Non-Pharmacological Treatments for Insulin Resistance: Effective Intervention of Plant-Based Diets—A Critical Review
Abstract
:1. Introduction
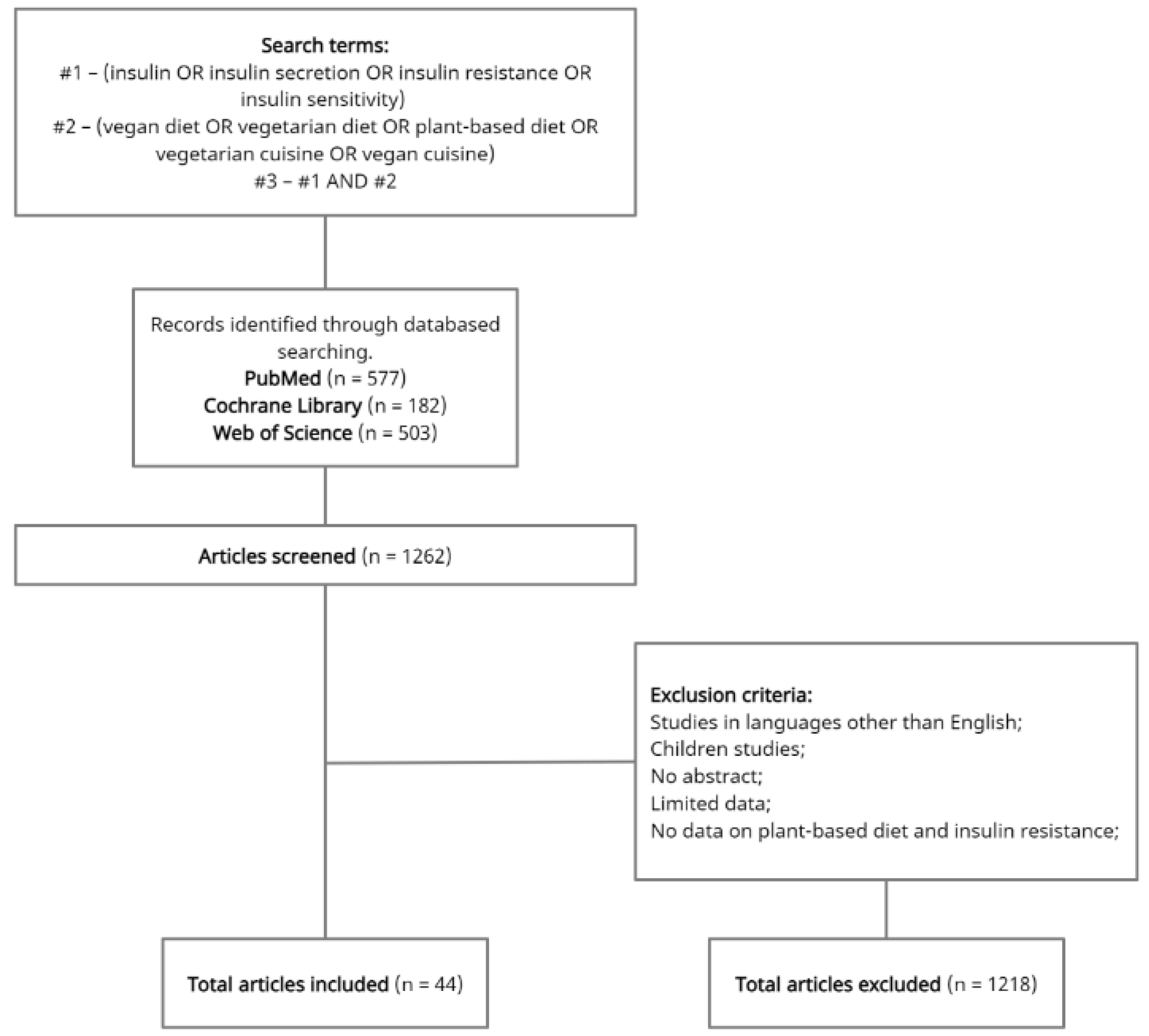
2. Materials and Methods
3. Plant-Based Diets
4. Impact of a Vegetarian Diet on Insulin Resistance
5. Impact of a Vegan Diet on Insulin Resistance
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wilcox, G. Insulin and Insulin Resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar] [PubMed]
- Reaven, G. The Metabolic Syndrome or the Insulin Resistance Syndrome? Different Names, Different Concepts, and Different Goals. Endocrinol. Metab. Clin. N. Am. 2004, 33, 283–303. [Google Scholar] [CrossRef] [PubMed]
- Cefalu, W.T. Insulin Resistance: Cellular and Clinical Concepts. Exp. Biol. Med. 2001, 226, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.E.; Walker, M. Genetics of Insulin Resistance and the Metabolic Syndrome. Curr. Cardiol. Rep. 2016, 18, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Freeman, A.M.; Pennings, N. Insulin Resistance; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Pacini, G.; Mari, A. Methods for Clinical Assessment of Insulin Sensitivity and Beta-Cell Function. Best Pract. Res. Clin. Endocrinol. Metab. 2003, 17, 305–322. [Google Scholar] [CrossRef]
- Gołąbek, K.; Regulska-Ilow, B. Dietary Support in Insulin Resistance: An Overview of Current Scientific Reports. Adv. Clin. Exp. Med. 2019, 28, 1577–1585. [Google Scholar] [CrossRef]
- Mirabelli, M.; Russo, D.; Brunetti, A. The Role of Diet on Insulin Sensitivity. Nutrients 2020, 12, 3042. [Google Scholar] [CrossRef]
- Leitzmann, C. Vegetarian Nutrition: Past, Present, Future. Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 496S–502S. [Google Scholar] [CrossRef] [Green Version]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef]
- Dinu, M.; Abbate, R.; Gensini, G.F.; Casini, A.; Sofi, F. Vegetarian, Vegan Diets and Multiple Health Outcomes: A Systematic Review with Meta-Analysis of Observational Studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3640–3649. [Google Scholar] [CrossRef]
- Bradbury, K.E.; Crowe, F.L.; Appleby, P.N.; Schmidt, J.A.; Travis, R.C.; Key, T.J. Serum Concentrations of Cholesterol, Apolipoprotein A-I and Apolipoprotein B in a Total of 1694 Meat-Eaters, Fish-Eaters, Vegetarians and Vegans. Eur. J. Clin. Nutr. 2014, 68, 178–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robberecht, H.; De Bruyne, T.; Hermans, N. Effect of Various Diets on Biomarkers of the Metabolic Syndrome. Int. J. Food Sci. Nutr. 2017, 68, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Reginster, J.-Y. The Effects of Calorie Restriction, Intermittent Fasting and Vegetarian Diets on Bone Health. Aging Clin. Exp. Res. 2019, 31, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, H.; Bozidis, P.; Touzios, C.; Kolios, D.; Athanasiou, G.; Athanasopoulou, E.; Gerou, I.; Gartzonika, C. Nutritional Status and the Influence of the Vegan Diet on the Gut Microbiota and Human Health. Medicina 2020, 56, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrone, G.; Guerriero, C.; Palazzetti, D.; Lido, P.; Marolla, A.; Di Daniele, F.; Noce, A. Vegan Diet Health Benefits in Metabolic Syndrome. Nutrients 2021, 13, 817. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Pinho, J.; Borges, C.; Teixeira Santos, M.; Santos, A.; Graça, P. Guidelines for a Healthy Vegetarian Diet; National Programme for the Promotion of Healthy Eating: Lisbon, Portugal, 2015. [Google Scholar]
- Le, L.T.; Sabaté, J. Beyond Meatless, the Health Effects of Vegan Diets: Findings from the Adventist Cohorts. Nutrients 2014, 6, 2131–2147. [Google Scholar] [CrossRef] [Green Version]
- Orlich, M.J.; Singh, P.N.; Sabaté, J.; Jaceldo-Siegl, K.; Fan, J.; Knutsen, S.; Beeson, W.L.; Fraser, G.E. Vegetarian Dietary Patterns and Mortality in Adventist Health Study 2. JAMA Intern. Med. 2013, 173, 1230–1238. [Google Scholar] [CrossRef]
- Orlich, M.J.; Fraser, G.E. Vegetarian Diets in the Adventist Health Study 2: A Review of Initial Published Findings1234. Am. J. Clin. Nutr. 2014, 100, 353S–358S. [Google Scholar] [CrossRef] [Green Version]
- Mariotti, F. Vegetarian and Plant-Based Diets in Health and Disease Prevention; Elsevier/Academic Press: London, UK, 2017. [Google Scholar]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and Management of Type 2 Diabetes: Dietary Components and Nutritional Strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef] [Green Version]
