Resveratrol for Weight Loss in Obesity: An Assessment of Randomized Control Trial Designs in ClinicalTrials.gov
Abstract
1. Introduction
2. Materials and Methods
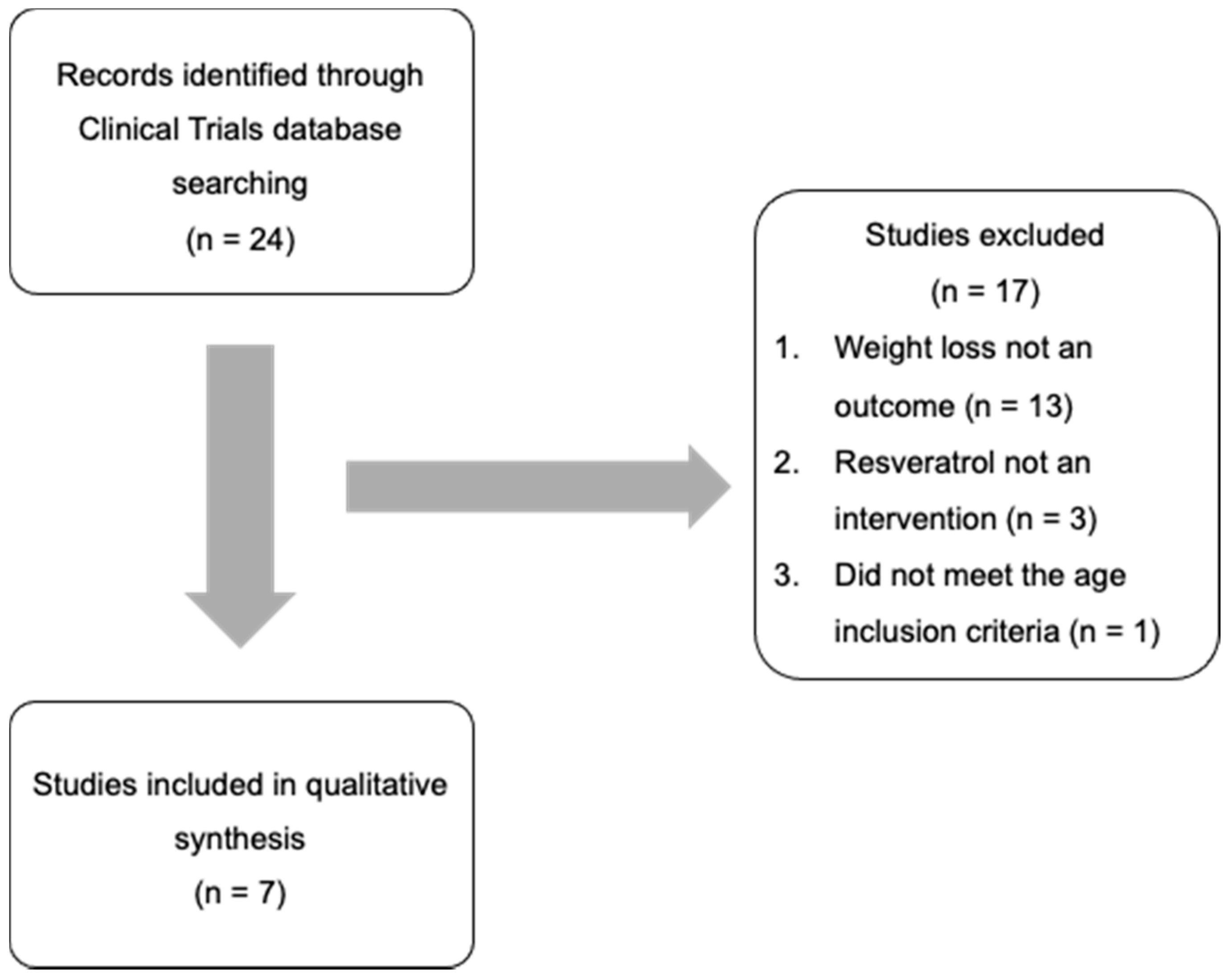
2.1. RCT Identification, Screening, and Eligibility
2.2. Comparison of Trial Elements
2.3. Comparison of ClinicalTrials.gov Trial Elements versus Those Included in the Most Recent Published Meta-Analysis
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.-A.; Rezaei-Tavirani, M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef] [PubMed]
- Craveiro, M.; Cretenet, G.; Mongellaz, C.; Matias, M.I.; Caron, O.; de Lima, M.C.P.; Zimmermann, V.S.; Solary, E.; Dardalhon, V.; Dulić, V.; et al. Resveratrol stimulates the metabolic reprogramming of human CD4+ T cells to enhance effector function. Sci. Signal. 2017, 10, eaal3024. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.-Y.; Tain, Y.-L.; Yu, H.-R.; Huang, L.-T. The Effects of Resveratrol in the Treatment of Metabolic Syndrome. Int. J. Mol. Sci. 2019, 20, 535. [Google Scholar] [CrossRef] [PubMed]
- Dyck, G.J.B.; Raj, P.; Zieroth, S.; Dyck, J.R.B.; Ezekowitz, J.A. The Effects of Resveratrol in Patients with Cardiovascular Disease and Heart Failure: A Narrative Review. Int. J. Mol. Sci. 2019, 20, 904. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-D.; Shi, G.; Jiang, Y.; Yao, C.; Zhu, C. A review on the potential of Resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed. Pharmacother. 2020, 125, 109767. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xiao, X.; Zhang, Q.; Zheng, J.; Deng, M. Deciphering the Anti-obesity Benefits of Resveratrol: The “Gut Microbiota-Adipose Tissue” Axis. Front. Endocrinol. 2019, 10, 413. [Google Scholar] [CrossRef] [PubMed]
- Arzola-Paniagua, M.A.; Garcia-Salgado Lopez, E.R.; Calvo-Vargas, C.G.; Guevara-Cruz, M. Efficacy of an orlistat-resveratrol combination for weight loss in subjects with obesity: A randomized controlled trial. Obesity 2016, 24, 1454–1463. [Google Scholar] [CrossRef]
- Faghihzadeh, F.; Adibi, P.; Rafiei, R.; Hekmatdoost, A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr. Res. 2014, 34, 837–843. [Google Scholar] [CrossRef]
