Antioxidant and Anti-Inflammatory Properties of Rubber Seed Oil in Lipopolysaccharide-Induced RAW 267.4 Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Characterization of Physicochemical Properties of RSO
2.3. Cell Culture and Cell Viability Testing
2.4. Determination of Intracellular ROS Level
2.5. Determination of NO, MDA, and T-AOC
2.6. Determination of Inflammatory Cytokine
2.7. RNA Extraction and qRT-PCR
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
3.1. Physicochemical Characteristics of RSO
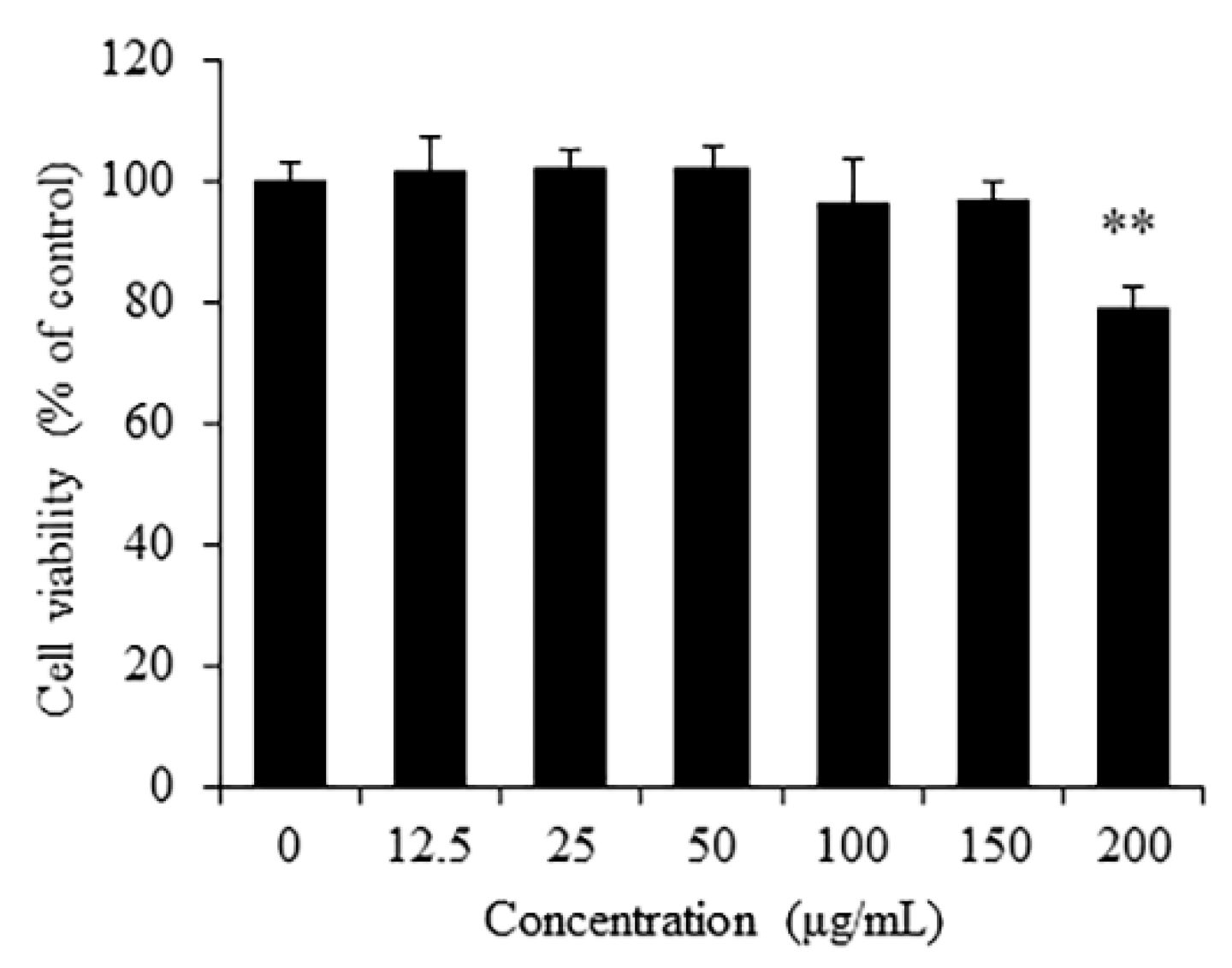
3.2. Cytotoxicity of RSO on RAW 264.7 Cells
3.3. Antioxidant Properties of RSO
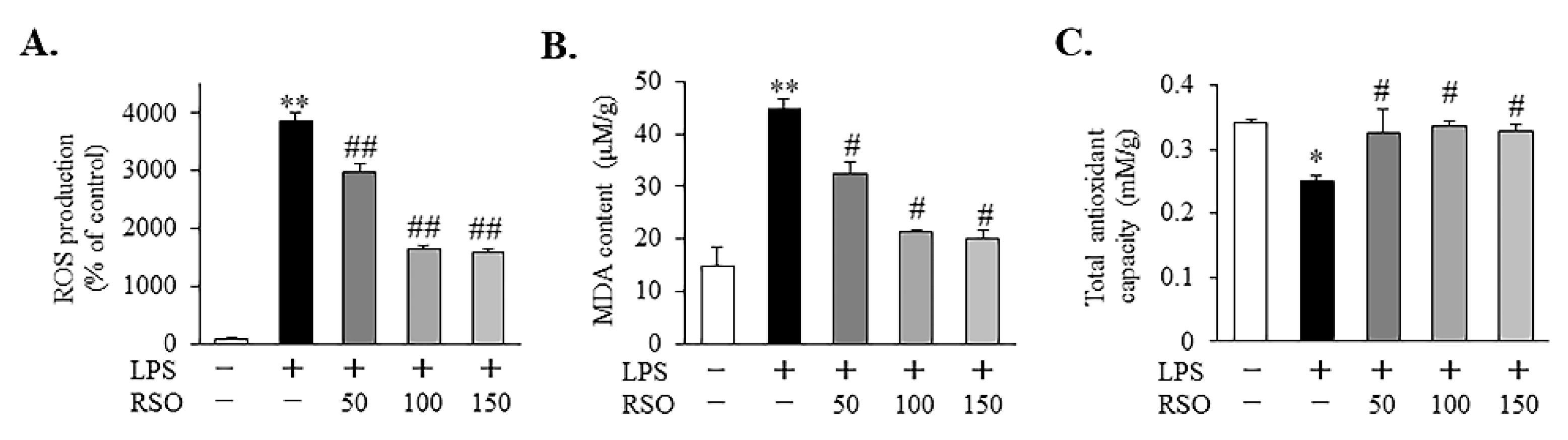
3.3.1. Effect of RSO on ROS Levels of LPS-Induced RAW 264.7 Cells
3.3.2. Effect of RSO on MDA Levels of LPS-Induced RAW 264.7 Cells
3.3.3. Effect of RSO on T-AOC of LPS-Induced RAW 264.7 Cells
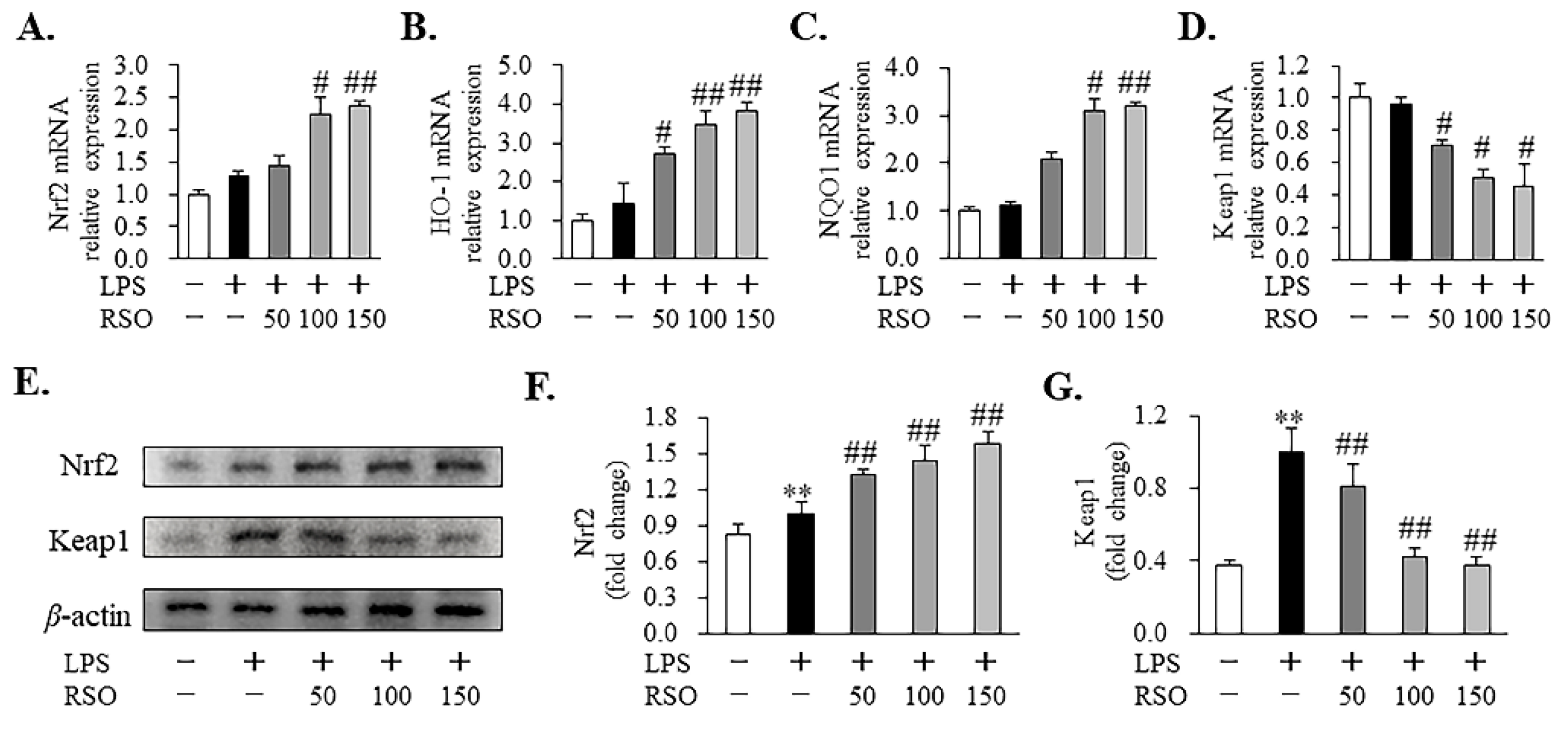
3.3.4. Effect of RSO on Nrf2-Keap1 Pathway of LPS-Induced RAW 264.7 Cells
3.4. Anti-Inflammatory Properties of RSO
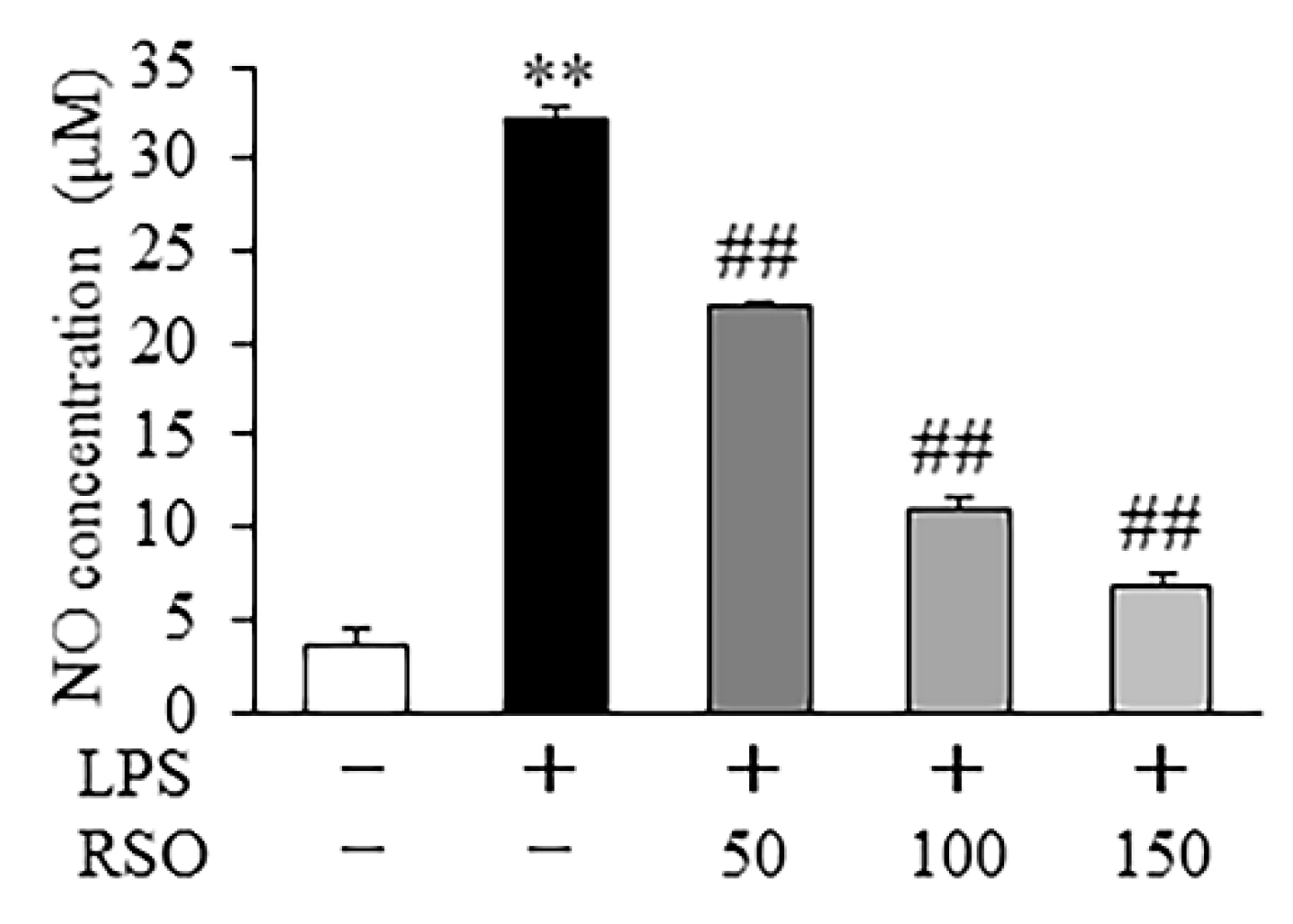
3.4.1. Effect of RSO on NO Contents of LPS-Induced RAW 264.7 Cells
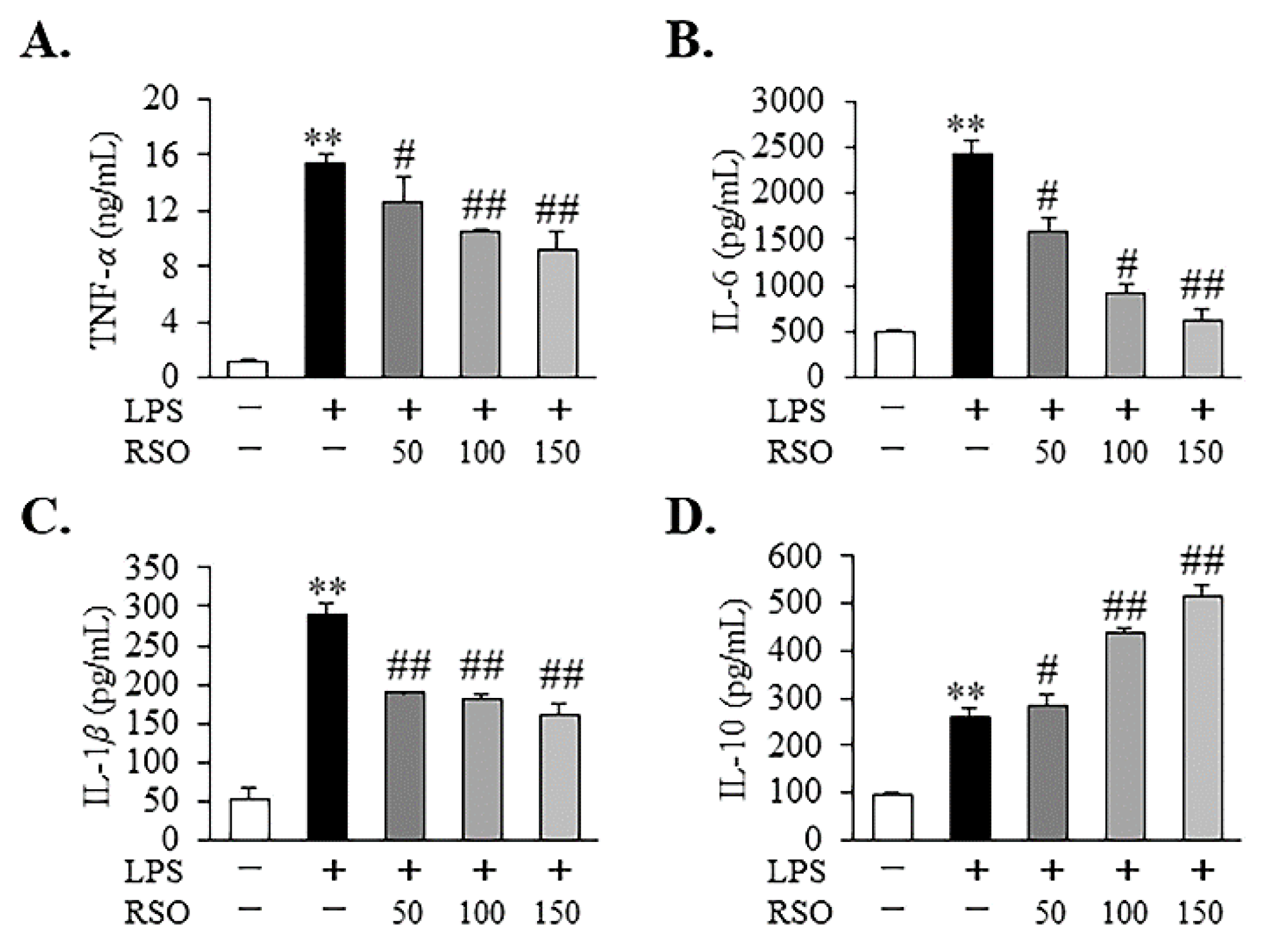
3.4.2. Effect of RSO on Inflammatory Cytokine of LPS-Induced RAW 264.7 Cells
3.4.3. Effect of RSO on Inflammatory-Related Gene Expression of LPS-Induced RAW 264.7 Cells
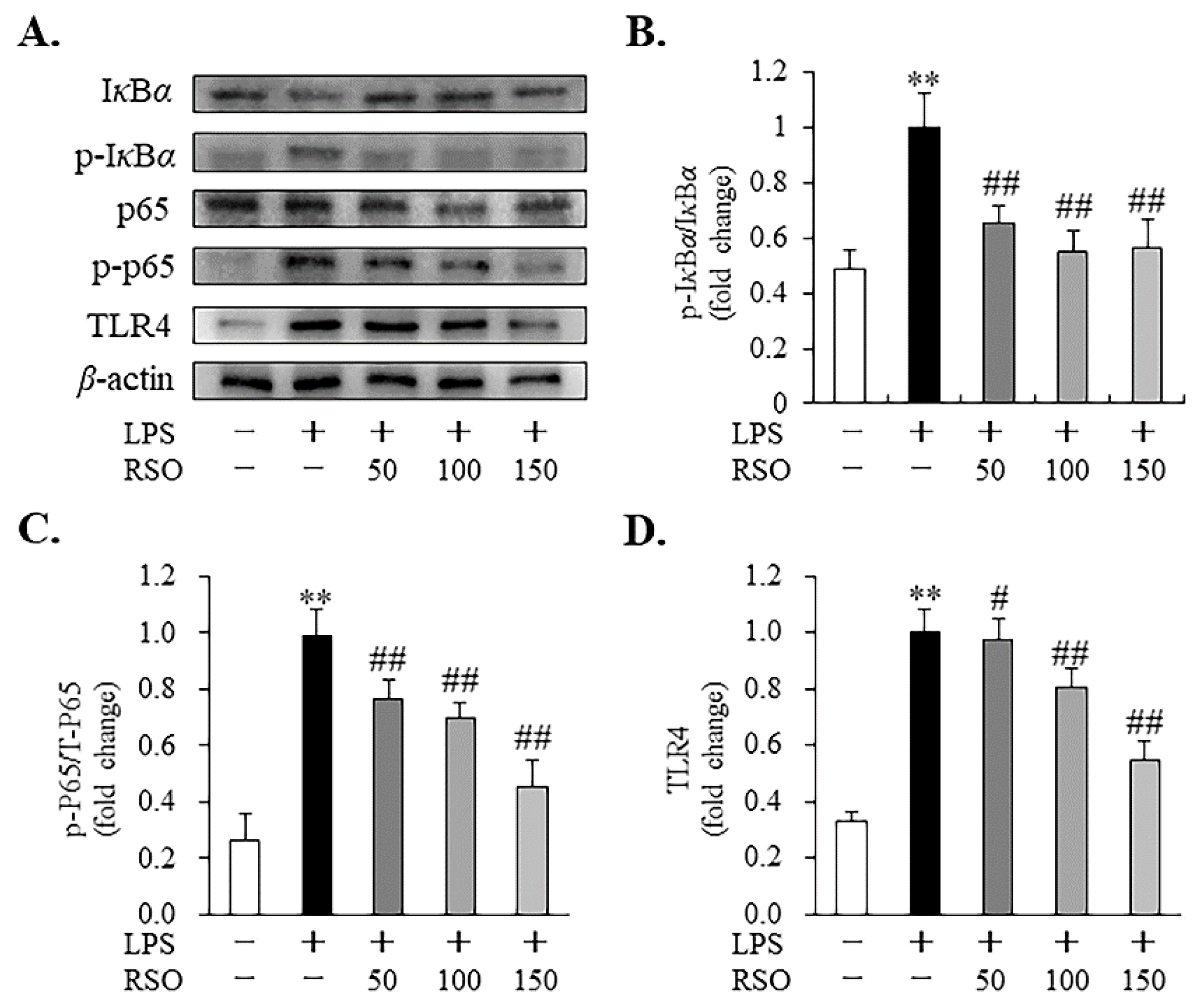
3.4.4. Effect of RSO on TLR4/NF-κB Pathway of LPS-Induced RAW 264.7 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azócar, L.; Ciudad, G.; Heipieper, H.J.; Navia, R. Biotechnological processes for biodiesel production using alternative oils. Appl. Microbiol. Biotechnol. 2010, 88, 621–636. [Google Scholar] [PubMed]