- Mann, J.I. Nutrition Recommendations for the Treatment and Prevention of Type 2 Diabetes and the Metabolic Syndrome: An Evidenced-Based Review. Nutr. Rev. 2006, 64, 422–427. [Google Scholar] [CrossRef]
- Hosseinpour-Niazi, S.; Mirmiran, P.; Hedayati, M.; Azizi, F. Substitution of Red Meat with Legumes in the Therapeutic Lifestyle Change Diet based on Dietary Advice Improves Cardiometabolic Risk Factors in Overweight Type 2 Diabetes Patients: A Cross-over Randomized Clinical Trial. Eur. J. Clin. Nutr. 2015, 69, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Kendall, C.W.C.; Augustin, L.S.A.; Mitchell, S.; Sahye-Pudaruth, S.; Blanco Mejia, S.; Chiavaroli, L.; Mirrahimi, A.; Ireland, C.; Bashyam, B.; et al. Effect of Legumes as Part of a Low Glycemic Index Diet on Glycemic Control and Cardiovascular Risk Factors in Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Arch. Intern. Med. 2012, 172, 1653–1660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittaway, J.K.; Robertson, I.K.; Ball, M.J. Chickpeas May Influence Fatty Acid and Fiber Intake in an Ad Libitum Diet, Leading to Small Improvements in Serum Lipid Profile and Glycemic Control. J. Am. Diet. Assoc. 2008, 108, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Tucker, L.A.; LeCheminant, J.D.; Bailey, B.W. Meat Intake and Insulin Resistance in Women without Type 2 Diabetes. J. Diabetes Res. 2015, 2015, 174742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, S.H.; Sun, Q.; Willett, W.C.; Eliassen, A.H.; Wu, K.; Pan, A.; Grodstein, F.; Hu, F.B. Associations between Red Meat Intake and Biomarkers of Inflammation and Glucose Metabolism in Women. Am. J. Clin. Nutr. 2014, 99, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Wang, B.; Wu, Y.; Xie, L.; Xun, P.; Tang, Q.; Cai, W.; Shen, X. Vegetarians Have a Lower Fasting Insulin Level and Higher Insulin Sensitivity than Matched Omnivores: A Cross-Sectional Study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 467–473. [Google Scholar] [CrossRef]
- Kim, M.-H.; Bae, Y.-J. Comparative Study of Serum Leptin and Insulin Resistance Levels between Korean Postmenopausal Vegetarian and Non-Vegetarian Women. Clin. Nutr. Res. 2015, 4, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-Y.; Li, X.-J.; Zhang, W.; Liu, C.-Q.; Zhang, H.-J.; Lin, J.-R.; Yan, B.; Yu, Y.-X.; Shi, X.-L.; Li, C.-D.; et al. Chinese Lacto-Vegetarian Diet Exerts Favorable Effects on Metabolic Parameters, Intima-Media Thickness, and Cardiovascular Risks in Healthy Men. Nutr. Clin. Pract. 2012, 27, 392–398. [Google Scholar] [CrossRef]
- Gammon, C.S.; von Hurst, P.R.; Coad, J.; Kruger, R.; Stonehouse, W. Vegetarianism, Vitamin B12 Status, and Insulin Resistance in a Group of Predominantly Overweight/Obese South Asian Women. Nutrition 2012, 28, 20–24. [Google Scholar] [CrossRef]
- Hung, C.-J.; Huang, P.-C.; Li, Y.-H.; Lu, S.-C.; Ho, L.-T.; Chou, H.-F. Taiwanese Vegetarians Have Higher Insulin Sensitivity than Omnivores. Br. J. Nutr. 2006, 95, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.-S.; Lai, N.-S.; Ho, L.-T.; Lin, C.-L. Insulin Sensitivity in Chinese Ovo-Lactovegetarians Compared with Omnivores. Eur. J. Clin. Nutr. 2004, 58, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Matoulek, M.; Malinska, H.; Oliyarnik, O.; Kazdova, L.; Neskudla, T.; Skoch, A.; Hajek, M.; Hill, M.; Kahle, M.; et al. Vegetarian Diet Improves Insulin Resistance and Oxidative Stress Markers More than Conventional Diet in Subjects with Type 2 Diabetes. Diabet. Med. 2011, 28, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Valachovicová, M.; Krajcovicová-Kudlácková, M.; Blazícek, P.; Babinská, K. No Evidence of Insulin Resistance in Normal Weight Vegetarians. A Case Control Study. Eur. J. Nutr. 2006, 45, 52–54. [Google Scholar] [CrossRef]