- Mendez-del Villar, M.; Gonzalez-Ortiz, M.; Martinez-Abundis, E.; Perez-Rubio, K.G.; Lizarraga-Valdez, R. Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab. Syndr. Relat. Disord. 2014, 12, 497–501. [Google Scholar] [CrossRef]
- Most, J.; Timmers, S.; Warnke, I.; Jocken, J.W.; van Boekschoten, M.; de Groot, P.; Bendik, I.; Schrauwen, P.; Goossens, G.H.; Blaak, E.E. Combined epigallocatechin-3-gallate and resveratrol supplementation for 12 wk increases mitochondrial capacity and fat oxidation, but not insulin sensitivity, in obese humans: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 215–227. [Google Scholar] [CrossRef]
- Tabrizi, R.; Tamtaji, O.R.; Lankarani, K.B.; Akbari, M.; Dadgostar, E.; Dabbaghmanesh, M.H.; Kolahdooz, F.; Shamshirian, A.; Momen-Heravi, M.; Asemi, Z. The effects of resveratrol intake on weight loss: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Batista-Jorge, G.C.; Barcala-Jorge, A.S.; Silveira, M.F.; Lelis, D.F.; Andrade, J.M.O.; de Paula, A.M.B.; Guimarães, A.L.S.; Santos, S.H.S. Oral resveratrol supplementation improves Metabolic Syndrome features in obese patients submitted to a lifestyle-changing program. Life Sci. 2020, 256, 117962. [Google Scholar] [CrossRef] [PubMed]
- Chachay, V.S.; Macdonald, G.A.; Martin, J.H.; Whitehead, J.P.; O’Moore–Sullivan, T.M.; Lee, P.; Franklin, M.; Klein, K.; Taylor, P.J.; Ferguson, M.; et al. Resveratrol Does Not Benefit Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2014, 12, 2092–2103.e2096. [Google Scholar] [CrossRef] [PubMed]
- de Ligt, M.; Bergman, M.; Fuentes, R.M.; Essers, H.; Moonen-Kornips, E.; Havekes, B.; Schrauwen-Hinderling, V.B.; Schrauwen, P. No effect of resveratrol supplementation after 6 months on insulin sensitivity in overweight adults: A randomized trial. Am. J. Clin. Nutr. 2020, 112, 1029–1038. [Google Scholar] [CrossRef]
- Delpino, F.M.; Figueiredo, L.M.; Caputo, E.L.; Mintem, G.C.; Gigante, D.P. What is the effect of resveratrol on obesity? A systematic review and meta-analysis. Clin. Nutr. ESPEN 2021, 41, 59–67. [Google Scholar] [CrossRef]