- Gandhi, V.M.; Cherian, K.M.; Mulky, M.J. Nutritional and toxicological evaluation of rubber seed oil. J. Am. Oil. Chem. Soc. 1990, 67, 883–886. [Google Scholar]
- Zhu, Y.; Xu, J.; Li, Q.; Mortimer, P.E. Investigation of rubber seed yield in xishuangbanna and estimation of rubber seed oil based biodiesel potential in southeast asia. Energy 2014, 69, 837–842. [Google Scholar]
- Aigbodion, A.I.; Pillai, C.K.S. Preparation, Analysis and Applications of Rubber Seed Oil and Its Derivatives in Surface Coatings. Prog. Org. Coat. 2000, 38, 187–192. [Google Scholar]
- Balköse, D.; Egbuchunam, T.; Okieimen, F. Thermal behaviour of metal soaps from biodegradable rubber seed oil. J. Therm. Anal. Calorim. 2010, 101, 795–799. [Google Scholar]
- Wen, Z.; Wu, Y.; Qi, Z.; Li, X.; Li, F.; Wu, X.; Yang, P. Rubber seed oil supplementation enriches n-3 polyunsaturated fatty acids and reduces cholesterol contents of egg yolks in laying hens. Food Chem. 2019, 301, 125198. [Google Scholar] [PubMed]
- Pi, Y.; Ma, L.; Wang, H.; Wang, J.; Xu, J.; Bu, D. Rubber seed oil and flaxseed oil supplementation on serum fatty acid profile, oxidation stability of serum and milk, and immune function of dairy cows. Asian Austral. J. Anim. 2019, 32, 1363–1372. [Google Scholar]
- Deng, J.; Mai, K.; Chen, L.; Mi, H.; Zhang, L. Effects of replacing soybean meal with rubber seed meal on growth, antioxidant capacity, non-specific immune response, and resistance to Aeromonas hydrophila in tilapia (Oreochromis niloticus × O. aureus). Fish Shellfish Immune. 2015, 44, 436–444. [Google Scholar]