- Chiang, J.-K.; Lin, Y.-L.; Chen, C.-L.; Ouyang, C.-M.; Wu, Y.-T.; Chi, Y.-C.; Huang, K.-C.; Yang, W.-S. Reduced Risk for Metabolic Syndrome and Insulin Resistance Associated with Ovo-Lacto-Vegetarian Behavior in Female Buddhists: A Case-Control Study. PLoS ONE 2013, 8, e71799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vučić Lovrenčić, M.; Gerić, M.; Košuta, I.; Dragičević, M.; Garaj-Vrhovac, V.; Gajski, G. Sex-Specific Effects of Vegetarian Diet on Adiponectin Levels and Insulin Sensitivity in Healthy Non-Obese Individuals. Nutrition 2020, 79, 110862. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, D.L.; Costantino, N.S.; Blackburn, H.L.; Engler, R.J.M.; Kashani, M.; Vernalis, M.N. Lifestyle Modification Interventions Differing in Intensity and Dietary Stringency Improve Insulin Resistance through Changes in Lipoprotein Profiles. Obes. Sci. Pract. 2016, 2, 282–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garousi, N.; Tamizifar, B.; Pourmasoumi, M.; Feizi, A.; Askari, G.; Clark, C.C.T.; Entezari, M.H. Effects of Lacto-Ovo-Vegetarian Diet vs. Standard-Weight-Loss Diet on Obese and Overweight Adults with Non-Alcoholic Fatty Liver Disease: A Randomised Clinical Trial. Arch. Physiol. Biochem. 2021, 1–9. [Google Scholar] [CrossRef]
- Chen, Z.; Zuurmond, M.G.; van der Schaft, N.; Nano, J.; Wijnhoven, H.A.H.; Ikram, M.A.; Franco, O.H.; Voortman, T. Plant versus Animal based Diets and Insulin Resistance, Prediabetes and Type 2 Diabetes: The Rotterdam Study. Eur. J. Epidemiol. 2018, 33, 883–893. [Google Scholar] [CrossRef] [Green Version]
- Satija, A.; Hu, F.B. Plant-Based Diets and Cardiovascular Health. Trends Cardiovasc. Med. 2018, 28, 437–441. [Google Scholar] [CrossRef]
- Nolan, C.J.; Larter, C.Z. Lipotoxicity: Why do Saturated Fatty Acids Cause and Monounsaturates Protect against It? J. Gastroenterol. Hepatol. 2009, 24, 703–706. [Google Scholar] [CrossRef]
- Najjar, R.S.; Feresin, R.G. Plant-Based Diets in the Reduction of Body Fat: Physiological Effects and Biochemical Insights. Nutrients 2019, 11, 2712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risérus, U.; Willett, W.C.; Hu, F.B. Dietary Fats and Prevention of Type 2 Diabetes. Prog. Lipid Res. 2009, 48, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizkalla, S.W.; Bellisle, F.; Slama, G. Health Benefits of Low Glycaemic Index Foods, Such as Pulses, in Diabetic Patients and Healthy Individuals. Br. J. Nutr. 2002, 88 (Suppl. 3), 255–262. [Google Scholar] [CrossRef] [PubMed]
- Polak, R.; Phillips, E.M.; Campbell, A. Legumes: Health Benefits and Culinary Approaches to Increase Intake. Clin. Diabetes 2015, 33, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Schulze, M.B.; Manson, J.E.; Willett, W.C.; Hu, F.B. Red Meat Consumption and Risk of Type 2 Diabetes: 3 Cohorts of US Adults and an Updated Meta-Analysis123. Am. J. Clin. Nutr. 2011, 94, 1088–1096. [Google Scholar] [CrossRef]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Manson, J.E.; Willett, W.C.; Hu, F.B. Changes in Red Meat Consumption and Subsequent Risk of Type 2 Diabetes: Three Cohorts of US Men and Women. JAMA Intern. Med. 2013, 173, 1328–1335. [Google Scholar] [CrossRef]
- Barnard, N.; Levin, S.; Trapp, C. Meat Consumption as a Risk Factor for Type 2 Diabetes. Nutrients 2014, 6, 897–910. [Google Scholar] [CrossRef] [Green Version]
- Fretts, A.M.; Follis, J.L.; Nettleton, J.A.; Lemaitre, R.N.; Ngwa, J.S.; Wojczynski, M.K.; Kalafati, I.P.; Varga, T.V.; Frazier-Wood, A.C.; Houston, D.K.; et al. Consumption of Meat is Associated with Higher Fasting Glucose and Insulin Concentrations Regardless of Glucose and Insulin Genetic Risk Scores: A Meta-Analysis of 50,345 Caucasians. Am. J. Clin. Nutr. 2015, 102, 1266–1278. [Google Scholar] [CrossRef] [Green Version]
- Frazier-Wood, A.C.; Garvey, W.T.; Dall, T.; Honigberg, R.; Pourfarzib, R. Opportunities for Using Lipoprotein Subclass Profile by Nuclear Magnetic Resonance Spectroscopy in Assessing Insulin Resistance and Diabetes Prediction. Metab. Syndr. Relat. Disord. 2012, 10, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Craig, W.J. Health Effects of Vegan Diets. Am. J. Clin. Nutr. 2009, 89, 1627S–1633S. [Google Scholar] [CrossRef] [Green Version]
- Kahleova, H.; Fleeman, R.; Hlozkova, A.; Holubkov, R.; Barnard, N.D. A Plant-Based Diet in Overweight Individuals in a 16-Week Randomized Clinical Trial: Metabolic Benefits of Plant Protein. Nutr. Diabetes 2018, 8, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnard, N.D.; Alwarith, J.; Rembert, E.; Brandon, L.; Nguyen, M.; Goergen, A.; Horne, T.; do Nascimento, G.F.; Lakkadi, K.; Tura, A.; et al. A Mediterranean Diet and Low-Fat Vegan Diet to Improve Body Weight and Cardiometabolic Risk Factors: A Randomized, Cross-over Trial. J. Am. Coll. Nutr. 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Dort, S.; Holubkov, R.; Barnard, N.D. A Plant-Based High-Carbohydrate, Low-Fat Diet in Overweight Individuals in a 16-Week Randomized Clinical Trial: The Role of Carbohydrates. Nutrients 2018, 10, 1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahleova, H.; Rembert, E.; Alwarith, J.; Yonas, W.N.; Tura, A.; Holubkov, R.; Agnello, M.; Chutkan, R.; Barnard, N.D. Effects of a Low-Fat Vegan Diet on Gut Microbiota in Overweight Individuals and Relationships with Body Weight, Body Composition, and Insulin Sensitivity. A Randomized Clinical Trial. Nutrients 2020, 12, 2917. [Google Scholar] [CrossRef]
