Century Wide Changes in Macronutrient Levels in Indian Mothers’ Milk: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
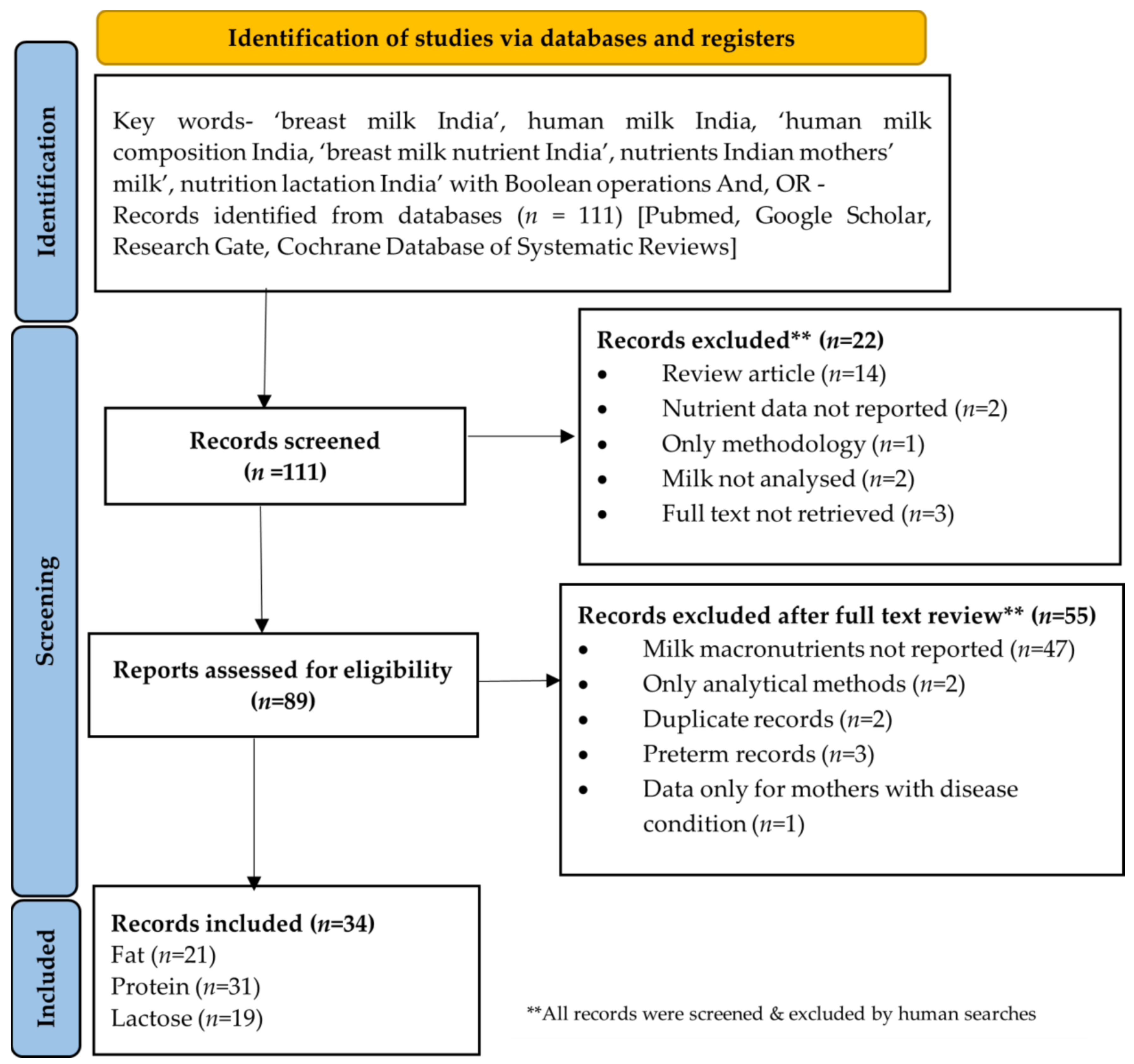
2.1. Search Strategy and Selection Criteria
- All observational, interventional or supplementation studies involving any nutrient in human milk of Indian mothers/in mothers of Indian origin published or accepted in peer reviewed journals were included during initial screening and assessed in detail for eligibility;
- Only studies which reported any macronutrient (fat, protein, lactose) were included for detailed assessment;
- Studies conducted on human milk in Indian mothers of term infants were included. Those records which reported both term and preterm mothers were also included but only term data was included in this review;
- Studies reporting any type of milk (colostrum/transitional/mature) from Indian mothers were included;
- Studies based on a milk macronutrients composition in maternal disease condition but had a control group as well were included. Data from control group from such publications was referred to for this review;
- Publications based on conferences, technical reports, Letters to the Editor with study findings, and data reported in Indian Medical Gazette on nutrient composition of Indian mother’s milk were also included.
- Animal and laboratory in vitro studies were excluded;
- Human milk nutrient composition of mothers of any other nationality other than Indian were excluded;
- All analytical/methodology papers, review articles, duplicate papers, or the records not reporting macronutrients were excluded;
- Publications where milk macronutrient composition was only from mothers with a disease condition were excluded.
