The Impact of Inpatient Multimodal Treatment or Family-Based Treatment on Six-Month Weight Outcomes in Youth with Anorexia Nervosa: A Naturalistic, Cross-Continental Comparison
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Centers and Participants
2.2. Models of Care
2.2.1. San Francisco and Chicago, USA: Family-Based Treatment for Anorexia Nervosa with/without Inpatient Medical Stabilization as Needed (FBT)
2.2.2. Berlin, Germany: Inpatient Multimodal Treatment Followed by Outpatient Care (IMT)
2.3. Patient Inclusion Criteria
2.4. Study Assessments
2.4.1. Clinical Characteristics
2.4.2. Outcome Assessments
2.5. Statistical Analysis
3. Results
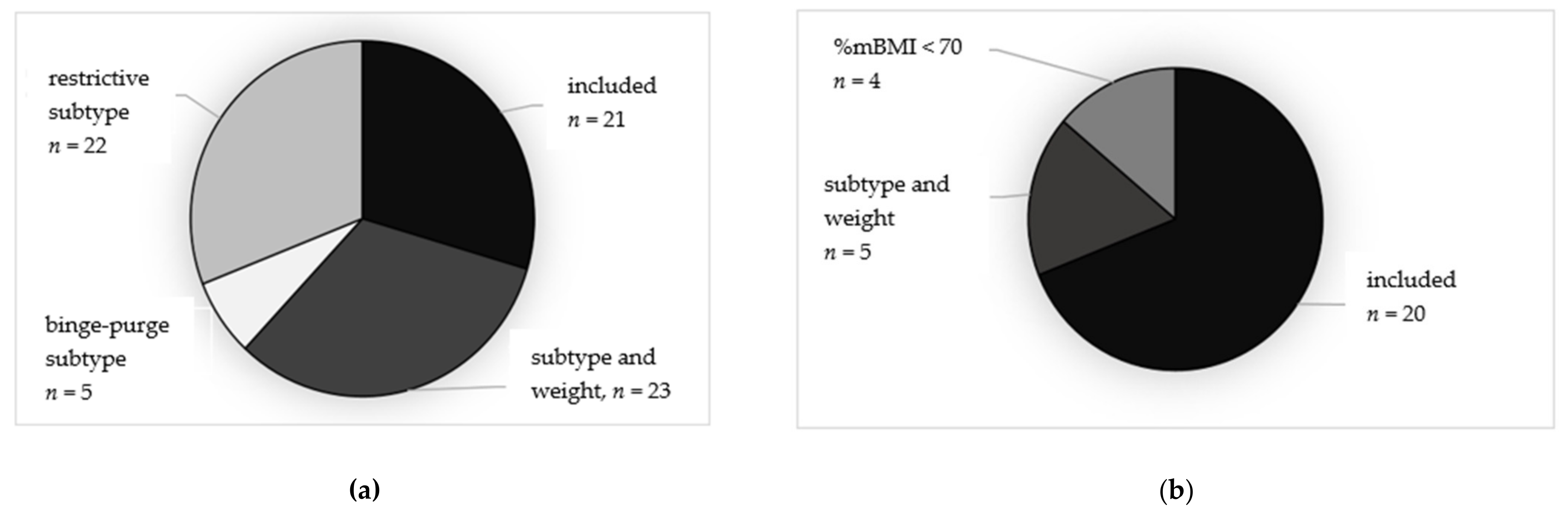
3.1. Representativeness of the Samples
3.2. Patient Baseline Characteristics of the Samples
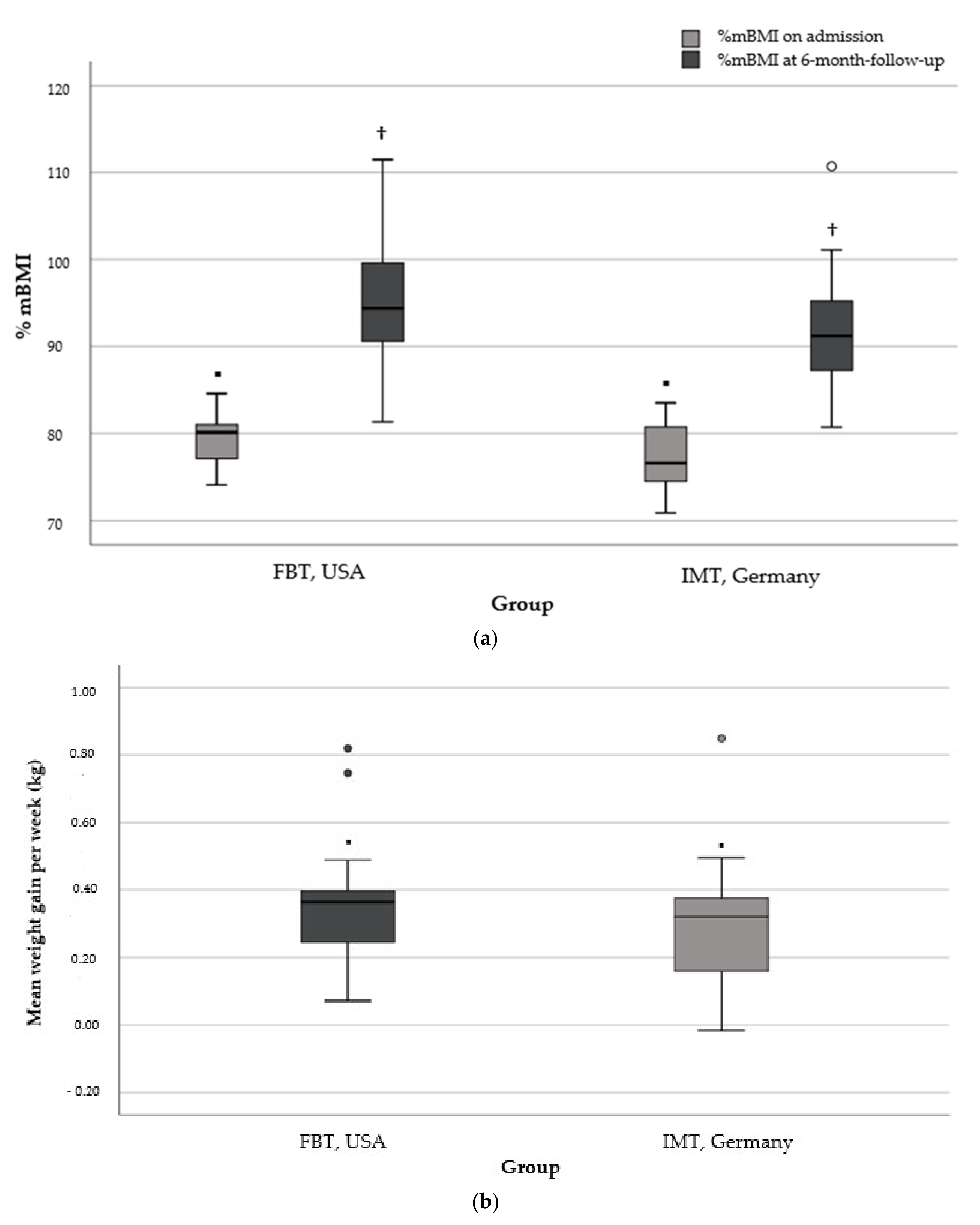
3.3. Weight Gain and Days in Hospital at 6-Month Follow-Up in the More Restricted Subsamples
3.4. Impact of Treatment Group on Weight- and Hospitalization Outcomes
4. Discussion
4.1. Differences in Baseline Characteristics of Cohorts and Limited Validity to Compare Treatment Outcomes
4.2. In Comparable Subgroups, Weekly Weight Gain Did Not Differ at 6-Month Follow-Up but Was Achieved with Fewer Days in Hospital
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Golden, N.H.; Schneider, M.; Wood, C. Preventing Obesity and Eating Disorders in Adolescents. Pediatrics 2016, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herpertz-Dahlmann, B.; Müller, B.; Herpertz, S.; Heussen, N.; Hebebrand, J.; Remschmidt, H. Prospective 10-year follow-up in adolescent anorexia nervosa—Course, outcome, psychiatric comorbidity, and psychosocial adaptation. J. Child Psychol. Psychiatry 2001, 42, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.; Golden, N.H.; Katzman, D.K.; Kreipe, R.E.; Rees, J.; Schebendach, J.; Sigman, G.; Ammerman, S.; Hoberman, H.M. Eating disorders in adolescents: A background paper. J. Adolesc. Health 1995, 16, 420–437. [Google Scholar] [CrossRef]