- Kahleova, H.; Hlozkova, A.; Fleeman, R.; Fletcher, K.; Holubkov, R.; Barnard, N.D. Fat Quantity and Quality, as Part of a Low-Fat, Vegan Diet, are Associated with Changes in Body Composition, Insulin Resistance, and Insulin Secretion. A 16-Week Randomized Controlled Trial. Nutrients 2019, 11, 615. [Google Scholar] [CrossRef] [Green Version]
- Kahleova, H.; Tura, A.; Hill, M.; Holubkov, R.; Barnard, N.D. A Plant-Based Dietary Intervention Improves Beta-Cell Function and Insulin Resistance in Overweight Adults: A 16-Week Randomized Clinical Trial. Nutrients 2018, 10, 189. [Google Scholar] [CrossRef] [Green Version]
- Kahleova, H.; McCann, J.; Alwarith, J.; Rembert, E.; Tura, A.; Holubkov, R.; Barnard, N.D. A Plant-Based Diet in Overweight Adults in a 16-Week Randomized Clinical Trial: The Role of Dietary Acid Load. Clin. Nutr. ESPEN 2021, 44, 150–158. [Google Scholar] [CrossRef]
- Kahleova, H.; Petersen, K.F.; Shulman, G.I.; Alwarith, J.; Rembert, E.; Tura, A.; Hill, M.; Holubkov, R.; Barnard, N.D. Effect of a Low-Fat Vegan Diet on Body Weight, Insulin Sensitivity, Postprandial Metabolism, and Intramyocellular and Hepatocellular Lipid Levels in Overweight Adults: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, 2025454. [Google Scholar] [CrossRef]
- Barnard, N.D.; Scialli, A.R.; Turner-McGrievy, G.; Lanou, A.J.; Glass, J. The Effects of a Low-Fat, Plant-Based Dietary Intervention on Body Weight, Metabolism, and Insulin Sensitivity. Am. J. Med. 2005, 118, 991–997. [Google Scholar] [CrossRef]
- Śliż, D.; Parol, D.; Wełnicki, M.; Chomiuk, T.; Grabowska, I.; Dąbrowska, D.; Król, W.; Price, S.; Braksator, W.; Mamcarz, A. Macronutrient Intake, Carbohydrate Metabolism and Cholesterol in Polish Male Amateur Athletes on a Vegan Diet. Nutr. Bull. 2021, 46, 120–127. [Google Scholar] [CrossRef]
- Krajcovicova-Kudlackova, M.; Babinska, K.; Valachovicova, M. Health Benefits and Risks of Plant Proteins. Bratisl. Lek. Listy 2005, 106, 231–234. [Google Scholar] [PubMed]
- McCarty, M.F. Vegan Proteins May Reduce Risk of Cancer, Obesity, and Cardiovascular Disease by Promoting Increased Glucagon Activity. Med. Hypotheses 1999, 53, 459–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lea, E.J.; Crawford, D.; Worsley, A. Public Views of the Benefits and Barriers to the Consumption of a Plant-Based Diet. Eur. J. Clin. Nutr. 2006, 60, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Lea, E.J.; Crawford, D.; Worsley, A. Consumers’ Readiness to Eat a Plant-Based Diet. Eur. J. Clin. Nutr. 2006, 60, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Fehér, A.; Gazdecki, M.; Véha, M.; Szakály, M.; Szakály, Z. A Comprehensive Review of the Benefits of and the Barriers to the Switch to a Plant-Based Diet. Sustainability 2020, 12, 4136. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef]
- Lea, E.; Worsley, A. Influences on Meat Consumption in Australia. Appetite 2001, 36, 127–136. [Google Scholar] [CrossRef]
- Śmiglak-Krajewska, M.; Wojciechowska-Solis, J. Consumption Preferences of Pulses in the Diet of Polish People: Motives and Barriers to Replace Animal Protein with Vegetable Protein. Nutrients 2021, 13, 454. [Google Scholar] [CrossRef]
- Caire-Juvera, G.; Vázquez-Ortiz, F.A.; Grijalva-Haro, M.I. Amino Acid Composition, Score and in Vitro Protein Digestibility of Foods Commonly Consumed in Northwest Mexico. Nutr. Hosp. 2013, 28, 365–371. [Google Scholar] [CrossRef]
- Watanabe, F. Vitamin B12 Sources and Bioavailability. Exp. Biol. Med. 2007, 232, 1266–1274. [Google Scholar] [CrossRef]
- Wickramasinghe, K.; Breda, J.; Berdzuli, N.; Rippin, H.; Farrand, C.; Halloran, A. The Shift to Plant-Based Diets: Are We Missing the Point? Glob. Food Secur. 2021, 29, 100530. [Google Scholar] [CrossRef]
- Jabs, J.; Devine, C.M.; Sobal, J. Model of the Process of Adopting Vegetarian Diets: Health Vegetarians and Ethical Vegetarians. J. Nutr. Educ. 1998, 30, 196–202. [Google Scholar] [CrossRef]