- Onoji, S.; Iyuke, S.; Igbafe, A.; Nkazi, D. Rubber seed oil: A potential renewable source of biodiesel for sustainable development in sub-Saharan Africa. Energy Convers. Manage. 2016, 110, 125–134. [Google Scholar]
- Ramadhas, A.; Jayaraj, S.; Muraleedharan, C. Biodiesel production from high FFA rubber seed oil. Fuel 2005, 84, 335–340. [Google Scholar]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [PubMed]
- Kumar, N.G.; Contaifer, D.; Madurantakam, P.; Carbone, S.; Price, E.T.; Van Tassell, B.; Brophy, D.F.; Wijesinghe, D.S. Dietary Bioactive Fatty Acids as Modulators of Immune Function: Implications on Human Health. Nutrients 2019, 11, 2974. [Google Scholar]
- Musazadeh, V.; Dehghan, P.; Saleh-Ghadimi, S.; Abbasalizad Farhangi, M. Omega 3-rich Camelina sativa oil in the context of a weight loss program improves glucose homeostasis, inflammation and oxidative stress in patients with NAFLD: A randomised placebo-controlled clinical trial. Int. J. Clin. Pract. 2021, 75, e14744. [Google Scholar] [PubMed]
- Williams, C.D.; Whitley, B.M.; Hoyo, C.; Grant, D.J.; Iraggi, J.D.; Newman, K.A.; Gerber, L.; Taylor, L.A.; McKeever, M.G.; Freedland, S.J. A high ratio of dietary n-6/n-3 polyunsaturated fatty acids is associated with increased risk of prostate cancer. Nutr. Res. 2011, 31, 1–8. [Google Scholar] [PubMed]
- Kunnumakkara, A.B.; Sailo, B.L.; Banik, K.; Harsha, C.; Prasad, S.; Gupta, S.C.; Bharti, A.C.; Aggarwal, B.B. Chronic diseases, inflammation, and spices: How are they linked? J. Transl. Med. 2018, 16, 14. [Google Scholar] [PubMed] [Green Version]
- Ambrozova, G.; Pekarova, M.; Lojek, A. Effect of polyunsaturated fatty acids on the reactive oxygen and nitrogen species production by raw 264.7 macrophages. Eur. J. Nutr. 2010, 49, 133–139. [Google Scholar] [PubMed]