- Herpertz-Dahlmann, B.; van Elburg, A.; Castro-Fornieles, J.; Schmidt, U. ESCAP Expert Paper: New developments in the diagnosis and treatment of adolescent anorexia nervosa—A European perspective. Eur. Child Adolesc. Psychiatry 2015, 24, 1153–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcelus, J.; Mitchell, A.J.; Wales, J.; Nielsen, S. Mortality rates in patients with anorexia nervosa and other eating disorders: A meta-analysis of 36 studies. Arch. Gen. Psychiatry 2011, 68, 724–731. [Google Scholar] [CrossRef] [Green Version]
- Westmoreland, P.; Krantz, M.J.; Mehler, P.S. Medical complications of anorexia nervosa and bulimia. Am. J. Med. 2016, 129, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Blank, S.; Zadik, Z.; Katz, I.; Mahazri, Y.; Toker, I.; Barak, I. The emergence and treatment of anorexia and bulimia nervosa. A comprehensive and practical model. Int. J. Adolesc. Med. Health 2002, 14, 257–260. [Google Scholar] [CrossRef]
- Madden, S.; Hay, P.; Touyz, S. Systematic review of evidence for different treatment settings in anorexia nervosa. World J. Psychiatry 2015, 5, 147–153. [Google Scholar] [CrossRef]
- Hartmann, A.; Weber, S.; Herpertz, S.; Zeeck, A. Psychological treatment for anorexia nervosa: A meta-analysis of standardized mean change. Psychother. Psychosom. 2011, 80, 216–226. [Google Scholar] [CrossRef]
- Zeeck, A.; Herpertz-Dahlmann, B.; Friederich, H.C.; Brockmeyer, T.; Resmark, G.; Hagenah, U.; Ehrlich, S.; Cuntz, U.; Zipfel, S.; Hartmann, A. Psychotherapeutic Treatment for Anorexia Nervosa: A Systematic Review and Network Meta-Analysis. Front Psychiatry 2018, 9, 158. [Google Scholar] [CrossRef] [Green Version]
- Couturier, J.; Kimber, M.; Szatmari, P. Efficacy of family-based treatment for adolescents with eating disorders: A systematic review and meta-analysis. Int. J. Eat. Disord. 2013, 46, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, S.; Giel, K.E.; Bulik, C.M.; Hay, P.; Schmidt, U. Anorexia nervosa: Aetiology, assessment, and treatment. Lancet Psychiatry 2015, 2, 1099–1111. [Google Scholar] [CrossRef]
