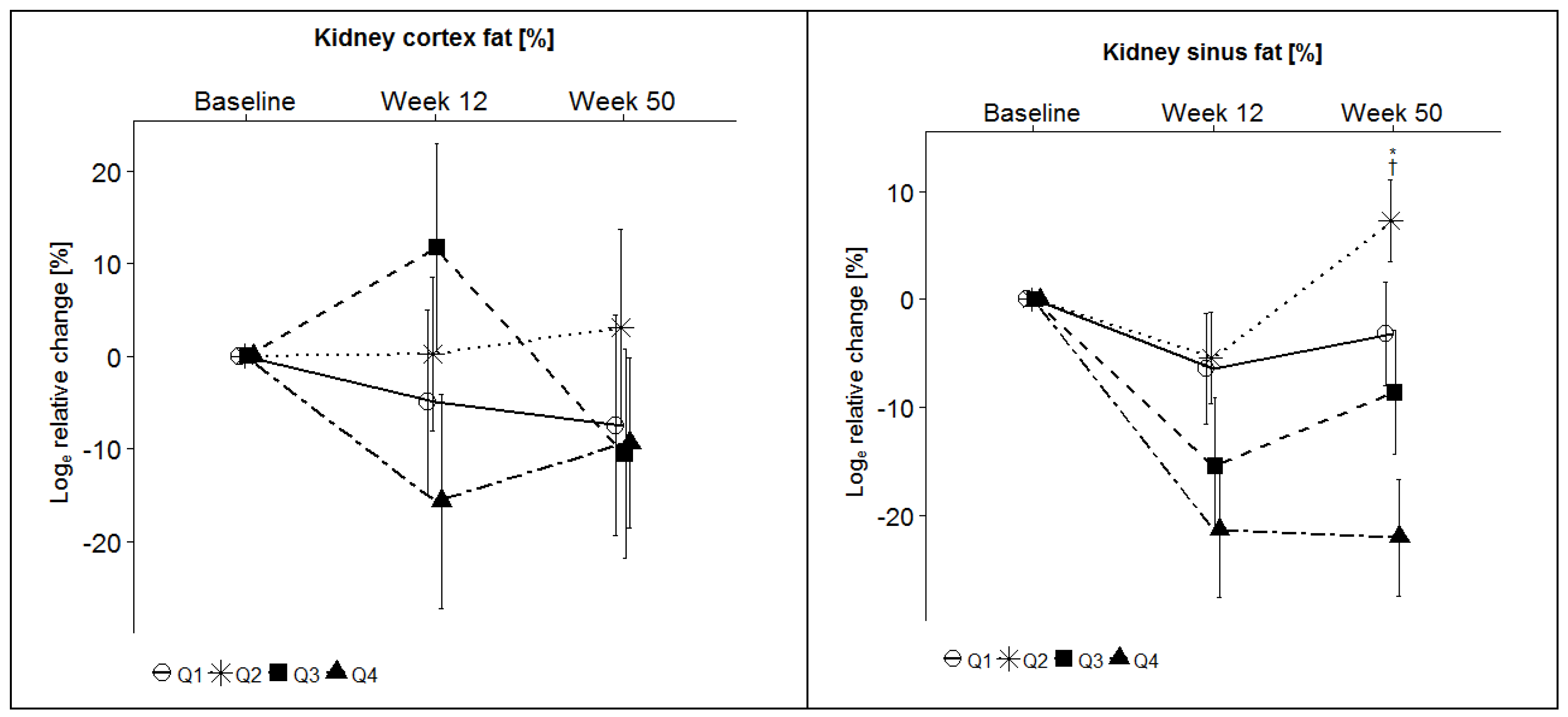
| Kidney cortex fat content (%) | Q1 | 3.6 ± 1.8 | 3.4 ± 1.5 | −5.0 ± 10.0 | 0.97 | 3.5 ± 1.7 | −7.5 ± 11.9 | 0.77 |
| Q2 | 3.2 ± 1.2 | 3.1 ± 1.1 | 0.2 ± 8.3 | 3.3 ± 1.7 | 3.1 ± 10.6 | |||
| Q3 | 2.9 ± 1.2 | 3.3 ± 1.8 | 11.7 ± 11.2 | 2.6 ± 1.1 | −10.5 ± 11.3 | |||
| Q4 | 3.4 ± 1.8 | 2.9 ± 1.8 | −15.7 ± 11.6 | 2.9 ± 1.0 | −9.4 ± 9.2 | |||
| Kidney sinus fat content (%) | Q1 | 55.3 ± 18.3 | 51.5 ± 18.3 | −6.4 ± 5.1 | 0.02 * | 53.9 ± 19.5 | −3.2 ± 4.8 | 0.001 * |
| Q2 | 49.2 ± 18.4 | 46.7 ± 16.9 | −5.4 ± 4.2 | 52.0 ± 17.3 | 7.3 ± 3.8 | |||
| Q3 | 49.1 ± 16.8 | 43.4 ± 16.1 | −15.4 ± 6.3 | 46.5 ± 18.5 | −8.6 ± 5.7 | |||
| Q4 | 49.4 ± 13.2 | 40.7 ± 13.0 | −21.3 ± 6.3 | 42.7 ± 16.1 | −22.0 ± 5.4 | |||
| Creatinine | Q1 | 0.783 ± 0.133 | 0.809 ± 0.136 | 2.8 ± 1.4 | 0.02 * | 0.805 ± 0.131 | 3.0 ± 1.6 | 0.77 |
| Q2 | 0.831 ± 0.125 | 0.84 ± 0.127 | 2.0 ± 1.3 | 0.833 ± 0.134 | −1.1 ± 1.2 | |||
| Q3 | 0.771 ± 0.122 | 0.795 ± 0.108 | 3.4 ± 1.3 | 0.809 ± 0.144 | 4.8 ± 1.4 | |||
| Q4 | 0.789 ± 0.103 | 0.773 ± 0.132 | −2.5 ± 1.8 | 0.815 ± 0.107 | 2.2 ± 2.0 | |||
| GFR | Q1 | 100.6 ± 6.9 | 99.6 ± 7.2 | −1.0 ± 0.5 | 0.04 * | 99.6 ± 6.7 | −1.0 ± 0.6 | 0.65 |
| Q2 | 99.2 ± 6.2 | 98.5 ± 5.9 | −0.8 ± 0.5 | 99.8 ±7.1 | 0.8 ± 0.7 | |||
| Q3 | 100.3 ± 12.4 | 99.1 ± 10.1 | −0.8 ± 0.8 | 97.9 ± 12.3 | −2.1 ± 0.7 | |||