- Abrescia, P.; Treppiccione, L.; Rossi, M.; Bergamo, P. Modulatory role of dietary polyunsaturated fatty acids in Nrf2-mediated redox homeostasis. Prog. Lipid Res. 2020, 80, 101066. [Google Scholar]
- Monmai, C.; Go, S.H.; Shin, I.S.; You, S.G.; Lee, H.; Kang, S.B.; Park, W.J. Immune-Enhancement and Anti-Inflammatory Activities of Fatty Acids Extracted from Halocynthia aurantium Tunic in RAW264.7 Cells. Mar. Drugs 2018, 16, 309. [Google Scholar]
- Wang, H.; Khor, T.O.; Saw, C.L.; Lin, W.; Wu, T.; Huang, Y.; Kong, A.N. Role of Nrf2 in suppressing LPS-induced inflammation in mouse peritoneal macrophages by polyunsaturated fatty acids docosahexaenoic acid and eicosapentaenoic acid. Mol. Pharmaceut. 2010, 7, 2185–2193. [Google Scholar]
- Dai, Y.; Zhang, L.; Yan, Z.; Li, Z.; Fu, M.; Xue, C.; Wang, J. A low proportion n-6/n-3 PUFA diet supplemented with Antarctic krill (Euphausia superba) oil protects against osteoarthritis by attenuating inflammation in ovariectomized mice. Food Funct. 2021, 12, 6766–6779. [Google Scholar] [PubMed]
- Marventano, S.; Kolacz, P.; Castellano, S.; Galvano, F.; Buscemi, S.; Mistretta, A.; Grosso, G. A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: Does the ratio really matter? Int. J. Food Sci. Nutr. 2015, 66, 611–622. [Google Scholar]
- Lourith, N.; Kanlayavattanakul, M.; Sucontphunt, A.; Ondee, T. Para rubber seed oil: New promising unconventional oil for cosmetics. J. Oleo Sci. 2014, 63, 709–716. [Google Scholar] [PubMed] [Green Version]
- Gu, J. Hypolipidaemic foods in China. Asia Pac. J. Clin. Nutr. 1996, 5, 249–253. [Google Scholar] [PubMed]