2.2. Screening and Retrieval of Full Text
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
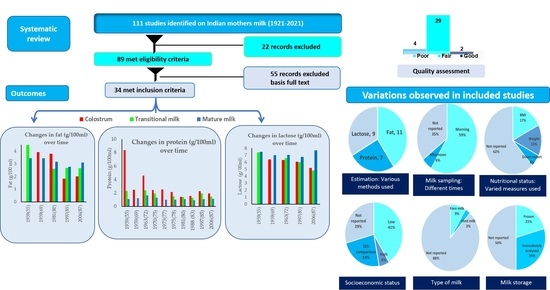
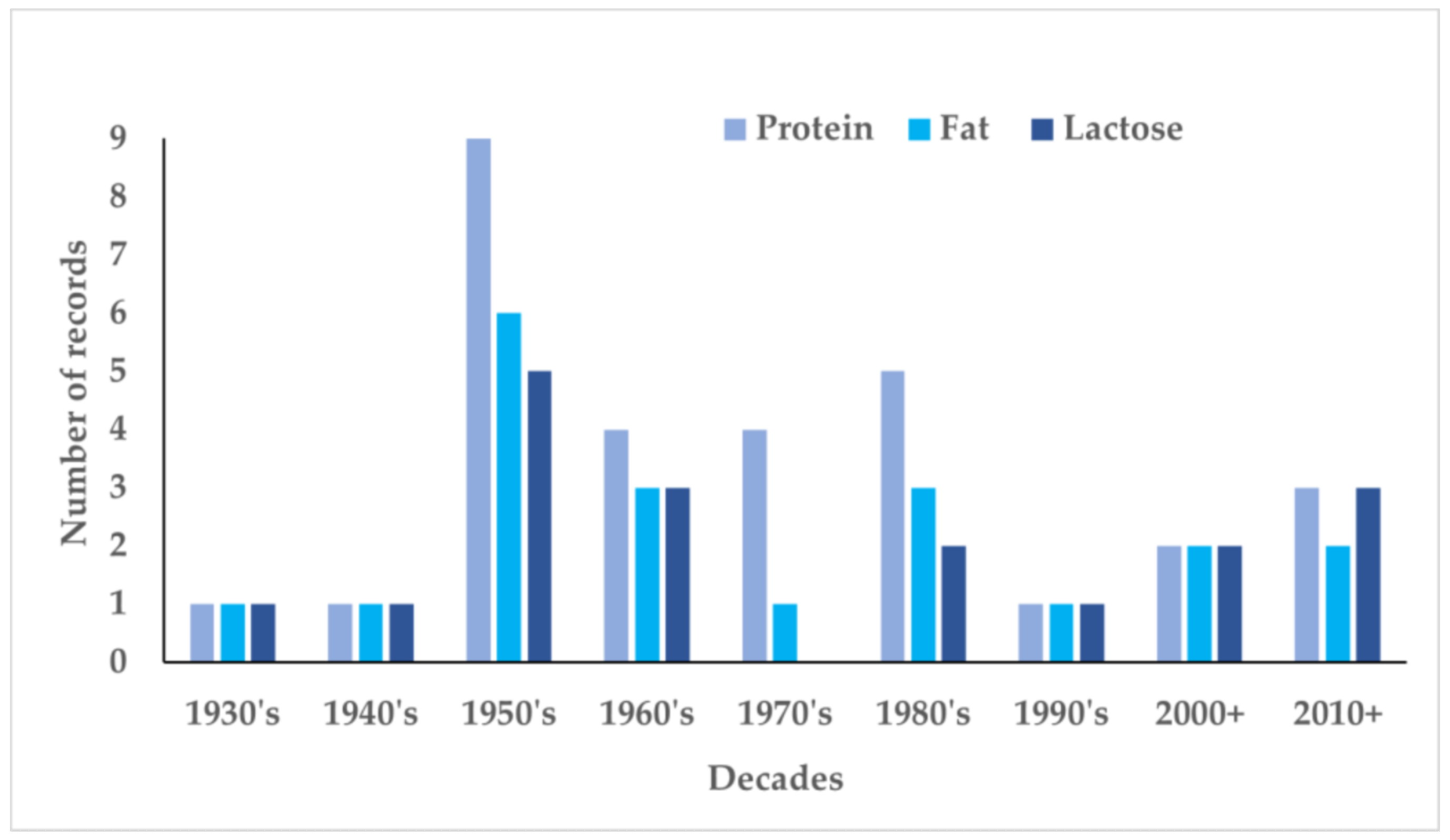
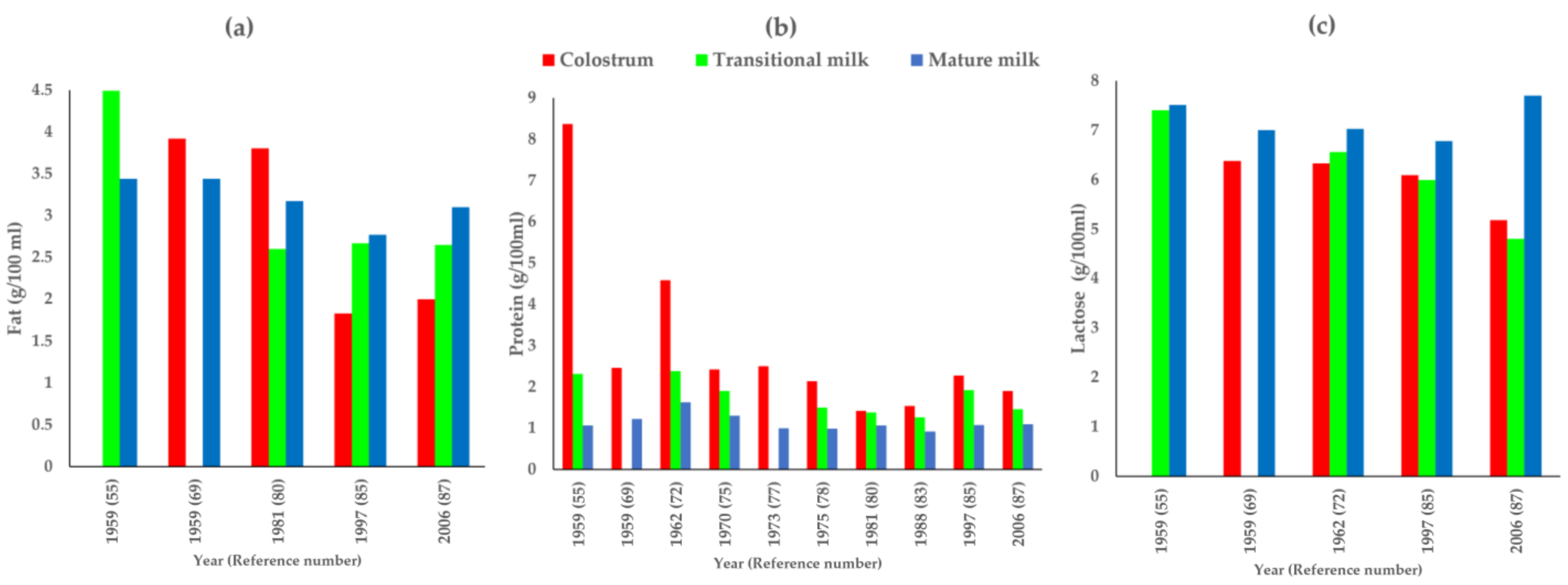
3. Results
4. Discussion
Strengths and Limitations of this Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Family Health Survey (NFHS-5), 2019–2021: India Fact Sheet. Available online: http://rchiips.org/nfhs/factsheet_NFHS-5.shtml (accessed on 26 November 2021).