- Le Grange, D.; Eckhardt, S.; Dalle Grave, R.; Crosby, R.D.; Peterson, C.B.; Keery, H.; Lesser, J.; Martell, C. Enhanced cognitive-behavior therapy and family-based treatment for adolescents with an eating disorder: A non-randomized effectiveness trial. Psychol. Med. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Toulany, A.; Wong, M.; Katzman, D.K.; Akseer, N.; Steinegger, C.; Hancock-Howard, R.L.; Coyte, P.C. Cost analysis of inpatient treatment of anorexia nervosa in adolescents: Hospital and caregiver perspectives. CMAJ Open 2015, 3, E192. [Google Scholar] [CrossRef] [Green Version]
- Hillege, S.; Beale, B.; McMaster, R. Impact of eating disorders on family life: Individual parents’ stories. J. Clin. Nurs. 2006, 15, 1016–1022. [Google Scholar] [CrossRef]
- Findlay, S.; Pinzon, J.; Goldberg, E.; Frappier, J.-Y.; Society, C.P.; Committee, A.H. Issues of care for hospitalized youth. Paediatr. Child Health 2008, 13, 61–64. [Google Scholar] [CrossRef]
- Hay, P.J.; Touyz, S.; Claudino, A.M.; Lujic, S.; Smith, C.A.; Madden, S. Inpatient versus outpatient care, partial hospitalisation and waiting list for people with eating disorders. Cochrane Database Syst. Rev. 2019, 1, CD010827. [Google Scholar] [CrossRef]
- Herpertz-Dahlmann, B.; Borzikowsky, C.; Altdorf, S.; Heider, K.; Dempfle, A.; Dahmen, B. ‘Therapists in action’—Home treatment in adolescent anorexia nervosa: A stepped care approach to shorten inpatient treatment. Eur. Eat. Disord. Rev. 2020, 29, 427–442. [Google Scholar] [CrossRef]
- Dalle Grave, R.; Eckhardt, S.; Calugi, S.; Le Grange, D. A conceptual comparison of family-based treatment and enhanced cognitive behavior therapy in the treatment of adolescents with eating disorders. J. Eat. Disord. 2019, 7, 42. [Google Scholar] [CrossRef]
- Lock, J.; Le Grange, D.; Agras, W.S.; Moye, A.; Bryson, S.W.; Jo, B. Randomized clinical trial comparing family-based treatment with adolescent-focused individual therapy for adolescents with anorexia nervosa. Arch. Gen. Psychiatry 2010, 67, 1025–1032. [Google Scholar] [CrossRef]