- AOCS. Official Methods and Recommended Practices of the AOCS; American Oil Chemists’ Society: Urbana, IL, USA, 1998. [Google Scholar]
- Ikediobi, C.; Olugboji, O.; Okoh, P. Cyanide profile of component parts of sorghum (Sorghum bicolor L. Moench) sprouts. Food Chem. 1988, 27, 167–175. [Google Scholar]
- Liu, G.; Zhu, W.; Zhang, J.; Song, D.; Zhuang, L.; Ma, Q.; Yang, X.; Liu, X.; Zhang, J.; Zhang, H.; et al. Antioxidant capacity of phenolic compounds separated from tea seed oil in vitro and in vivo. Food Chem. 2022, 371, 131122. [Google Scholar]
- Onoji, S.; Iyuke, S.; Igbafe, A. Hevea brasiliensis (Rubber Seed) Oil: Extraction, Characterization, and Kinetics of Thermo-oxidative Degradation Using Classical Chemical Methods. Energy Fuels 2016, 30, 10555–10567. [Google Scholar]
- World Health Organization. Evaluation of Certain Contaminants in Food; World Health Organization Technical Report Series: Geneva, Switzerland, 2017; pp. 1–166. [Google Scholar]
- Chaikul, P.; Lourith, N.; Kanlayavattanakul, M. Antimelanogenesis and cellular antioxidant activities of rubber (Hevea brasiliensis) seed oil for cosmetics. Ind. Crop. Prod. 2017, 108, 56–62. [Google Scholar]
- Che, H.; Fu, X.; Zhang, L.; Gao, X.; Wen, M.; Du, L.; Xue, C.; Xu, J.; Wang, Y. Neuroprotective Effects of n-3 Polyunsaturated Fatty Acid-Enriched Phosphatidylserine Against Oxidative Damage in PC12 Cells. Cell. Mol. Neurobiol. 2018, 38, 657–668. [Google Scholar]
- Wen, C.; Jiang, M.; Huang, W.; Liu, S. Antarctic Krill Oil Attenuates Oxidative Stress via the KEAP1-NRF2 Signaling in Patients with Coronary Heart Disease. Evid-Based Complement. Alternat. Med. 2020, 2020, 9534137. [Google Scholar] [PubMed]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [PubMed]