- Victora, C.G.; Christian, P.; Vidaletti, L.P.; Gatica-Domínguez, G.; Menon, P.; Black, R.E. Revisiting maternal and child undernutrition in low-income and middle-income countries: Variable progress towards an unfinished agenda. Lancet 2021, 397, 1388–1399. [Google Scholar] [CrossRef]
- Office of the Registrar General and Census Commissioner. Sample Registration System Statistical Report 2018; Office of the Registrar General and Census Commissioner: New Delhi, India, 2018. [Google Scholar]
- UNICEF. Levels and Trends in Child Mortality Report 2017. UN Inter-Agency Group for Child Mortality Estimation; UNICEF: New York, NY, USA, 2017. [Google Scholar]
- United Nations Children’s Fund. Levels & Trends in Child Mortality: Report 2021, Estimates Developed by the United Nations Inter-Agency Group for Child Mortality Estimation; United Nations Children’s Fund: New York, NY, USA, 2021. [Google Scholar]
- Perin, J.; Mulick, A.; Yeung, D.; Villavicencio, F.; Lopez, G.; Strong, K.L.; Prieto-Merino, D.; Cousens, S.; Black, R.E.; Liu, L. Global, regional, and national causes of under-5 mortality in 2000-19: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc. Health 2022, 6, 106–115. [Google Scholar] [CrossRef]
- Lassi, Z.S.; Middleton, P.F.; Crowther, C.; Bhutta, Z.A. Interventions to Improve Neonatal Health and Later Survival: An Overview of Systematic Reviews. EBioMedicine 2015, 2, 985–1000. [Google Scholar] [CrossRef] [PubMed]
- Phukan, D.; Ranjan, M.; Dwivedi, L.K. Impact of timing of breastfeeding initiation on neonatal mortality in India. Int. Breastfeed. J. 2018, 13, 27. [Google Scholar] [CrossRef]
- Gartner, L.M.; Morton, J.; Lawrence, R.A.; Naylor, A.J.; O’Hare, D.; Schanler, R.J.; Eidelman, A.I. Breastfeeding and the use of human milk. Pediatrics 2005, 115, 496–506. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Protecting, Promoting, and Supporting Breastfeeding in Facilities Providing Maternal and Newborn Services; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- National Institute of Nutrition, Indian Council of Medical Research. Dietary Guidelines for Indians; National Institute of Nutrition: Hyderabad, Indian, 2011. [Google Scholar]
- Oftedal, O.T. The evolution of milk secretion and its ancient origins. Animal 2012, 6, 355–368. [Google Scholar] [CrossRef]
- Jensen, R.G. Lipids in human milk. Lipids 1999, 34, 1243–1271. [Google Scholar] [CrossRef]
- Perrella, S.; Gridneva, Z.; Lai, C.T.; Stinson, L.; George, A.; Bilston-John, S.; Geddes, D. Human milk composition promotes optimal infant growth, development and health. Semin. Perinatol. 2021, 45, 151380. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Fields, D.A.; George, B.; Williams, M.; Whitaker, K.; Allison, D.B.; Teague, A.; Demerath, E.W. Associations between human breast milk hormones and adipocytokines and infant growth and body composition in the first 6 months of life. Pediatr. Obes. 2017, 12 (Suppl. 1), 78–85. [Google Scholar] [CrossRef]
- Haschke, F.; Binder, C.; Huber-Dangl, M.; Haiden, N. Early-Life Nutrition, Growth Trajectories, and Long-Term Outcome. Nestle Nutr. Inst. Workshop Ser. 2019, 90, 107–120. [Google Scholar] [CrossRef] [PubMed]
- George, A.D.; Gay, M.C.L.; Wlodek, M.E.; Murray, K.; Geddes, D.T. The Fatty Acid Species and Quantity Consumed by the Breastfed Infant Are Important for Growth and Development. Nutrients 2021, 13, 4183. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Esparza, N.C.; Vásquez-Garibay, E.M.; Guzmán-Mercado, E.; Larrosa-Haro, A.; Comas-Basté, O.; Latorre-Moratalla, M.L.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Influence of the Type of Breastfeeding and Human Milk Polyamines on Infant Anthropometric Parameters. Front. Nutr. 2021, 8, 815477. [Google Scholar] [CrossRef] [PubMed]
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- Fields, D.A.; Schneider, C.R.; Pavela, G. A narrative review of the associations between six bioactive components in breast milk and infant adiposity. Obesity 2016, 24, 1213–1221. [Google Scholar] [CrossRef]
- Khan, J.; Vesel, L.; Bahl, R.; Martines, J.C. Timing of Breastfeeding Initiation and Exclusivity of Breastfeeding During the First Month of Life: Effects on Neonatal Mortality and Morbidity—A Systematic Review and Meta-analysis. Matern. Child Health J. 2015, 19, 468–479. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.; França, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Morrow, A.L.; Rangel, J.M. Human milk protection against infectious diarrhea: Implications for prevention and clinical care. Semin. Pediatr. Infect. Dis. 2004, 15, 221–228. [Google Scholar] [CrossRef]