- Agras, W.S.; Lock, J.; Brandt, H.; Bryson, S.W.; Dodge, E.; Halmi, K.A.; Jo, B.; Johnson, C.; Kaye, W.; Wilfley, D.; et al. Comparison of 2 family therapies for adolescent anorexia nervosa: A randomized parallel trial. JAMA Psychiatry 2014, 71, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Le Grange, D.; Hughes, E.K.; Court, A.; Yeo, M.; Crosby, R.D.; Sawyer, S.M. Randomized Clinical Trial of Parent-Focused Treatment and Family-Based Treatment for Adolescent Anorexia Nervosa. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Herpertz-Dahlmann, B.; Schwarte, R.; Krei, M.; Egberts, K.; Warnke, A.; Wewetzer, C.; Pfeiffer, E.; Fleischhaker, C.; Scherag, A.; Holtkamp, K.; et al. Day-patient treatment after short inpatient care versus continued inpatient treatment in adolescents with anorexia nervosa (ANDI): A multicentre, randomised, open-label, non-inferiority trial. Lancet 2014, 383, 1222–1229. [Google Scholar] [CrossRef]
- Yager, J.; Devlin, M.J.; Halmi, K.A.; Herzog, D.B.; Mitchell III, J.E.; Powers, P.; Zerbe, K.J. Guideline watch (August 2012): Practice guideline for the treatment of patients with eating disorders. Focus 2014, 12, 416–431. [Google Scholar] [CrossRef] [Green Version]
- Society for Adolescent Health and Medicine; Katzman, D.K.; Sawyer, S.M.; Ornstein, R.M.; Rome, E.S.; Garber, A.K.; Kohn, M.; Kreipe, R.E. Position Paper of the Society for Adolescent Health and Medicine: Medical management of restrictive eating disorders in adolescents and young adults. J. Adolesc. Health 2015, 56, 121–125. [Google Scholar]
- Lock, J.; Le Grange, D. Treatment Manual for Anorexia Nervosa: A Family-Based Approach; Paperback Edition; Guilford Press: New York, NY, USA, 2013; p. 289. [Google Scholar]
- Herpertz, S.; Herpertz-Dahlmann, B.; Fichter, M.; Tuschen-Caffier, B.; Zeeck, A. S3 Leitlinie Diagnostik und Behandlung der Essstörungen; Springer: Berlin, Germany, 2018. [Google Scholar]
- Sheehan, D.V.; Sheehan, K.H.; Shytle, R.D.; Janavs, J.; Bannon, Y.; Rogers, J.E.; Milo, K.M.; Stock, S.L.; Wilkinson, B. Reliability and validity of the mini international neuropsychiatric interview for children and adolescents (MINI-KID). J. Clin. Psychiatry 2010, 71, 17393. [Google Scholar] [CrossRef]
- Fairburn, C.G.; Beglin, S.J. Eating disorder examination questionnaire. Cogn. Behav. Ther. Eat. Disord. 2008, 309, 313. [Google Scholar]
- Kuczmarski, R.J. CDC Growth Charts: United States; US Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2000.
