Curcumin Enhances Fed-State Muscle Microvascular Perfusion but Not Leg Glucose Uptake in Older Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
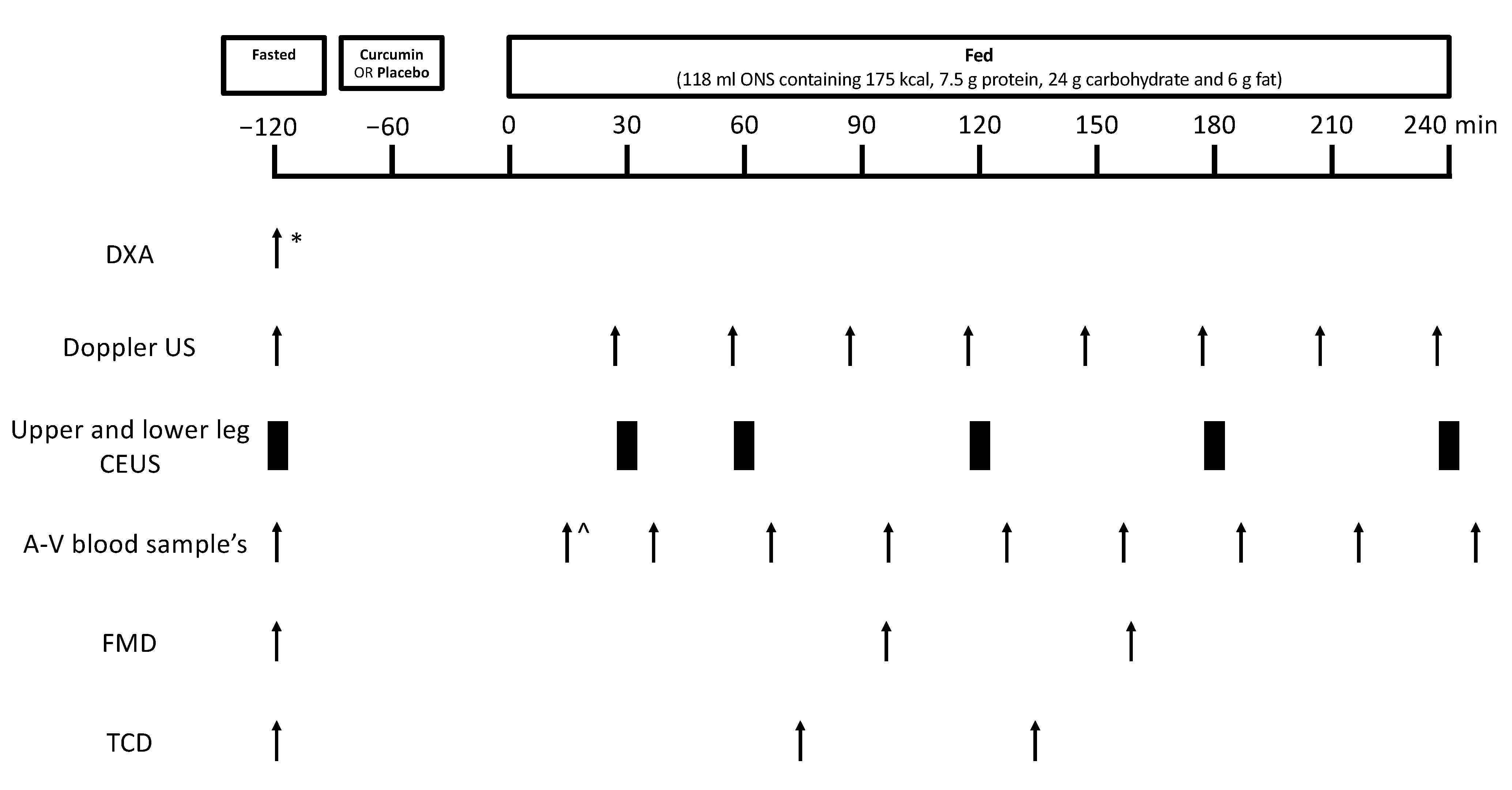
2.2. Volunteers and Study Design
2.3. ONS Feeding and Study Supplements
2.4. Measurement of MBF Using CEUS
2.5. Measurement of LBF Using Doppler Ultrasound
2.6. Measurement of Systemic Endothelial Function and Cerebrovascular Function