| Q4 | 103.1 ± 8.0 | 104.3 ± 8.7 | 1.1 ± 0.7 | 102.3 ± 7.3 | −0.8 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spurny, M.; Jiang, Y.; Sowah, S.A.; Nonnenmacher, T.; Schübel, R.; Kirsten, R.; Johnson, T.; von Stackelberg, O.; Ulrich, C.M.; Kaaks, R.; et al. Changes in Kidney Fat upon Dietary-Induced Weight Loss. Nutrients 2022, 14, 1437. https://doi.org/10.3390/nu14071437
Spurny M, Jiang Y, Sowah SA, Nonnenmacher T, Schübel R, Kirsten R, Johnson T, von Stackelberg O, Ulrich CM, Kaaks R, et al. Changes in Kidney Fat upon Dietary-Induced Weight Loss. Nutrients. 2022; 14(7):1437. https://doi.org/10.3390/nu14071437
Chicago/Turabian StyleSpurny, Manuela, Yixin Jiang, Solomon A. Sowah, Tobias Nonnenmacher, Ruth Schübel, Romy Kirsten, Theron Johnson, Oyunbileg von Stackelberg, Cornelia M. Ulrich, Rudolf Kaaks, and et al. 2022. "Changes in Kidney Fat upon Dietary-Induced Weight Loss" Nutrients 14, no. 7: 1437. https://doi.org/10.3390/nu14071437
APA StyleSpurny, M., Jiang, Y., Sowah, S. A., Nonnenmacher, T., Schübel, R., Kirsten, R., Johnson, T., von Stackelberg, O., Ulrich, C. M., Kaaks, R., Kauczor, H.-U., Kühn, T., & Nattenmüller, J. (2022). Changes in Kidney Fat upon Dietary-Induced Weight Loss. Nutrients, 14(7), 1437. https://doi.org/10.3390/nu14071437