- Kromeyer-Hauschild, K.; Wabitsch, M.; Kunze, D.; Geller, F.; Geiß, H.C.; Hesse, V.; Hippel, A.; Jaeger, U.; Johnsen, D.; Korte, W.; et al. Perzentile für den Body-mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Mon. Kinderheilkd. 2001, 149, 807–818. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Taylor & Francis: New York, NY, USA, 1988. [Google Scholar]
- Fisher, C.A.; Skocic, S.; Rutherford, K.A.; Hetrick, S.E. Family therapy approaches for anorexia nervosa. Cochrane Database Syst. Rev. 2019, 5, CD004780. [Google Scholar] [CrossRef]
- Hübel, C.; Yilmaz, Z.; Schaumberg, K.E.; Breithaupt, L.; Hunjan, A.; Horne, E.; García-González, J.; O’Reilly, P.F.; Bulik, C.M.; Breen, G. Body composition in anorexia nervosa: Meta-analysis and meta-regression of cross-sectional and longitudinal studies. Int. J. Eat. Disord. 2019, 52, 1205–1223. [Google Scholar] [CrossRef] [Green Version]
- Dei, M.; Seravalli, V.; Bruni, V.; Balzi, D.; Pasqua, A. Predictors of recovery of ovarian function after weight gain in subjects with amenorrhea related to restrictive eating disorders. Gynecol. Endocrinol. 2008, 24, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Zito, J.M.; Tobi, H.; de Jong-van den Berg, L.T.; Fegert, J.M.; Safer, D.J.; Janhsen, K.; Hansen, D.G.; Gardner, J.F.; Glaeske, G. Antidepressant prevalence for youths: A multi-national comparison. Pharmacoepidemiol. Drug Saf. 2006, 15, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Wales, J.; Brewin, N.; Cashmore, R.; Haycraft, E.; Baggott, J.; Cooper, A.; Arcelus, J. Predictors of positive treatment outcome in people with anorexia nervosa treated in a specialized inpatient unit: The role of early response to treatment. Eur. Eat. Disord. Rev. 2016, 24, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.A.; Murray, S.B.; Anderson, L.K.; Kaye, W.H. Early predictors of treatment outcome in a partial hospital program for adolescent anorexia nervosa. Int. J. Eat. Disord. 2020, 53, 1550–1555. [Google Scholar] [CrossRef] [PubMed]
- Föcker, M.; Bühren, K.; Timmesfeld, N.; Dempfle, A.; Knoll, S.; Schwarte, R.; Egberts, K.M.; Pfeiffer, E.; Fleischhaker, C.; Wewetzer, C. The relationship between premorbid body weight and weight at referral, at discharge and at 1-year follow-up in anorexia nervosa. Eur. Child Adolesc. Psychiatry 2015, 24, 537–544. [Google Scholar] [CrossRef]
- Gregertsen, E.C.; Mandy, W.; Kanakam, N.; Armstrong, S.; Serpell, L. Pre-treatment patient characteristics as predictors of drop-out and treatment outcome in individual and family therapy for adolescents and adults with anorexia nervosa: A systematic review and meta-analysis. Psychiatry Res. 2019, 271, 484–501. [Google Scholar] [CrossRef]
- Nogal, P.; Pniewska-Siark, B.; Lewinski, A. Analysis of treatment efficacy in girls with anorexia nervosa (III). Neuroendocr. Lett. 2009, 30, 32–38. [Google Scholar]
- Couturier, J.; Lock, J. What is recovery in adolescent anorexia nervosa? Int. J. Eat. Disord. 2006, 39, 550–555. [Google Scholar] [CrossRef]