- Oddy, W.H. Breastfeeding, Childhood Asthma, and Allergic Disease. Ann. Nutr. Metab. 2017, 70 (Suppl. 2), 26–36. [Google Scholar] [CrossRef]
- Rajani, P.S.; Seppo, A.E.; Järvinen, K.M. Immunologically Active Components in Human Milk and Development of Atopic Disease, With Emphasis on Food Allergy, in the Pediatric Population. Front. Pediatr. 2018, 6, 218. [Google Scholar] [CrossRef]
- Duijts, L.; Jaddoe, V.W.; Hofman, A.; Moll, H.A. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics 2010, 126, e18–e25. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Lochhead, P.; Ko, Y.; Claggett, B.; Leong, R.W.; Ananthakrishnan, A.N. Systematic review with meta-analysis: Breastfeeding and the risk of Crohn’s disease and ulcerative colitis. Aliment. Pharmacol. Ther. 2017, 46, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.A.; Wagner, C.L.; Feldman, H.A.; Shypailo, R.J.; Belfort, M.B. Associations of infant feeding with trajectories of body composition and growth. Am. J. Clin. Nutr. 2017, 106, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Demmelmair, H.; Grote, V.; Totzauer, M. Optimized protein intakes in term infants support physiological growth and promote long-term health. Semin. Perinatol. 2019, 43, 151153. [Google Scholar] [CrossRef] [PubMed]
- Rodekamp, E.; Harder, T.; Kohlhoff, R.; Franke, K.; Dudenhausen, J.W.; Plagemann, A. Long-term impact of breast-feeding on body weight and glucose tolerance in children of diabetic mothers: Role of the late neonatal period and early infancy. Diabetes Care 2005, 28, 1457–1462. [Google Scholar] [CrossRef][Green Version]
- Schneider-Worthington, C.R.; Bahorski, J.S.; Fields, D.A.; Gower, B.A.; Fernández, J.R.; Chandler-Laney, P.C. Associations Among Maternal Adiposity, Insulin, and Adipokines in Circulation and Human Milk. J. Hum. Lact. 2021, 37, 714–722. [Google Scholar] [CrossRef]
- Mitoulas, L.R.; Gurrin, L.C.; Doherty, D.A.; Sherriff, J.L.; Hartmann, P.E. Infant intake of fatty acids from human milk over the first year of lactation. Br. J. Nutr. 2003, 90, 979–986. [Google Scholar] [CrossRef]
- Koletzko, B.; Baker, S.; Cleghorn, G.; Neto, U.F.; Gopalan, S.; Hernell, O.; Hock, Q.S.; Jirapinyo, P.; Lonnerdal, B.; Pencharz, P.; et al. Global standard for the composition of infant formula: Recommendations of an ESPGHAN coordinated international ex-pert group. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 584–599. [Google Scholar] [CrossRef]
- Innis, S.M. Impact of maternal diet on human milk composition and neurological development of infants. Am. J. Clin. Nutr. 2014, 99, 734S–741S. [Google Scholar] [CrossRef]
- Daly, S.E.; Di Rosso, A.; Owens, R.A.; Hartmann, P.E. Degree of breast emptying explains changes in the fat content, but not fatty acid composition, of human milk. Exp. Physiol. 1993, 78, 741–755. [Google Scholar] [CrossRef]
- Minda, H.; Kovács, A.; Funke, S.; Szász, M.; Burus, I.; Molnár, S.; Marosvölgyi, T.; Decsi, T. Changes of fatty acid composition of human milk during the first month of lactation: A day-to-day approach in the first week. Ann. Nutr. Metab. 2004, 48, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Agostoni, C.; Bergmann, R.; Ritzenthaler, K.; Shamir, R. Physiological aspects of human milk lipids and implications for infant feeding: A workshop report. Acta Paediatr. 2011, 100, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of maternal nutrition on breast-milk composition: A systematic review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef] [PubMed]
- Leghi, G.E.; Netting, M.J.; Middleton, P.F.; Wlodek, M.E.; Geddes, D.T.; Muhlhausler, A.B.S. The impact of maternal obesity on human milk macronutrient composition: A systematic review and meta-analysis. Nutrients 2020, 12, 934. [Google Scholar] [CrossRef]
- Adhikari, S.; Kudla, U.; Nyakayiru, J.; Brouwer-Brolsma, E.M. Maternal dietary intake, nutritional status and macronutrient composition of human breast milk: Systematic review. Br. J. Nutr. 2021, 23, 1–25. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Lin, T.; Zhao, J.; Luo, Z.; Hou, J.; Sun, B.; Chen, L. Relationship between traditional maternal diet pattern and breastmilk composition of rural lactating women during the first month postpartum in Shigatse, Tibet. Food Sci. Nutr. 2021, 9, 4185–4198. [Google Scholar] [CrossRef]
- Gopalan, C. Studies on lactation in poor Indian Communities. J. Trop. Pediatr. 1958, 4, 87–91. [Google Scholar] [CrossRef]
- Jelliffe, D.B.; Jelliffe, E.F. The volume and composition of human milk in poorly nourished communities. A review. Am. J. Clin. Nutr. 1978, 31, 492–515. [Google Scholar] [CrossRef]