- Novichkova, E.; Chumin, K.; Eretz-Kdosha, N.; Boussiba, S.; Gopas, J.; Cohen, G.; Khozin-Goldberg, I. DGLA from the Microalga Lobosphaera Incsa P127 Modulates Inflammatory Response, Inhibits iNOS Expression and Alleviates NO Secretion in RAW264.7 Murine Macrophages. Nutrients 2020, 12, 2892. [Google Scholar]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [PubMed]
- Koto, T.; Nagai, N.; Mochimaru, H.; Kurihara, T.; Izumi-Nagai, K.; Satofuka, S.; Shinoda, H.; Noda, K.; Ozawa, Y.; Inoue, M.; et al. Eicosapentaenoic acid is anti-inflammatory in preventing choroidal neovascularization in mice. Invest. Ophth. Vis. Sci. 2007, 48, 4328–4334. [Google Scholar]
- Pereira, H.; Barreira, L.; Figueiredo, F.; Custódio, L.; Vizetto-Duarte, C.; Polo, C.; Rešek, E.; Engelen, A.; Varela, J. Polyunsaturated fatty acids of marine macroalgae: Potential for nutritional and pharmaceutical applications. Mar. Drugs 2012, 10, 1920–1935. [Google Scholar]
- Messina, M.; Shearer, G.; Petersen, K. Soybean oil lowers circulating cholesterol levels and coronary heart disease risk and has no effect on markers of inflammation and oxidation. Nutrition 2021, 89, 111343. [Google Scholar] [PubMed]
- Salimon, J.; Abdullah, B.M.; Salih, N. Rubber (Hevea brasiliensis) seed oil toxicity effect and Linamarin compound analysis. Lipids Health Dis. 2012, 11, 74. [Google Scholar] [PubMed] [Green Version]
- Castaneda, O.A.; Lee, S.C.; Ho, C.T.; Huang, T.C. Macrophages in oxidative stress and models to evaluate the antioxidant function of dietary natural compounds. J. Food Drug Anal. 2017, 25, 111–118. [Google Scholar] [PubMed] [Green Version]