- Datta, N.; Matheson, B.E.; Le Grange, D.; Brandt, H.A.; Woodside, B.; Halmi, K.A.; Wilfley, D.E.; Lock, J.D. Exploring Differences in the Role of Hospitalization on Weight Gain Based on Treatment Type From Randomized Clinical Trials for Adolescent Anorexia Nervosa. Front. Psychiatry 2020, 11. [Google Scholar] [CrossRef]
- Sadeh-Sharvit, S.; Arnow, K.D.; Osipov, L.; Lock, J.D.; Jo, B.; Pajarito, S.; Brandt, H.; Dodge, E.; Halmi, K.A.; Johnson, C. Are parental self-efficacy and family flexibility mediators of treatment for anorexia nervosa? Int. J. Eat. Disord. 2018, 51, 275–280. [Google Scholar] [CrossRef]
- Byrne, C.E.; Accurso, E.C.; Arnow, K.D.; Lock, J.; Le Grange, D. An exploratory examination of patient and parental self-efficacy as predictors of weight gain in adolescents with anorexia nervosa. Int. J. Eat. Disord. 2015, 48, 883–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Striegel-Moore, R.H.; Leslie, D.; Petrill, S.A.; Garvin, V.; Rosenheck, R.A. One-year use and cost of inpatient and outpatient services among female and male patients with an eating disorder: Evidence from a national database of health insurance claims. Int. J. Eat. Disord. 2000, 27, 381–389. [Google Scholar] [CrossRef]
- Krauth, C.; Buser, K.; Vogel, H. How high are the costs of eating disorders - anorexia nervosa and bulimia nervosa—For German society? Eur. J. Health Econ. 2002, 3, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Ridic, G.; Gleason, S.; Ridic, O. Comparisons of health care systems in the United States, Germany and Canada. Mater Sociomed 2012, 24, 112–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| SAHM (USA) a | S3 (Germany) b |
|---|---|
| hypothermic (<36.3°) | rapid or sustained weight loss (>20% over six months) |
| Bradycardic (heart rate < 50 or QTc > 0.45) | severe underweight (BMI < 15 kg/m2, or below the 3rd sex- and age-adjusted BMI percentile in children and adolescents) |
| orthostatic (pulse increase > 35, systolic blood pressure decreases greater than 10 mm hg) | sustained weight loss or insufficient weight gain over three months (earlier for children and adolescents) despite outpatient or day-hospital treatment social or family factors, which strongly hamper the healing process (e.g., social isolation, problematic family situation, insufficient social support) |
| weight below 75% IBW | pronounced mental comorbidity |
| Suicidality | |
| severe bulimic symptoms (e.g., abuse of laxatives/diuretics, severe binge eating with vomiting) and/or excessive urge to exercise, which cannot be mastered in the outpatient setting | |
| physical risk or complications | |
| low insight into the illness | |
| excessive demands in the outpatient setting (too little structure in the guidelines regarding mealtime structure, amount of food, feedback on eating behavior; breakdown of family resources) | |