- Huffman, S.L.; Chowdhury, A.; Chakraborty, J.; Simpson, N.K. Breast-feeding patterns in rural Bangladesh. Am. J. Clin. Nutr. 1980, 33, 144–154. [Google Scholar] [CrossRef]
- Al-Tamer, Y.Y.; Mahmood, A.A. The influence of Iraqi mothers’ socioeconomic status on their milk-lipid content. Eur. J. Clin. Nutr. 2006, 60, 1400–1405. [Google Scholar] [CrossRef][Green Version]
- Peng, Y.; Zhou, T.; Wang, Q.; Liu, P.; Zhang, T.; Zetterström, R.; Strandvik, B. Fatty acid composition of diet, cord blood and breast milk in Chinese mothers with different dietary habits. Prostaglandins Leukot Essent Fat. Acids 2009, 81, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Su, L.L.; Sk, T.C.; Lim, S.L.; Chen, Y.; Tan, E.A.; Pai, N.N.; Gong, Y.H.; Foo, J.; Rauff, M.; Chong, Y.S. The influence of maternal ethnic group and diet on breast milk fatty acid composition. Ann. Acad. Med. Singap. 2010, 39, 675. [Google Scholar] [PubMed]
- Roy, S.; Ghosh, S.; Dhar, P.; Ghosh, M. Studies on the fluidity of milk lipids of mothers from three socioeconomic groups of West Bengal, India. J. Trop. Pediatr. 2013, 59, 407–412. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nayak, U.; Kanungo, S.; Zhang, D.; Ross Colgate, E.; Carmolli, M.P.; Dey, A.; Alam, M.; Manna, B.; Nandy, R.K.; Kim, D.R.; et al. Influence of maternal and socioeconomic factors on breast milk fatty acid composition in urban, low-income families. Matern Child Nutr. 2017, 13, e12423. [Google Scholar] [CrossRef]
- Butts, C.A.; Hedderley, D.I.; Herath, T.D.; Paturi, G.; Glyn-Jones, S.; Wiens, F.; Stahl, B.; Gopal, P. Human Milk Composition and Dietary Intakes of Breastfeeding Women of Different Ethnicity from the Manawatu-Wanganui Region of New Zealand. Nutrients 2018, 10, 1231. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Liu, G.; Whitfield, K.C.; Kroeun, H.; Green, T.J.; Gibson, R.A.; Makrides, M.; Zhou, S.J. Comparison of Human Milk Fatty Acid Composition of Women From Cambodia and Australia. J. Hum. Lact. 2018, 34, 585–591. [Google Scholar] [CrossRef]
- Kumar, H.; du Toit, E.; Kulkarni, A.; Aakko, J.; Linderborg, K.M.; Zhang, Y.; Nicol, M.P.; Isolauri, E.; Yang, B.; Collado, M.C.; et al. Distinct Patterns in Human Milk Microbiota and Fatty Acid Profiles Across Specific Geographic Locations. Front. Microbiol. 2016, 7, 1619. [Google Scholar] [CrossRef]
- Brown, K.H.; Akhtar, N.A.; Robertson, A.D.; Ahmed, M.G. Lactational capacity of marginally nourished mothers: Relationships between maternal nutritional status and quantity and proximate composition of milk. Pediatrics 1986, 78, 909–919. [Google Scholar] [CrossRef]
- Belavady, B.; Gopalan, C. Chemical composition of human milk in poor Indian women. Indian J. Med. Res. 1959, 47, 234–245. [Google Scholar]
- Reddy, V.; Bhaskaram, C.; Raghuramulu, N.; Jagadeesan, V. Antimicrobial factors in human milk. Acta Paediatr. Scand. 1977, 66, 229–232. [Google Scholar] [CrossRef]
- Narula, P.; Mittal, S.K.; Gupta, S.; Saha, K. Cellular and humoral factors of human milk in relation to nutritional status in lactating mothers. Indian J. Med. Res. 1982, 76, 415–423. [Google Scholar] [PubMed]
- Garg, M.; Thirupuram, S.; Saha, K. Colostrum composition, maternal diet and nutrition in north India. J. Trop. Pediatr. 1988, 34, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Christian, P.; Smith, E.R.; Lee, S.E.; Vargas, A.J.; Bremer, A.A.; Raiten, D.J. The need to study human milk as a biological system. Am. J. Clin. Nutr. 2021, 113, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Rodriguez-Palmero, M.; Demmelmair, H.; Fidler, N.; Jensen, R.; Sauerwald, T. Physiological aspects of human milk lipids. Early Hum. Dev. 2001, 65, S3–S18. [Google Scholar] [CrossRef]
- Bunce, E.H. Observations on the composition of human milk in Burma. Indian Med. Gaz. 1931, 66, 306. [Google Scholar]
- Sundararajan, A.R. The vitamin B1 content of human milk. Indian J. Med. Res. 1941, 29, 567–573. [Google Scholar]
- Srinivasan, P.R.; Ramanathan, M.K. Protein and sulphur amino acids in breast milk of poor class Indian women in the Nilgiris. Indian J. Med. Res. 1954, 42, 51–54. [Google Scholar]
- Karmarkar, M.G.; Deodhar, A.D.; Kapur, J.; Ramakrishnan, C.V. Effect of dietary fat on fat content of human milk. Lancet 1958, 272, 909. [Google Scholar] [CrossRef]
- Belavady, B. Distribution of nitrogen in breast milk of Indian mothers. Indian J. Med. Res. 1959, 47, 217–221. [Google Scholar]
- Belavady, B.; Pasricha, S.; Shankar, K. Studies on lactation and dietary habits of Nilgiri Hill Tribes. Indian J. Med. Res. 1959, 47, 221–233. [Google Scholar] [PubMed]