- Heeboll, S.; Kreuzfeldt, M.; Hamilton-Dutoit, S.; Kjaer Poulsen, M.; Stodkilde-Jorgensen, H.; Moller, H.J.; Jessen, N.; Thorsen, K.; Kristina Hellberg, Y.; Bonlokke Pedersen, S.; et al. Placebo-controlled, randomised clinical trial: High-dose resveratrol treatment for non-alcoholic fatty liver disease. Scand. J. Gastroenterol. 2016, 51, 456–464. [Google Scholar] [CrossRef]
- Konings, E.; Timmers, S.; Boekschoten, M.V.; Goossens, G.H.; Jocken, J.W.; Afman, L.A.; Muller, M.; Schrauwen, P.; Mariman, E.C.; Blaak, E.E. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int. J. Obes. 2014, 38, 470–473. [Google Scholar] [CrossRef]
- Poulsen, M.M.; Vestergaard, P.F.; Clasen, B.F.; Radko, Y.; Christensen, L.P.; Stodkilde-Jorgensen, H.; Moller, N.; Jessen, N.; Pedersen, S.B.; Jorgensen, J.O. High-dose resveratrol supplementation in obese men: An investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes 2013, 62, 1186–1195. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- González-Muniesa, P.; Mártinez-González, M.-A.; Hu, F.B.; Després, J.-P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Primers 2017, 3, 17034. [Google Scholar] [CrossRef]
- Button, K.S.; Ioannidis, J.P.; Mokrysz, C.; Nosek, B.A.; Flint, J.; Robinson, E.S.; Munafo, M.R. Power failure: Why small sample size undermines the reliability of neuroscience. Nat. Rev. NeuroSci. 2013, 14, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Gambineri, A.; Pelusi, C. Sex hormones, obesity and type 2 diabetes: Is there a link? Endocr. Connect. 2019, 8, R1–R9. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.M.; Jones, T.H. Testosterone and obesity. Obes. Rev. 2015, 16, 581–606. [Google Scholar] [CrossRef] [PubMed]
- Lizcano, F.; Guzmán, G. Estrogen Deficiency and the Origin of Obesity during Menopause. Biomed. Res. Int. 2014, 2014, 757461. [Google Scholar] [CrossRef]
- Salinero, A.E.; Anderson, B.M.; Zuloaga, K.L. Sex differences in the metabolic effects of diet-induced obesity vary by age of onset. Int. J. Obes. 2018, 42, 1088–1091. [Google Scholar] [CrossRef]
- Zore, T.; Palafox, M.; Reue, K. Sex differences in obesity, lipid metabolism, and inflammation-A role for the sex chromosomes? Mol. Metab. 2018, 15, 35–44. [Google Scholar] [CrossRef]