- Graça, J.; Oliveira, A.; Calheiros, M.M. Meat, beyond the Plate. Data-Driven Hypotheses for Understanding Consumer Willingness to Adopt a More Plant-Based Diet. Appetite 2015, 90, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Lea, E.; Worsley, A. Benefits and Barriers to the Consumption of a Vegetarian Diet in Australia. Public Health Nutr. 2003, 6, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Pohjolainen, P.; Vinnari, M.; Jokinen, P. Consumers’ Perceived Barriers to following a Plant-Based Diet. Br. Food J. 2015, 117, 1150–1167. [Google Scholar] [CrossRef]
- Vanhonacker, F.; Van Loo, E.J.; Gellynck, X.; Verbeke, W. Flemish Consumer Attitudes towards More Sustainable Food Choices. Appetite 2013, 62, 7–16. [Google Scholar] [CrossRef]
- Mullee, A.; Vermeire, L.; Vanaelst, B.; Mullie, P.; Deriemaeker, P.; Leenaert, T.; De Henauw, S.; Dunne, A.; Gunter, M.J.; Clarys, P.; et al. Vegetarianism and Meat Consumption: A Comparison of Attitudes and Beliefs between Vegetarian, Semi-Vegetarian, and Omnivorous Subjects in Belgium. Appetite 2017, 114, 299–305. [Google Scholar] [CrossRef]
- Vainio, A.; Niva, M.; Jallinoja, P.; Latvala, T. From Beef to Beans: Eating Motives and the Replacement of Animal Proteins with Plant Proteins among Finnish Consumers. Appetite 2016, 106, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Melendrez-Ruiz, J.; Buatois, Q.; Chambaron, S.; Monnery-Patris, S.; Arvisenet, G. French Consumers Know the Benefits of Pulses, but do not Choose Them: An Exploratory Study Combining Indirect and Direct Approaches. Appetite 2019, 141, 104311. [Google Scholar] [CrossRef]
- Havemeier, S.; Erickson, J.; Slavin, J. Dietary Guidance for Pulses: The Challenge and Opportunity to Be Part of Both the Vegetable and Protein Food Groups. Ann. N. Y. Acad. Sci. 2017, 1392, 58–66. [Google Scholar] [CrossRef]
- Figueira, N.; Curtain, F.; Beck, E.; Grafenauer, S. Consumer Understanding and Culinary Use of Legumes in Australia. Nutrients 2019, 11, 1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Dietary Approach | Type of Diet | Characteristics |
|---|---|---|
| Plant-based diet | Vegetarian | Does not contain meat, fish or seafood. Contains fruit, vegetables, whole grains, pulses, nuts and seeds. May or may not include egg and/or dairy products. |
| Lacto-ovo-vegetarian | Contains eggs and dairy products. | |
| Lacto-vegetarian | Includes dairy products but excludes eggs. | |
| Ovo-vegetarian | Includes eggs but excludes dairy products. | |
| Vegan | Does not contain any animal products. May exclude honey. | |
| Raw vegan | Includes uncooked vegetables, fruits, nuts, seeds, legumes/beans and whole grains. The amount of uncooked food varies from 75% to 100%. |
| Study | Year | Country | Cohort | Analysed Groups | Time of Intervention | Results |
|---|---|---|---|---|---|---|
| Hosseinpour-Niazi et al. [24] | 2015 | Iran | 31 participants (24 women and 7 men; age: 58.1 ± 6.0 years, with type 2 diabetes) | Legume-based Therapeutic Lifestyle Change (TLC) diet Control diet (legume-free TLC diet) | 8 weeks | Decreased fasting blood glucose (p = 0.04), fasting insulin (p = 0.04), triglyceride concentrations (p = 0.04) and low-density lipoprotein cholesterol (p = 0.02) for TLC group. No change in body mass index (BMI), waist circumference. |
| Jenkins et al. [25] | 2012 | Canada | 121 participants with type 2 diabetes (60 women, 61 men, aged 59.5 ± 1 | (1) Low-GI diet (diet rich in legume) (2) High wheat fiber diet (diet rich in high wheat fiber foods) | 3 months | Decreased HbA1c (p < 0.001), body weight) (p = 0.002), fasting glucose level (p = 0.001), systolic BP (p < 0.001), diastolic BP (p < 0.001), heart rate (p < 0.001), absolute CHD risk (10 years) (p = 0.003) in both groups. |
| Pittaway et al. [26] | 2008 | Australia | 45 participants (13 premenopausal women, 19 postmenopausal women, 13 men; age: 52.2 ± 6.1 years) | A diet consisting of a minimum of 728 g of chickpeas per week as part of traditional diet for 12 weeks (chickpea phase), followed by 4 weeks of the traditional diet without chickpeas (usual phase). | 20 weeks | Significant decrease in mean serum total cholesterol of 7.7 mg/dL (p = 0.002), LDL cholesterol of 7.3 mg/dL (p = 0.01), fasting insulin of 0.75 IU/mL (p = 0.045) and in HOMA-IR of 0.21 (p = 0.01). |