- Mukherji, P.S.; Anwikar, A.K. Protein content of breast milk of Indian mothers with malnourished children. Indian J. Pediatr. 1959, 26, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.P.; Saran, A.; Sinha, A.S. Composition of human milk. Indian J. Physiol. Pharmacol. 1959, 3, 173–176. [Google Scholar] [PubMed]
- Karmarkar, M.G.; Kapur, J.; Deodhar, A.D.; Ramakrishnan, C.V. Studies on human lactation Part I. Diet survey of lactating women in different socio-economic status and stage of lactation of the proximate principles and essential amino acids of human milk. Indian J. Med. Res. 1959, 47, 344–351. [Google Scholar]
- Karmarkar, M.G.; Ramakrishnan, C.V. Relation between the dietary intake of lactating women and the chemical composition of milk with regard to principal and certain inorganic constituents. Acta Pediatr. 1960, 49, 599–604. [Google Scholar] [CrossRef]
- Ashdhir, S.; Puri, B. Human milk studies—Chemical composition of human milk at three different stages. Indian J. Pediatrics 1962, 29, 99–109. [Google Scholar] [CrossRef]
- Deb, A.K.; Cama, H.R. Studies on human lactation. Dietary nitrogen utilization during lactation, and distribution of nitrogen in mother’s milk. Br. J. Nutr. 1962, 16, 65–73. [Google Scholar] [CrossRef]
- Karmarkar, M.G.; Rajalakshmi, R.; Ramakrishnan, C.V. Effect of Dietary Protein and Fat Supplementation on Protein, Fat and Essential Amino acid Contents of Breast Milk. Acta Paediatr. Scand. 1963, 52, 473–480. [Google Scholar] [CrossRef]
- Khurana, V.; Agarwal, K.N.; Gupta, S.; Nath, T. Estimation of total protein in breast milk of Indian lactating mothers. Indian Pediatr. 1970, 7, 156–158. [Google Scholar]
- Jathar, V.S.; Kamath, S.A.; Parikh, M.N.; Rege, D.V.; Satoskar, R.S. Maternal milk and serum vitamin B12, folic acid, and protein levels in Indian subjects. Arch. Dis. Child 1970, 45, 236–241. [Google Scholar] [CrossRef]
- Rao, P.U.; Belavady, B. Protein fractions in human milk: Part II-Isolation and characterization of basic protein from human milk & lytic activity of milk samples. Indian J. Biochem. Biophys. 1973, 10, 87–90. [Google Scholar] [PubMed]
- Agarwal, K.N.; Khurana, V.; Agarwal, D.K. Protein and free amino acids content of human milk. Indian Pediatr. 1975, 12, 415–417. [Google Scholar] [PubMed]
- Belavady, B. Lipid and trace element composition of human milk. Acta Paediatr. Scand. 1978, 67, 566–571. [Google Scholar] [CrossRef]
- Rao, P.S.; Belavady, B. Lipids and bile salt stimulated lipase in human milk of Indian mothers. Indian J. Med. Res. 1981, 73, 363–368. [Google Scholar]
- Bijur, A.M.; Desai, A.G. Composition of breast milk with reference to vitamin B12 and folic acid in Indian mothers. Indian J. Pediatr. 1985, 52, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Kumbhat, M.M.; Khanna, S.A.; Bijur, A.M.; Jadhav, C.S. Breast milk composition in relation to gestation. Indian Pediatr. 1985, 22, 229–233. [Google Scholar] [PubMed]
- Raghuvanshi, R.S.; Agarwal, K.N.; Fransson, G.B.; Hambreus, L. Breastmilk total nitrogen, non-protein nitrogen and lactoferrin content. Indian Pediatr. 1988, 25, 149–159. [Google Scholar]
- Patil, N.D.; Phadke, M.S. Proximate principle and energy content of human milk in well-nourished urban mother. Indian Pediatr. 1989, 26, 1211–1213. [Google Scholar]
- Paul, V.K.; Singh, M.; Srivastava, L.M.; Arora, N.K.; Deorari, A.K. Macronutrient and energy content of breast milk of mothers delivering prematurely. Indian J. Pediatr. 1997, 64, 379–382. [Google Scholar] [CrossRef]
- Kaushik, S.; Trivedi, S.S.; Jain, A.; Bhattacharjee, J. Unusual changes in colostrum composition in lactating Indian women having medical complications during pregnancy-A pilot study. Indian J. Clin. Biochem. 2002, 17, 68–73. [Google Scholar] [CrossRef][Green Version]
- Narang, A.P.; Bains, H.S.; Kansal, S.; Singh, D. Serial composition of human milk in preterm and term mothers. Indian J. Clin. Biochem. 2006, 21, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Dias, B.; Nakhawa, P. Effect of maternal nutritional status on the biochemical composition of human milk. Int. J. Res. Med. Sci. 2016, 4, 4541–4543. [Google Scholar] [CrossRef][Green Version]
- Kothari, N.; Kothari, P.K.; Mondkar, J. Effect of maternal nutritional status on human milk composition. New Indian J. Pediatr. 2018, 7, 94–100. [Google Scholar]
- Divedi, P.; Maheshwari, P.; Seth, S.; Mishra, A. Analysis of Transitional Milk of Post-Natal Cases in Non-Anaemic Mothers and Its Comparison with Anaemic Mothers in Rural Western Uttar Pradesh. Cureus 2020, 12, e11818. [Google Scholar] [CrossRef]
- Study Quality Assessment Tools | NHLBI, NIH. 2021, 18, 1897. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 1 February 2022).