- Nash, K.M.; Ahmed, S. Nanomedicine in the ROS-mediated pathophysiology: Applications and clinical advances. Nanomedicine 2015, 11, 2033–2040. [Google Scholar]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta. Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [PubMed]
- Soskić, S.S.; Dobutović, B.D.; Sudar, E.M.; Obradović, M.M.; Nikolić, D.M.; Djordjevic, J.D.; Radak, D.J.; Mikhailidis, D.P.; Isenović, E.R. Regulation of Inducible Nitric Oxide Synthase (iNOS) and its Potential Role in Insulin Resistance, Diabetes and Heart Failure. Open Cardiovasc. Med. J. 2011, 5, 153–163. [Google Scholar] [PubMed] [Green Version]
- Miao, F.; Shan, C.; Shah, S.; Akhtar, R.W.; Geng, S.; Ning, D.; Wang, X. The protective effect of walnut oil on lipopolysaccharide-induced acute intestinal injury in mice. Food Sci. Nutr. 2020, 9, 711–718. [Google Scholar] [PubMed]
- Oliveira, J.; Reygaert, W.C. Gram Negative Bacteria; StatPearls Publishing: Treasure Island, FL, USA, 2021; pp. 1–3. [Google Scholar]
- Tan, Y.; Kagan, J.C. A cross-disciplinary perspective on the innate immune responses to bacterial lipopolysaccharide. Mol. Cell. 2014, 54, 212–223. [Google Scholar] [PubMed] [Green Version]

| Physicochemical Parameters 1 | Fatty Acids Composition (%) | ||
|---|---|---|---|
| L* | 87.72 ± 0.03 | Saturated | |
| a* | 2.28 ± 0.18 | Palmitic acid (C16:0) | 9.23 ± 1.26 |
| b* | 31.70 ± 1.11 | Stearic acid (C18:0) | 7.49 ± 0.20 |
| Refractive index (n20 D) | 1.4697 ± 0.0015 | Monounsaturated | |
| Acid value (mg KOH/g) | 0.75 ± 0.25 | Oleic acid (C18:1 n-9) | 25.26 ± 3.62 |
| Iodine value (g iodine/100 g) | 137.51 ± 0.72 | Polyunsaturated | |