- Chow, H.H.; Garland, L.L.; Heckman-Stoddard, B.M.; Hsu, C.H.; Butler, V.D.; Cordova, C.A.; Chew, W.M.; Cornelison, T.L. A pilot clinical study of resveratrol in postmenopausal women with high body mass index: Effects on systemic sex steroid hormones. J. Transl. Med. 2014, 12, 223. [Google Scholar] [CrossRef]
- American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Obesity Expert Panel. Expert panel report: Guidelines (2013) for the management of overweight and obesity in adults. Obesity 2014, 22, S41–S410. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Consultation, W.H.O.E. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Jih, J.; Mukherjea, A.; Vittinghoff, E.; Nguyen, T.T.; Tsoh, J.Y.; Fukuoka, Y.; Bender, M.S.; Tseng, W.; Kanaya, A.M. Using appropriate body mass index cut points for overweight and obesity among Asian Americans. Prev. Med. 2014, 65, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shiwaku, K.; Anuurad, E.; Enkhmaa, B.; Nogi, A.; Kitajima, K.; Shimono, K.; Yamane, Y.; Oyunsuren, T. Overweight Japanese with body mass indexes of 23.0–24.9 have higher risks for obesity-associated disorders: A comparison of Japanese and Mongolians. Int. J. Obes. 2004, 28, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Safety of synthetic trans-resveratrol as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2016, 14, 4368. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Dudley, J.I.; Das, D.K. Dose-dependency of resveratrol in providing health benefits. Dose Response 2010, 8, 478–500. [Google Scholar] [CrossRef] [PubMed]
- van der Made, S.M.; Plat, J.; Mensink, R.P. Resveratrol Does Not Influence Metabolic Risk Markers Related to Cardiovascular Health in Overweight and Slightly Obese Subjects: A Randomized, Placebo-Controlled Crossover Trial. PLoS ONE 2015, 10, e0118393. [Google Scholar] [CrossRef]
- Sakpal, T.V. Sample size estimation in clinical trial. Perspect. Clin. Res. 2010, 1, 67–69. [Google Scholar]
- Ponzo, V.; Soldati, L.; Bo, S. Resveratrol: A supplementation for men or for mice? J. Transl. Med. 2014, 12, 158. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Gugleva, V.; Zasheva, S.; Hristova, M.; Andonova, V. Topical use of resveratrol: Technological aspects. Pharmacia 2020, 67. [Google Scholar] [CrossRef]