- Jenness, R. The composition of human milk. Semin. Perinatol. 1979, 3, 225–239. [Google Scholar]
- Miller, E.M.; Aiello, M.O.; Fujita, M.; Hinde, K.; Milligan, L.; Quinn, E.A. Field and laboratory methods in human milk research. Am. J. Hum. Biol. 2013, 25, 1–11. [Google Scholar] [CrossRef]
- Khan, S.; Hepworth, A.R.; Prime, D.K.; Lai, C.T.; Trengove, N.J.; Hartmann, P.E. Variation in fat, lactose, and protein composition in breast milk over 24 h: Associations with infant feeding patterns. J. Hum. Lact. 2013, 29, 81–89. [Google Scholar] [CrossRef]
- Leghi, G.E.; Middleton, P.F.; Netting, M.J.; Wlodek, M.E.; Geddes, D.T.; Muhlhausler, B.S. A Systematic Review of Collection and Analysis of Human Milk for Macronutrient Composition. J. Nutr. 2020, 150, 1652–1670. [Google Scholar] [CrossRef]
- Samuel, T.M.; Zhou, Q.; Giuffrida, F.; Munblit, D.; Verhasselt, V.; Thakkar, S.K. Nutritional and Non-nutritional Composition of Human Milk Is Modulated by Maternal, Infant, and Methodological Factors. Front. Nutr. 2020, 7, 576133. [Google Scholar] [CrossRef]
- Agostoni, C.; Braegger, C.; Decsi, T.; Kolacek, S.; Koletzko, B.; Michaelsen, K.F.; Mihatsch, W.; Moreno, L.A.; Puntis, J.; Shamir, R.; et al. Breast-feeding: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 112–125. [Google Scholar] [CrossRef] [PubMed]
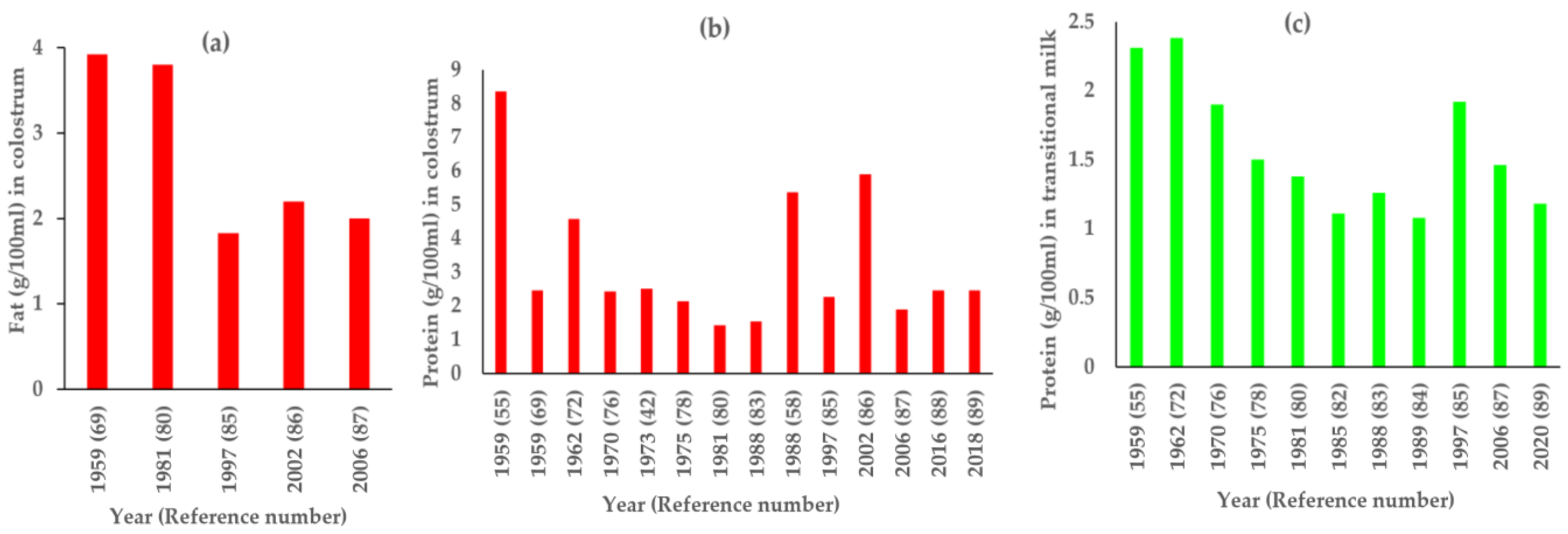
| S.No. | First Author, Year (Total N) | Fat (g/100 mL) | Protein (g/100 mL) | Lactose (g/100 mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Colostrum | Transitional | Mature | Colostrum | Transitional | Mature | Colostrum | Transitional | Mature | ||
| 1 | Bunce, 1931 [62] (N = 8) | 3.23 † (n = 3) | 2.33 † (n = 3) | 6.78 † (n = 3) | ||||||
| 2 | Sundararajan, 1941 [63] (N = 40) | 3.3 † (infants < 4 m–1.1–5.9, n = 17; infants > 4 m-1.0–6.9, n = 23) | 2.05 † (infants < 4 m–1.4–3.4, n = 15; infants > 4 m–0.5–3.6, n = 20) | 7.26 † (infants < 4 m–3.2–9.5, n = 21; infants > 4 m-4.4–9.0, n = 19) | ||||||
| 3 | Srinivasan, 1954 [64] (N = 31) | 1.27 * (0.89–1.80, n = 25) | ||||||||
| 4 | Karmarkar, 1958 [65] (N = 175) | 4.68 † (n = 175) | ||||||||
| 5 | Gopalan P1, 1958 [43] (N = 6) | 4.78 (n = 6) | 1.19 † (n = 6) | 6.99 † (n = 6) | ||||||
| 6 | Gopalan P2, 1958 [43] (N = 40) | 3.34 ± 0.242 * (n = 40) | 1.06 ± 0.036 * (n = 40) | 7.47 ± 0.072 * (n = 40) | ||||||
| 7 | Belavady, 1959 [66] (N = 29) | 0.96 † (n = 29) | ||||||||
| 8 | Belavady, 1959 [55] (N = 191) | 4.49 ± 0.47 * (n = 9) | 3.44 † (n = 109) | 8.36 ± 0.921 * (n = 18) | 2.31 ± 0.435 * (n = 74) | 1.07 † (n = 146) | 7.41 ± 0.161 * | 7.51 † (n = 146) | ||
| 9 | Belavady, 1959 [67] (N = 36) | 2.85 † (n = 20) | 1.05 † (n = 36) | 7.67 † (n = 20) | ||||||
| 10 | Mukherji, 1959 [68] (N = 35) | 1.24 † (0–1 year- 0.7–2.8, n = 25; 1–2 year-0.8–2.3, n = 10) | ||||||||
| 11 | Sinha, 1959 [69] (N = 63) | 3.92 * (1.6–5.8, n = 9) | 3.44 ± 1.50 * (1.0–7.0, n = 54) | 2.46 * (1.83–3.84, n = 9) | 1.22 ± 0.28 * (0.84–2.3, n = 54) | 6.38 * (5.82–7.0, n = 9) | 7 ± 0.17 * (67–7.6, n = 54) | |||
| 12 | Karmarkar, 1959 [70] (N = 232) | 4.6 † (n = 232) | 1.33 † (n = 232) | 7.17 † (n = 232) | ||||||
| 13 | Karmarkar, 1960 [71] (N = 60) | 4.49 † (n = 60) | 1.37 † (n = 60) | 7.15 † (n = 60) | ||||||
| 14 | Ashdhir, 1962 [72] (N = 10) | 4.58 ± 0.83 * (3.31–5.88, n = 10) | 2.38 ± 0.54 * (1.05–3.80, n = 10) | 1.63 ± 0.4 * (1.0–2.14, n = 10) | 6.33 ± 0.45 * (5.61–7.18, n = 10) | 6.56 ± 0.68 * (5.59–7.78, n = 10) | 7.03 ± 0.71 * (5.71–7.86, n = 10) | |||