| Tucker et al. [27] | 2015 | USA | 292 participants (nondiabetic women; age: 40.3 ± 3.1 years) | 3 groups: woman with low meat intake (n = 73), moderate meat intake (n = 164) and high meat intake (n = 73) | 7 days | Significantly higher HOMA scores in groups with high and moderate meat consumption (p = 0.007). |
| Ley et al. [28] | 2014 | USA | 3690 participants (nondiabetic women from Nurses’ Health Study; age 30–55 years) | - | - | Higher red meat consumption was associated with higher plasma CRP, ferritin, fasting insulin, and HbA1c, and lower adiponectin (p ≤ 0.03 for all). Substituting a serving of total red meat intake with alternative protein food showed improvement in lowering CRP, ferritin, HbA1c and fasting insulin levels(p ≤ 0.02 for all). |
| Cui et al. [29] | 2019 | China | 558 participants (healthy men and women, age 32–34 years old) | 279 vegetarians (73 vegans, 206 lacto-ovo-vegetarians) and 279 omnivores | 3 months | Vegan diet and lacto-ovo-vegetarian diet were negatively correlated with HOMA-IR after adjusting for BMI. |
| Kim et al. [30] | 2015 | Korea | 102 participants (postmenopausal women, age of 47 to 85 years old) | 54 vegetarian women and 48 non-vegetarian women | - | Significantly lower body weight (p < 0.01), body mass index (p < 0.001), % of body fat (p < 0.001), serum levels of leptin (p < 0.05), glucose (p < 0.001), insulin (p < 0.01) and HOMA-IR (p < 0.01) in the vegetarians group. |
| Yang et al. [31] | 2012 | China | 295 participants (men aged 21–76 years) | 169 lacto-vegetarians 126 omnivores | - | Remarkably lower body mass index (p = 0.049), triglyceride level (p = 0.016), total cholesterol (p < 0.001), LDL cholesterol (p < 0.001) and fasting blood glucose (p < 0.001) in lacto-vegetarians group. Higher homeostasis model assessment β cell function (p < 0.001) and insulin secretion index (p = 0.048) in lacto-vegetarians. |
| Gammon et al. [32] | 2012 | New Zealand | 124 participants (women at least 20 years old) | 90 non-vegetarians 34 vegetarians | - | Increased body mass index, waist circumference and HOMA2-IR levels in non-vegetarians group. Higher serum vitamin B12 levels in non-vegetarians (p < 0.001). |
| Hung et al. [33] | 2006 | Taiwan | 98 participants (healthy women, age 31–45 years old) | 49 lactovegetarians 49 omnivores | - | Significantly lower levels of fasting insulin (p < 0.001), plasma glucose (p < 0.001) and i resistance (HOMA-IR) (p < 0.001) in lactovegetarians group. No difference in beta-cell function between the two groups (p = 0.062). |
| Kuo et al. [34] | 2004 | Taiwan | 36 healthy participants (omnivore—55.7 ± 3.7; vegetarians—58.6 ± 3.6 years old) | 19 vegetarians 17 omnivores | - | Significantly lower levels of steady-state plasma glucose (SSPG) (p < 0.001), fasting insulin (p = 0.004), HOMA-IR (p = 0.002), HOMA %S (p = 0.018). |
| Kahleova et al. [35] | 2011 | Czech Republic | 74 participants with type 2 diabetes (experimental group—54.6 ± 7.8, control group—57.7 ± 4.9 years old) | (1) experimental group (n = 37; vegetarian diet) (2) the control group (n = 37; conventional diet) | 24 weeks | Reduced diabetes medication in the experimental group (43% participants; p < 0.001). Decreased body weight (p = 0.001) and visceral and subcutaneous fat in the experimental group (p = 0.007 and p = 0.02, respectively). Increased insulin sensitivity (p = 0.04) and plasma adiponectin (p = 0.02) in the experimental group. |
| Valachovicová et al. [36] | 2006 | Slovak Republic | 202 participant (healthy adult subjects (age range 19–64 years; BMI 18.6–25.0 kg/m2) | (1) a vegetarian group (95 long-term lacto-ovo-vegetarians) (2) a non-vegetarian control group (107 participants on a traditional western diet) | - | Significantly lower glucose (p < 0.001), insulin concentrations (p < 0.01) and IR (HOMA) (p < 0.01) in the vegetarian group. Significantly higher intake of whole grain products, pulses, products from oat and barley (p < 0.001) in the vegetarians group. |
| Chiang et al. [37] | 2013 | Taiwan | 706 female participants (age 56.4 ± 8.4 years old, overall healthy) | 391 vegetarians (~80% lacto-ovo-vegetarians) 315 non-vegetarians | - | Significantly lower body mass index (p < 0.001), waist circumference (p < 0.001), lower total cholesterol (p < 0.001), LDL cholesterol (LDL-C) (p < 0.001), glucose (p < 0.001), insulin (p < 0.001), HOMA-IR (p < 0.001) and the risks for the MetS (p = 0.006). |