| necessity for treatment by a multi-professional team (multi-modal treatment program integrating psychological and medical treatment methods as well as social work and creative arts therapies) within a hospital setting (psychosomatic/psychiatric hospital treatment) |
| Assessment | Variable | Method | Assessed by a |
|---|---|---|---|
| Body weight | kilogram | FBT: medical scale, gown-weighed or light clothing IMT: medical scale in underwear | FBT: 1 or 2 IMT: 3 |
| Body height | centimeter | stadiometer | FBT: 1 or 2 IMT: 3 |
| Menstrual status | amenorrhea: yes/no, type | Interview | FBT: 1 IMT: 4 |
| Psychotropic medication | yes/no, type | Interview | FBT: 2 IMT: 4 |
| Duration of illness | months since illness onset | Interview | FBT: 2 IMT: 4 |
| Psychiatric comorbidities | yes/no, type | M.I.N.I b | FBT: 2 IMT:4 supervised by 5 |
| Eating Disorder Pathology | Total score Subscale score | EDE-Q c | FBT: self-report IMT: self-report |
| Days in hospital | hospital days after the first day of study intervention | Medical Records | FBT: 4 IMT: 4 |
| Broad, Non-Matched Samples a | FBT (USA) (n = 71) | IMT (Germany) (n = 29) | pb |
|---|---|---|---|
| Age | 15.1 ± 1.4 (12.2–18.1) | 14.7 ± 1.5 (12.1–17.6) | 0.241 |
| Female (n, %) | 59 (83.1) | 27 (93.1) | 0.191 |
| %mBMI | 90.5 ± 12.9 (73.0–145.6) | 78.3 ± 9.1 (63.1–107.0) | ≤0.001 * |
| BMI percentile c | 23.3 ± 24.1 (0.0–90.7) | 7.0 ± 14.4 (0.0–72.0) | ≤0.001 * |
| Weight(kg) | 47.2 ± 8.5 (27.0–77.3) | 43.1 ± 8.6 (29.4–72.0) | 0.029 * |
| Atypical AN (n, %) | 21 (29.6) | 6 (20.7) | 0.364 |
| Amenorrhea d (n, %) | 26 (36.7) | 22 (75.9) | ≤0.001 * |
| Months of illness | 13.1 ± 10.6 (2.0–57.0) | 12.7 ± 7.7 (4.0–36.0) | 0.879 |
| EDE-Q Global Score | 2.9 ± 1.8 (0.0–5.8) | 3.1 ± 1.7 (0.5–5.7) | 0.552 |
| Restraint | 2.8 ± 1.9 (0.0–6.0) | 3.1 ± 1.9 (0.0–6.0) | 0.481 |
| Weight Concern | 3.1 ± 2.1 (0.0–6.0) | 3.2 ± 2.1 (0.6–6.4) | 0.592 |
| Shape Concern | 3.4 ± 2.0 (0.0–6.0) | 3.7 ± 1.9 (0.8–6.0) | 0.374 |
| Eating Concern | 2.8 ± 1.9 (0.0–6.0) | 2.4 ± 1.4 (0.2–5.4) | 0.374 |
| ≥1 psychiatric comorbidity (%) | 42 (59.2) | 20 (69.0) | 0.359 |
| Depressive Disorder | 27 (38.0) | 15 (51.7) | 0.208 |
| Anxiety Disorder | 19 (26.8) | 6 (20.7) | 0.525 |
| OCD | 3 (4.2) | 6 (20.7) | 0.009 * |
| Other | 3 (4.2) | 3 (10.3) | 0.242 |
| Intake of ≥1 medication (%) | 27 (38.0) | 11 (37.9) | 0.493 |
| SSRI | 21 (29.6) | 3 (10.3) | 0.041 * |
| SNRI | 3 (4.2) | 0 (0.0) | 0.261 |
| Second-generation antipsychotic | 7 (9.9) | 2 (6.9) | 0.639 |
| Other | 3 (4.2) | 1 (3.4) | 0.857 |
| Matched Subsamplesa | FBT, USA (n = 21) | IMT, Germany (n = 20) | pb |
|---|---|---|---|
| Age | 15.0 ± 1.5 (12.2–17.4) | 14.7 ± 1.5 (12.1–17.4) | 0.457 |
| Female (n, %) | 17 (81.0) | 18 (90.0) | 0.413 |
| %mBMI | 79.3 ± 3.2 (74.1–84.6) | 77.3 ± 3.9 (70.9–83.5) | 0.081 † |
| BMI percentile c | 2.8 ± 2.5 (0.0–8.7) | 2.7 ± 2.6 (0.0–8.0) | 0.773 |
| Weight(kg) | 41.1 ± 7.2(27.0–54.1) | 42.1 ± 5.0 (30.1–49.1) | 0.591 |
| Amenorrhea d (n, %) | 11 (52.4) | 16 (80.0) | 0.062 † |
| Months of illness | 11.9 ± 9.9 (2.0–48.0) | 13.1 ± 8.2 (4.0–36.0) | 0.678 |