- Huang, H.; Chen, G.; Liao, D.; Zhu, Y.; Pu, R.; Xue, X. The effects of resveratrol intervention on risk markers of cardiovascular health in overweight and obese subjects: A pooled analysis of randomized controlled trials. Obes. Rev. 2016, 17, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Conte, C.; Fontana, L.; Mittendorfer, B.; Imai, S.-i.; Schechtman, K.B.; Gu, C.; Kunz, I.; Fanelli, F.R.; Patterson, B.W.; et al. Resveratrol Supplementation Does Not Improve Metabolic Function in Nonobese Women with Normal Glucose Tolerance. Cell Metab. 2012, 16, 658–664. [Google Scholar] [CrossRef] [PubMed]
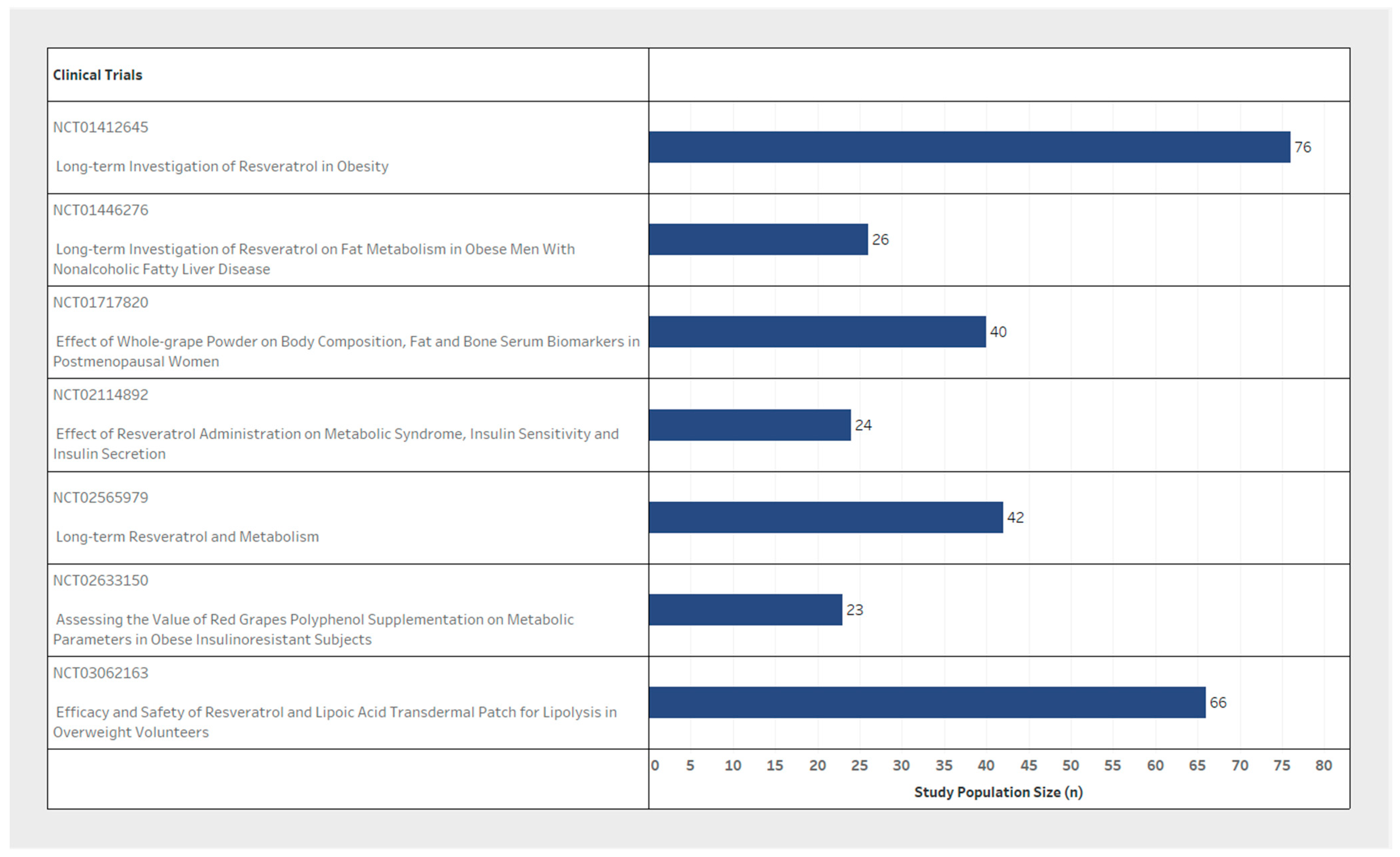
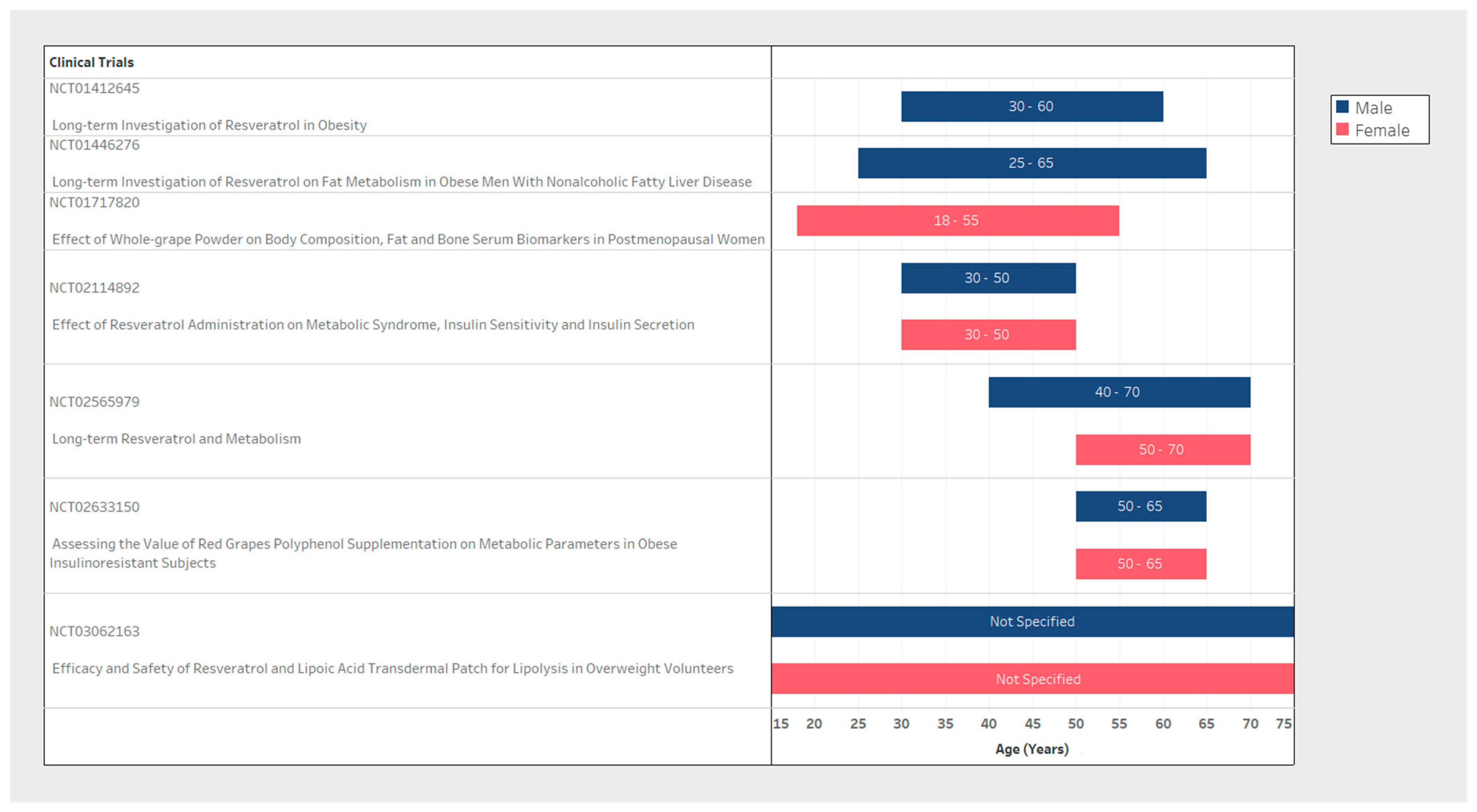
| NCT Number | n (Study Population Size) | Inclusion Criteria | Exclusion Criteria | Resveratrol | Weight Loss Measure | Biomarkers Tested (Related to Weight Loss) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Age Range (Years) | BMI (kg/m2) | Population | Population | Resveratrol Dosage (per Day) | Interval | Outcome Measures | Type of Weight Loss Measures | Timing/Interval of Measures | |||
| NCT01412645 | 76 | Male | 30–60 | Not Specified | MetS | T2DM/Chronic Condition/Malignancy | Resveratrol 1000 mg/Resveratrol 150 mg | 2 times/day for 120 days | Other: Body composition/Biomarkers | DXA/MR | 16 weeks | General: Inflammatory Biomarkers/Fat- and sugar-metabolism/Bone-metabolism. |
| NCT01446276 | 26 | Male | 25–65 | >28 | NAFLD | Malignancy | Resveratrol 1500 mg | 3 times/day for 180 days | Secondary: Body composition Other: Lipid turnover/Liver fat content/Lipase activity and fat cell size | DXA/MR | 24 weeks | Specific: Hepatic VLDL-TG secretion and peripheral VLDL-TG clearance/Basal and insulin stimulated free fatty acid and glucose turnover/VLDL-TG oxidation |
| NCT01717820 | 40 | Female | 18-55 | Not Specified | PMW | Whole grape powder 46 g | 2 times/day for 84 days | Primary: Body composition Secondary: Adipose metabolism | Anthropometric measurements/DXA | 12 weeks | ||