| Vučić Lovrenčić et al. [38] | 2020 | Croatia | 76 participants (healthy non-obese adult, age- and gender matched; BMI < 30 kg/m2; 18–60 years old) | Vegetarians (n = 40) Omnivore (n = 36) | - | Significantly higher levels of adiponectin in female (p = 0.03) and the HOMA2-%B in vegetarians group than omnivore controls (p = 0.04). No differences in HOMA2-IRI, inflammatory and metabolic biomarkers. |
| Ellsworth et al. [39] | 2016 | USA | 325 participants (subjects with diagnosed type-2 diabetes, CAD or significant risk factors; average age was 60.3 years (range 40.7–79.8)—intensive lifestyle and 61.5 years (range 33.9–86.2) in moderate lifestyle) | (1) intensive non-randomised program with a strict vegetarian diet (n = 90 participants, 90 matched controls) (2) moderate randomised trial following a Mediterranean-style diet (n = 89 subjects, 58 controls) | 1 year | Decrease in weight loss (−8.9% (95% CI, −10.3 to −7.4), intensive programme; −2.8% (95% CI, −3.8 to −1.9), moderate programme; adjusted p < 0.001) and the LPIR score (−13.3% (95% CI, −18.2 to −8.3), intensive; −8.8% (95% CI, −12.9 to −4.7), moderate; adjusted p < 0.01) in both intervention with an advantage in the vegetarian diet. |
| Garousi et al. [40] | 2021 | Iran | 75 participants (overweight/obese adults with NAFLD, aged between 20 and 55 years) | (1) lacto-ovo-vegetarian diet (LOV-D) (n = 37) (2) a standard weight-loss diet (SWL-D) (n = 38) | 3 months | Decreased levels of alanine aminotransferase (ALT) (p < 0.001), body weight (p < 0.001), waist circumference (p < 0.001), BMI (p < 0.001), fasting blood sugar (p < 0.001), insulin (p < 0.001), HOMA-IR (p < 0.001), triacylglycerol (TG) (p = 0.001), cholesterol (p < 0.001), LDL cholesterol (p < 0.001),and systolic blood pressure (p = 0.001) in LOV-D group. |
| Chen et al. [41] | 2018 | The Netherlands | 6798 participants (age 62.0 ± 7.8) | (1) 6514 participants for plant-based diet with insulin resistance (2) 5768 participants for a plant-based diet with prediabetes risk (3) 6770 participants for a plant-based diet with T2D risk | - | Higher score on the plant-based dietary index was associated with lower insulin resistance (per 10 units higher score: β = −0.09; 95% CI: −0.10; −0.08), lower prediabetes risk (HR = 0.89; 95% CI: 0.81; 0.98) and lower T2D risk (HR = 0.82 (0.73; 0.92)). |
| Study | Year | Country | Cohort | Analysed Groups | Time of Intervention | Results |
|---|---|---|---|---|---|---|
| Kahleova et al. [54] | 2018 | USA | 75 participants (healthy overweight or obese adult men and women, BMI between 28 and 40 kg/m2, age 53.2 ± 12.6 years old) | (1) a plant-based diet (n = 38) (2) a control diet (current participant’s diet) (n = 37) | 16 weeks | Significant reductions in body weight (−6.5 kg; p < 0.001), fat mass (−4.3 kg; p < 0.001) and HOMA-IR (−1.0; p = 0.004) in the vegan group. |
| Barnard et al. [55] | 2021 | USA | 62 participants (healthy, overweight adults, BMI between 28 and 40 kg/m2, group 1—56.6 ± 10.9 years old, group 2—58.3 ± 8.4 years old) | (1) group on the Mediterranean diet (2) group on a low-fat vegan diet | 16 weeks | Decreased weight (−6.0 kg; p < 0.001) and HOMA-IR (−0.7; p = 0.21) in vegan group. Increased oral glucose insulin sensitivity (OGIS) (+35.8 mL/min/m2; p = 0.003) in vegan group. No significant change in the Mediterranean diet group. |
| Kahleova et al. [56] | 2018 | USA | 75 participants (healthy, overweight adults with a BMI between 28 and 40 kg/m2, age 53.2 ± 12.6 years old) | (1) plant-based high-carbohydrate, low-fat (vegan) diet (n = 38) (2) control group (current participant’s diet) (n = 37) | 16 weeks | Significant reduction in body weight (−6.5 kg; p < 0.001), fat mass (−4.3 kg; p < 0.001) and HOMA-IR (−1.0; p = 0.004) in the vegan group. |
| Kahleova et al. [57] | 2020 | USA | 168 participants (overweight, but otherwise healthy adult men and women with a BMI between 28 and 40 kg/m2; vegan group—52.9 ± 11.7 years old, control group—57.5 ± 10.2 years old) | (1) vegan group (n = 84) (2) control group (current participant’s diet) (n = 84) | 16 weeks | Decreased body weight (−5.9 kg; p < 0.001), fat mass (−3.9 kg; p < 0.001) and visceral fat (−240 cm3; p < 0.001) in the vegan group. Increased PREDIcted M, insulin sensitivity index (PREDIM) in the vegan group (+0.83; p < 0.001). Significant changes in gut microbiota (p < 0.001) due to the low-fat vegan diet. |