| Peroxide value (mmol/kg) | 3.12 ± 0.05 | Linoleic acid (C18:2 n-6) | 37.26 ± 3.16 |
| Saponification value (mg KOH/g) | 194.35 ± 6.17 | Linolenic acid (C18:3 n-3) | 19.43 ± 1.59 |
| Unsaponifiable matter (%) | 1.02 ± 0.10 | Other fatty acids | 1.33 ± 3.51 |
| Cyanide | ND 2 | ∑SFA 3 | 17.33 ± 1.31 |
| Aflatoxin B1 (μg/kg) | <0.6 | ∑MUFA 3 | 25.79 ± 2.23 |
| Zearalenone (mg/kg) | 0.64 ± 0.01 | ∑PUFA 3 | 56.88 ± 1.09 |
| Deoxynivalenol (μg/kg) | 0.8 ± 0.1 | ∑n-3 PUFA | 19.48 ± 1.70 |
| TPC 3 (mg GAE/kg) | 1032.60 ± 21.55 | ∑n-6 PUFA | 37.40 ± 2.79 |
| TFC 3 (mg RE/kg) | 304.31 ± 3.24 | n-6/n-3 | 1.92 ± 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhao, L.; Cai, H.; Zhao, Z.; Wu, Y.; Wen, Z.; Yang, P. Antioxidant and Anti-Inflammatory Properties of Rubber Seed Oil in Lipopolysaccharide-Induced RAW 267.4 Macrophages. Nutrients 2022, 14, 1349. https://doi.org/10.3390/nu14071349
Liu J, Zhao L, Cai H, Zhao Z, Wu Y, Wen Z, Yang P. Antioxidant and Anti-Inflammatory Properties of Rubber Seed Oil in Lipopolysaccharide-Induced RAW 267.4 Macrophages. Nutrients. 2022; 14(7):1349. https://doi.org/10.3390/nu14071349
Chicago/Turabian StyleLiu, Jing, Lulu Zhao, Hongying Cai, Zitao Zhao, Yongbao Wu, Zhiguo Wen, and Peilong Yang. 2022. "Antioxidant and Anti-Inflammatory Properties of Rubber Seed Oil in Lipopolysaccharide-Induced RAW 267.4 Macrophages" Nutrients 14, no. 7: 1349. https://doi.org/10.3390/nu14071349
APA StyleLiu, J., Zhao, L., Cai, H., Zhao, Z., Wu, Y., Wen, Z., & Yang, P. (2022). Antioxidant and Anti-Inflammatory Properties of Rubber Seed Oil in Lipopolysaccharide-Induced RAW 267.4 Macrophages. Nutrients, 14(7), 1349. https://doi.org/10.3390/nu14071349