| 15 | Deb, 1962 [73] (N = 20) | 2.99 * (2.4–3.8, n = 20) | 0.88 * (0.70–1.18, n = 20) | 6.88 * (6.8–7.1, n = 20) | ||||||
| 16 | Karmarkar, 1963 [74] (N = 60) | 3.55 * (n = 5) | 1.08 * (n = 5) | |||||||
| 17 | Khurana, 1970 [75] (N = 194) | 2.425 ± 0.510 * (0.857–4.237, n = 28) | 1.904 ± 0.6 * (0.811–3.400, n = 30) | 1.3 † (0.250–3.600, n = 136) | ||||||
| 18 | Jathar, 1970 [76] (N = 48) | 1.58 ± 0.06 * (n = 48) | ||||||||
| 19 | Rao, 1973 [77] (N = 31) | 2.5 ± 0.54 * (n = 8) | 1 † (n = 24) | |||||||
| 20 | Agarwal, 1975 [78] (N = 97) | 2.14 ± 0.98 * (0.89–4.12, n = 21) | 1.5 ± 0.69 * (0.81–2.91, n = 17) | 0.99 ± 0.41 * (0.88–3.12, n = 59) | ||||||
| 21 | Belavady, 1978 [79] (N = NA) | 3.23 † | ||||||||
| 22 | Rao, 1981 [80] (N = 70) | 3.8 ± 1.28 * (n = 4) | 2.6 ± 1.27 * (n = 12) | 3.17 † (n = 52) | 1.42 ± 0.071 * (n = 3) | 1.38 ± 0.0537 * (n = 7) | 1.07 † (n = 34) | |||
| 23 | Bijur, 1985 [81] (N = 50) | 1.22 † (n = 50) | ||||||||
| 24 | Kumbhat, 1985 [82] (N = 50) | 3.079 † (n = 25) | 1.34 * (n = 25) | 1.27 † (n = 25) | 1.235 † (n = 25) | 6.9 † (n = 25) | ||||
| 25 | Raghuvanshi, 1988 [83] (N = 121) | 1.54 ± 1.16 * (n = 10) | 1.26 ± 0.17 * (n = 7) | 0.922 † (n = 237) | ||||||
| 26 | Garg, 1988 [58] (N = 35) | 5.36 † (WN- 4.5–6.8, n = 20; UN- 2.6–6.8, n = 15) | ||||||||
| 27 | Patil, 1989 [84] (N = 54) | 4.48 ± 1.5 * (1.54–9.0, n = 54) | 1.08 ± 0.42 * (0.70–3.44, n = 54) | 6.51 ± 1.28 * (4.0–9.4, n = 54) | ||||||
| 28 | Paul, 1997 [85] (N = 52) | 1.83 ± 0.58 * (n = 23) | 2.67 † (n = 23) | 2.77 ± 0.80 * (n = 23) | 2.27 ± 0.93 * (n = 23) | 1.92 † (n = 23) | 1.08 ± 0.63 * (n = 23) | 6.09 ± 1.49 * (n = 23) | 5.99 † (n = 23) | 6.78 ± 1.75 * (n = 23) |
| 29 | Kaushik, 2002 [86] (N = 80) | 2.2 ± 0.8 * (n = 20) | 5.9 ± 0.9 * (n = 20) | 4.5 ± 0.9 * (n = 20) | ||||||
| 30 | Narang, 2006 [87] (N = 86) | 2.0 ± 0.58 * (n = 41) | 2.65 † (n = 41) | 3.1 ± 0.69 * (n = 41) | 1.9 ± 0.69 * (n = 41) | 1.46 † (n = 41) | 1.1 ± 0.48 * (n = 41) | 5.18 ± 0.44 * (n = 41) | 4.8 † (n = 41) | 7.7 ± 1.3 * (n = 41) |
| 31 | Roy, 2013 [49] (N = 217) | 4.57 † (n = 209) | ||||||||
| 32 | Dias, 2016 [88] (N = 63) | 2.46 ± 1.25 * (0.6–7.1, n = 63) | 6.47 ± 1.45 * (4.2–9.7, n = 63) | |||||||
| 33 | Kothari, 2018 [89] (N = 63) | 2.46 ± 1.25 * (0.6–7.1, n = 63) | 6.47 ± 1.45 * (4.2–9.7, n = 63) | |||||||
| 34 | Divedi, 2020 [90] (N = 132) | 5.59 ± 0.54 * (n = 66) | 1.18 ± 0.15 * (n = 66) | 5.63 ± 0.41 * (n = 66) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanna, D.; Yalawar, M.; Verma, G.; Gupta, S. Century Wide Changes in Macronutrient Levels in Indian Mothers’ Milk: A Systematic Review. Nutrients 2022, 14, 1395. https://doi.org/10.3390/nu14071395
Khanna D, Yalawar M, Verma G, Gupta S. Century Wide Changes in Macronutrient Levels in Indian Mothers’ Milk: A Systematic Review. Nutrients. 2022; 14(7):1395. https://doi.org/10.3390/nu14071395
Chicago/Turabian StyleKhanna, Deepti, Menaka Yalawar, Gaurav Verma, and Shavika Gupta. 2022. "Century Wide Changes in Macronutrient Levels in Indian Mothers’ Milk: A Systematic Review" Nutrients 14, no. 7: 1395. https://doi.org/10.3390/nu14071395
APA StyleKhanna, D., Yalawar, M., Verma, G., & Gupta, S. (2022). Century Wide Changes in Macronutrient Levels in Indian Mothers’ Milk: A Systematic Review. Nutrients, 14(7), 1395. https://doi.org/10.3390/nu14071395