| Kahleova et al. [58] | 2019 | USA | 75 participants (healthy, overweight adults with a BMI between 28 and 40 kg/m2, age 53.2 ± 12.6 years old) | (1) low-fat vegan diet (n = 38) (2) control diet (current participant’s diet) (n = 37) | 16 weeks | Decreased intakes of C18:0 (p = 0.004) and CLA-trans-10-cis12 (p = 0.002) in the vegan group. Increased intake of C18:2 (p = 0.002) and C18:3 (p = 0.006). Changes in the consumption of fatty acids have caused a decrease in HOMA-IR (p = 0.02) in the vegan group. The main fatty acids associated with changes in fasting insulin secretion were C12:0 (p = 0.03) and TRANS 16:1 (p = 0.02). |
| Kahleova et al. [59] | 2018 | USA | 75 participants (healthy, overweight adults with a BMI between 28 and 40 kg/m2, age 53.2 ± 12.6 years old) | (1) vegan group (low-fat plant-based diet) (n = 38) (2) control group (current participant’s diet) (n = 37) | 16 weeks | Decreased significantly HOMA-IR in the vegan group (−1.0; p = 0.004). Changes in HOMA-IR correlated positively with changes in body mass index (p = 0.009) and visceral fat volume (p = 0.001). |
| Kahleova et al. [60] | 2021 | USA | 244 healthy participants (intervention group—52.6 ± 14.7 years old, control group—54.3 ± 9.9 years old) | (1) intervention group (vegan) (n = 122) (2) control group (current participant’s diet)(n = 122) | 16 weeks | Reduction in Potential Renal Acid Load (PRAL) (−24.7 mEq/day; p < 0.001) and Net Endogenous Acid Production (NEAP) (−23.8 mEq/day; p < 0.001), body weight (−5.9 kg; p < 0.001) and HOMA-IR (p = 0.008) in vegan group. Increased PREDIM in the vegan group (p < 0.001). |
| Kahleova et al. [61] | 2020 | USA | 244 healthy participants (BMI between 28 and 40 kg/m2, age 25 to 75 years) | (1) intervention group(low-fat vegan diet) (n = 122) (2) control group (current participant’s diet) (n = 122) | 16 weeks | Decreased body weight (−5.9 kg; p < 0.001), HOMA (−1.3; p < 0.001), hepatocellular lipid levels (−34.4%; p = 0.002) and intramyocellular lipid levels (−10.4%; p = 0.03) in the intervention group. Increased thermic effect of food (+14.1%; p < 0.001) and PREDIM (+0.9; p < 0.001) in the intervention group. No significant changes in the control group. |
| Barnard et al. [62] | 2005 | USA | 64 participants (overweight or obese, postmenopausal women; mean age for intervention group—57.4 y, for control group—55.6 y) | (1) intervention group (low-fat, vegan diet) (2) control group (control diet based on National Cholesterol Education Program guidelines) | 14 weeks | Decreased body weight (−5.8 ± 3.2 kg in the intervention group; −3.8 ± 2.8 kg in the control group; p = 0.012). Increased index of insulin sensitivity (from 4.6 ± 2.9 to 5.7 ± 3.9; p = 0.017) in the intervention group. |
| Śliż et al. [63] | 2021 | Poland | 98 participants (healthy Polish males, athletes, aged 20–39 years) | (1) vegan group (VEG; n = 44) (2) omnivore group (OMN; n = 54) | - | Higher intake of carbohydrate (p < 0.01), unsaturated fatty acids (p < 0.01) in the VEG group. Lover intake of protein (p < 0.01), fat (p < 0.01), saturated fatty acids (p < 0.01) and EPA + DHA (p < 0.01) in the VEG group. Significantly better outcomes in n-6/n-3 fatty acid ratio (6.5% ± 2.3% vs. 5.0% ± 2.1%; p < 0.01) insulin sensitivity (HOMA-IR), C-peptide and total blood cholesterol levels (p < 0.01) in the VEG group. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banaszak, M.; Górna, I.; Przysławski, J. Non-Pharmacological Treatments for Insulin Resistance: Effective Intervention of Plant-Based Diets—A Critical Review. Nutrients 2022, 14, 1400. https://doi.org/10.3390/nu14071400
Banaszak M, Górna I, Przysławski J. Non-Pharmacological Treatments for Insulin Resistance: Effective Intervention of Plant-Based Diets—A Critical Review. Nutrients. 2022; 14(7):1400. https://doi.org/10.3390/nu14071400
Chicago/Turabian StyleBanaszak, Michalina, Ilona Górna, and Juliusz Przysławski. 2022. "Non-Pharmacological Treatments for Insulin Resistance: Effective Intervention of Plant-Based Diets—A Critical Review" Nutrients 14, no. 7: 1400. https://doi.org/10.3390/nu14071400
APA StyleBanaszak, M., Górna, I., & Przysławski, J. (2022). Non-Pharmacological Treatments for Insulin Resistance: Effective Intervention of Plant-Based Diets—A Critical Review. Nutrients, 14(7), 1400. https://doi.org/10.3390/nu14071400