| NCT02633150 | 23 | Female & Male | 50–65 | >30 | PMW | T2DM/Chronic Condition | Supplementation with red grapes polyphenol (Volume not specified) | 56 days | Others: Body composition/Biomarkers | Anthropometric measurements/DXA | 8 weeks | Specific: Adipokines sensitivity/Clamp hyperinsulinemic-euglycemic/Lipopolysaccharides/Mitochondrial activity or cell characterization (adipocyte precursor and muscle) |
| NCT02114892 | 24 | Female & Male | 30–50 | <39.9 | MetS | Chronic Condition/Pregnancy | Resveratrol 1500 mg | 3 times/day for 90 days | Primary: Waist circumference/Biomarkers Secondary: Weight/BMI/Biomarkers | Anthropometric measurements | 12 weeks | Specific: Triglycerides levels/High density lipoprotein levels/Low density lipoproteins |
| NCT03062163 | 66 | Female & Male | Not Specified | >23 | Dermal Patch loaded with resveratrol (Volume not specified) | 14 days | Primary: Fat thickness | Ultrasound | 2 weeks | |||
| NCT02565979 | 42 | Female & Male | 50–70/ 40–70 | 27–35 | PMW | T2DM/Hypertension/Chronic Condition | Resveratrol 150 mg | 2 times/day for 180 days | Primary: Body composition/Biomarkers | DXA | 24 weeks | General: Glucose Tolerance/Intra-hepatic lipid content/Blood plasma markers |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hillsley, A.; Chin, V.; Li, A.; McLachlan, C.S. Resveratrol for Weight Loss in Obesity: An Assessment of Randomized Control Trial Designs in ClinicalTrials.gov. Nutrients 2022, 14, 1424. https://doi.org/10.3390/nu14071424
Hillsley A, Chin V, Li A, McLachlan CS. Resveratrol for Weight Loss in Obesity: An Assessment of Randomized Control Trial Designs in ClinicalTrials.gov. Nutrients. 2022; 14(7):1424. https://doi.org/10.3390/nu14071424
Chicago/Turabian StyleHillsley, Ashley, Vanessa Chin, Amy Li, and Craig S. McLachlan. 2022. "Resveratrol for Weight Loss in Obesity: An Assessment of Randomized Control Trial Designs in ClinicalTrials.gov" Nutrients 14, no. 7: 1424. https://doi.org/10.3390/nu14071424
APA StyleHillsley, A., Chin, V., Li, A., & McLachlan, C. S. (2022). Resveratrol for Weight Loss in Obesity: An Assessment of Randomized Control Trial Designs in ClinicalTrials.gov. Nutrients, 14(7), 1424. https://doi.org/10.3390/nu14071424