| EDE-Q (Global Score) | 2.0 ± 1.9 (0.0–5.8) | 2.8 ± 1.7 (0.5–5.3) | 0.200 |
| Restraint | 2.2 ± 2.1 (0.0-5.8) | 2.7 ± 1.9 (0.0–5.8) | 0.407 |
| Weight Concern | 2.2 ± 2.2 (0.0–6.0) | 2.8 ± 2.1 (0.6–6.4) | 0.170 |
| Shape Concern | 2.2 ± 2.2 (0.0–6.0) | 3.5 ± 1.9 (0.0–5.8) | 0.041 † |
| Eating Concern | 1.6 ± 1.8 (0.0–5.4) | 2.1 ± 1.3 (0.2–5.0) | 0.400 |
| Any psychiatric comorbidity (%) | 8 (38.1) | 14 (70.0) | 0.041 † |
| Depressive Disorder | 6 (28.6) | 10 (50.0) | 0.160 |
| Anxiety Disorder | 4 (19.0) | 2 (10.0) | 0.413 |
| OCD | 2 (9.5) | 6 (30.0) | 0.098 † |
| Other | 1 (4.8) | 3 (15.0) | 0.269 |
| Any psychotropic medication (%) | 4 (19.0) | 4 (20.0) | 0.939 |
| SSRI | 3 (14.3) | 1 (5.0) | 0.317 |
| SNRI | 0 (0.0) | 0(0.0) | 1.000 |
| Second-generation antipsychotic | 2 (9.5) | 2 (10.0) | 0.959 |
| Other | 1 (4.8) | 1 (5.0) | 0.972 |
| Matched Subsamples a | FBT, USA (n = 21) | IMT, Germany (n = 20) | pb | Cohen’s d |
|---|---|---|---|---|
| Months of observation | 6.0 ± 0.3 (5.5–6.5) | 6.3 ± 0.5 (5.5–7.2) | 0.005 * | 0.73 |
| Weight at baseline (kg) | 41.1 ± 7.2 (27.0–54.1) | 42.1 ± 5.0 (30.1–49.1) | 0.591 | 0.16 |
| Weekly weight gain (kg) c | 0.35 ± 0.18 (0.07–0.82) | 0.30 ± 0.18 (−0.01–0.85) | 0.407 | 0.28 |
| Weight at follow-up (kg) | 50.2 ± 8.0 (38.6–66.6) | 50.5 ± 8.2 (34.8–71.0) | 0.903 | 0.04 |
| %mBMI baseline | 79.3 ± 3.2 (74.1–84.6) | 77.3 ± 3.9 (70.9–83.5) | 0.081 | 0.56 |
| %mBMI at follow-up | 95.4 ± 6.8 (81.3–111.5) | 91.9 ± 7.1 (80.7–110.7) | 0.131 | 0.50 |
| %mBMI change | 16.0 ± 8.3 (2.7–33.8) | 14.3 ± 8.5 (−2.8–36.7) | 0.528 | 0.20 |
| Days in hospital d | 3 ± 5 (0–11) | 121 ± 42 (58–218) | <0.0001 * | 3.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadler, J.; Correll, C.U.; Le Grange, D.; Accurso, E.C.; Haas, V. The Impact of Inpatient Multimodal Treatment or Family-Based Treatment on Six-Month Weight Outcomes in Youth with Anorexia Nervosa: A Naturalistic, Cross-Continental Comparison. Nutrients 2022, 14, 1396. https://doi.org/10.3390/nu14071396
Nadler J, Correll CU, Le Grange D, Accurso EC, Haas V. The Impact of Inpatient Multimodal Treatment or Family-Based Treatment on Six-Month Weight Outcomes in Youth with Anorexia Nervosa: A Naturalistic, Cross-Continental Comparison. Nutrients. 2022; 14(7):1396. https://doi.org/10.3390/nu14071396
Chicago/Turabian StyleNadler, Janine, Christoph U. Correll, Daniel Le Grange, Erin C. Accurso, and Verena Haas. 2022. "The Impact of Inpatient Multimodal Treatment or Family-Based Treatment on Six-Month Weight Outcomes in Youth with Anorexia Nervosa: A Naturalistic, Cross-Continental Comparison" Nutrients 14, no. 7: 1396. https://doi.org/10.3390/nu14071396
APA StyleNadler, J., Correll, C. U., Le Grange, D., Accurso, E. C., & Haas, V. (2022). The Impact of Inpatient Multimodal Treatment or Family-Based Treatment on Six-Month Weight Outcomes in Youth with Anorexia Nervosa: A Naturalistic, Cross-Continental Comparison. Nutrients, 14(7), 1396. https://doi.org/10.3390/nu14071396