2.7. Blood Sampling
2.8. Statistical Analysis
3. Results
3.1. Volunteer Characteristics
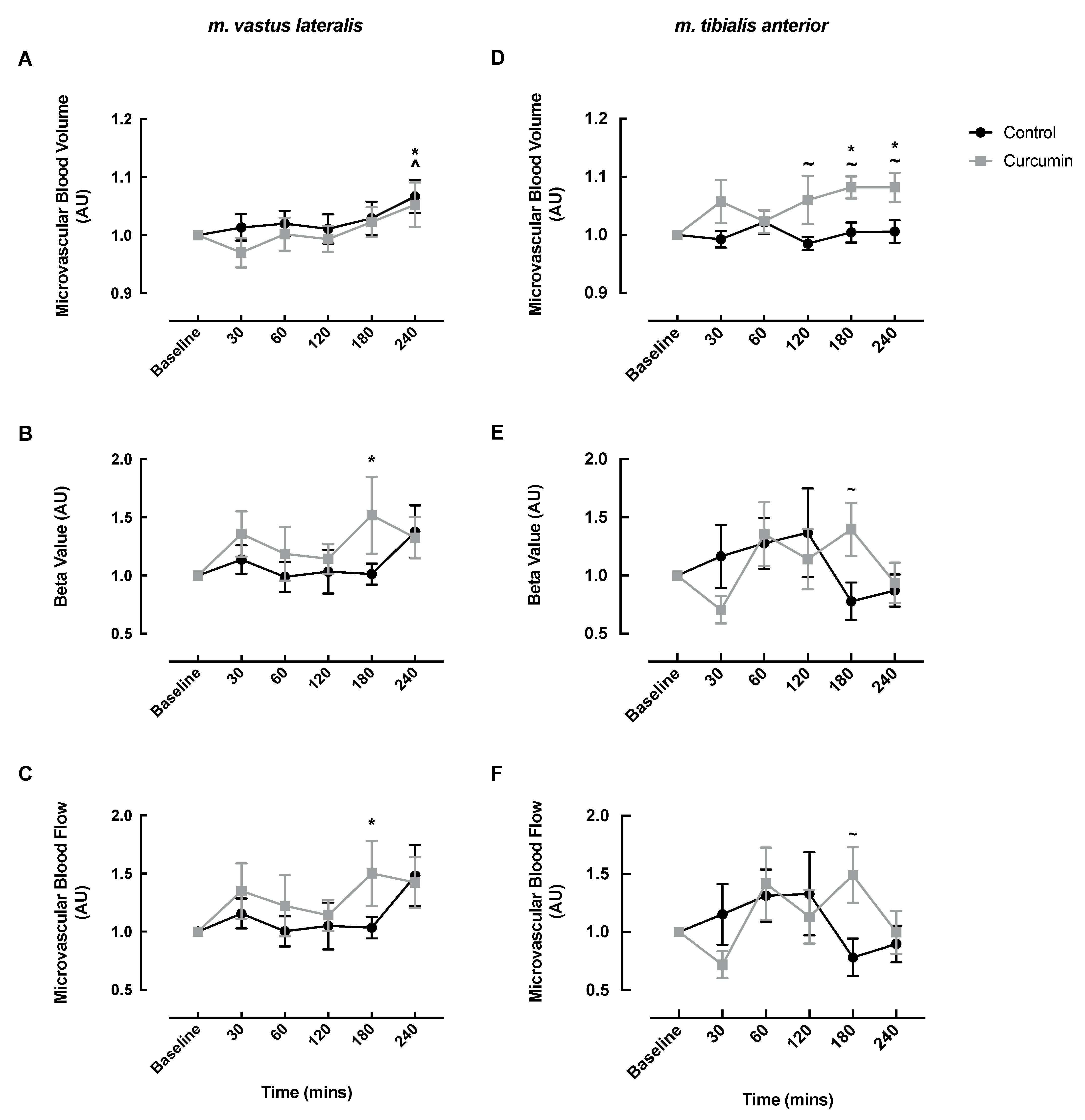
3.2. Microvascular Responses to Curcumin Supplementation
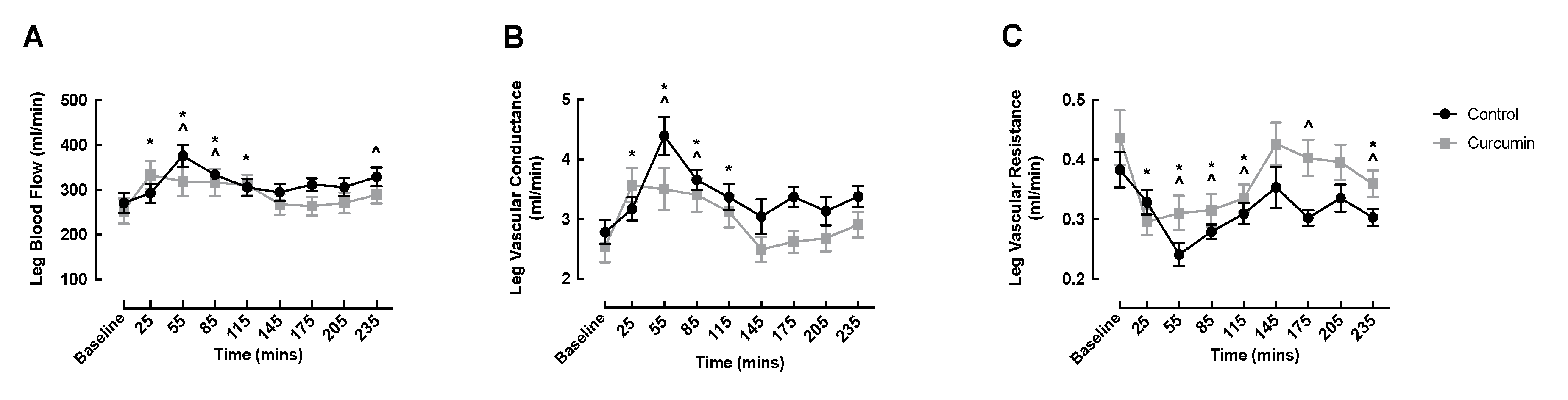
3.3. Macrovascular Responses to Curcumin Supplementation
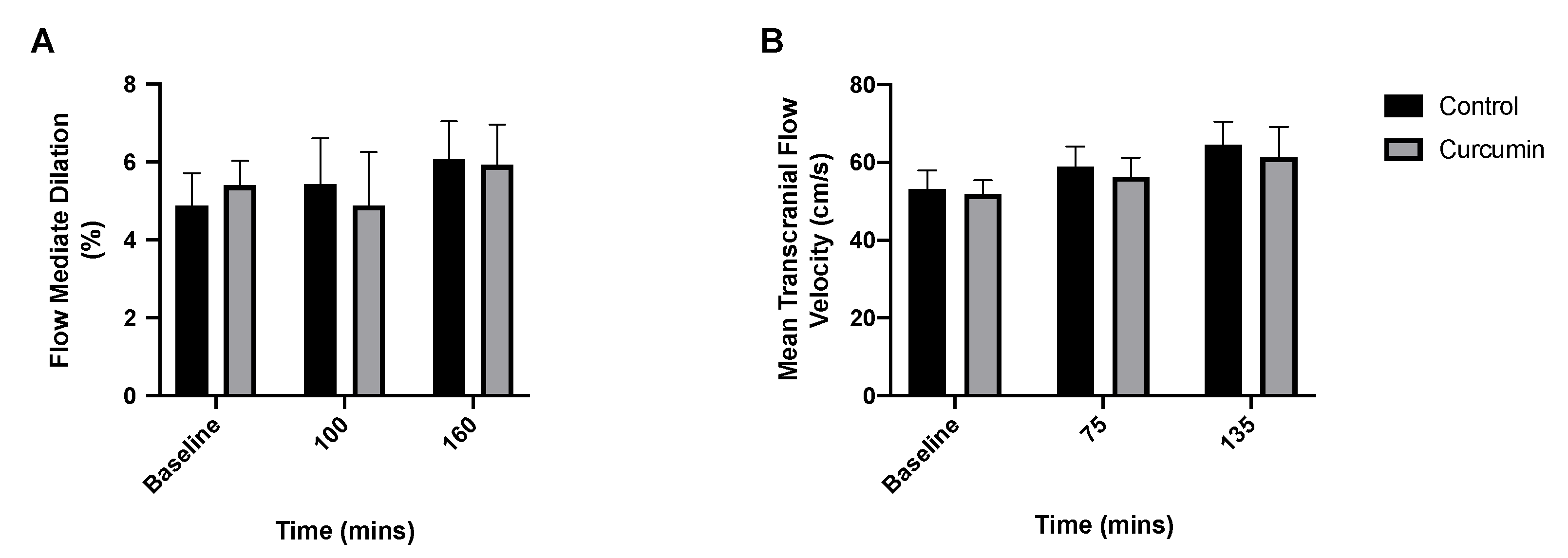
3.4. Endothelial and Cerebrovascular Responses to Curcumin Supplementation
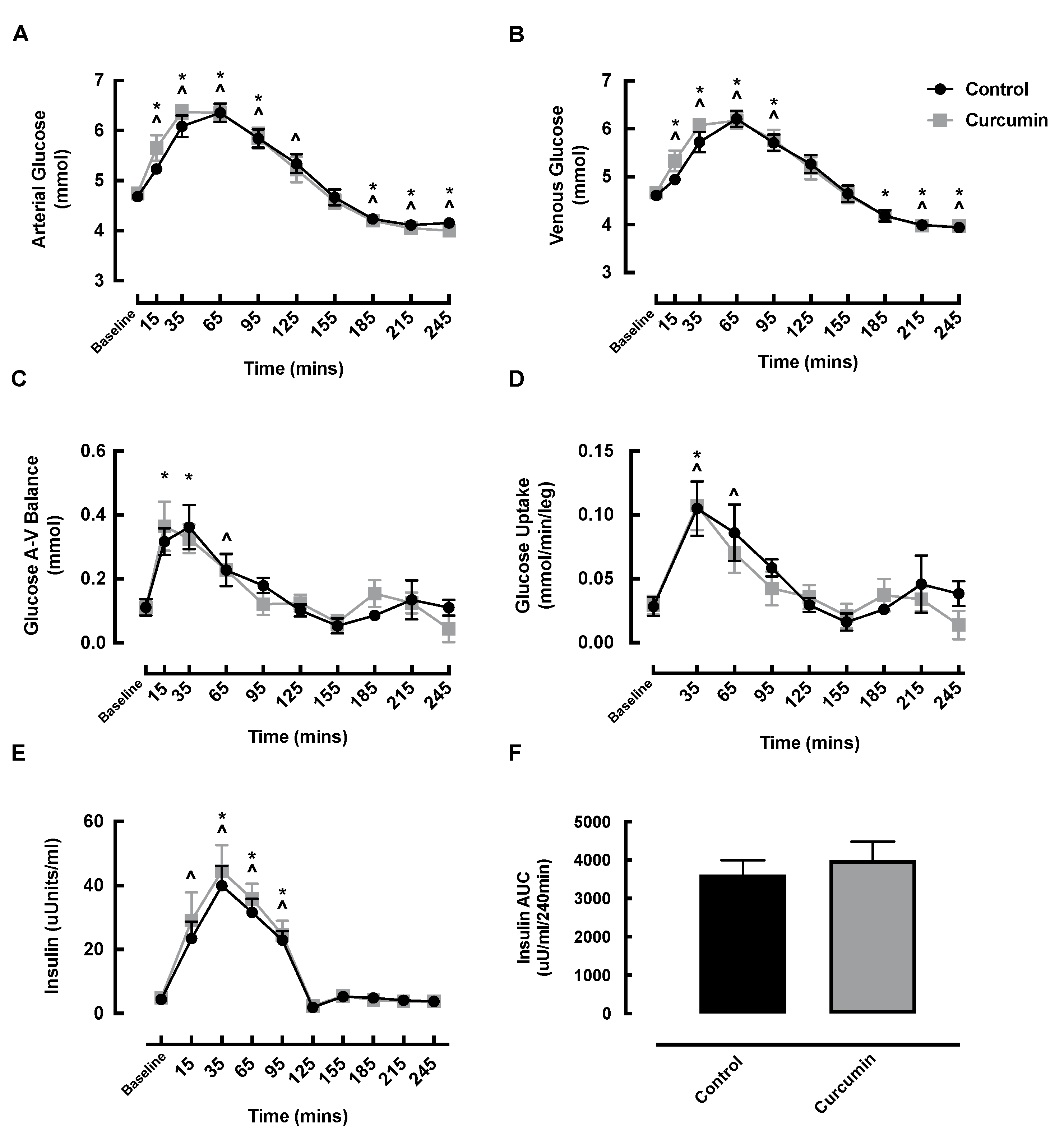
3.5. Blood Glucose and Insulin Responses to Curcumin Supplementation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lonn, E.; Bosch, J.; Teo, K.K.; Pais, P.; Xavier, D.; Yusuf, S. The polypill in the prevention of cardiovascular diseases: Key concepts, current status, challenges, and future directions. Circulation 2010, 122, 2078–2088. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, W.S.; Daniels, S.R.; Burke, L.E.; Franklin, B.A.; Goff, D.C., Jr.; Hayman, L.L.; Lloyd-Jones, D.; Pandey, D.K.; Sanchez, E.J.; Schram, A.P.; et al. Value of primordial and primary prevention for cardiovascular disease: A policy statement from the American Heart Association. Circulation 2011, 124, 967–990. [Google Scholar] [CrossRef] [PubMed]
- Writing Group, M.; Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A “set up” for vascular disease. Circulation 2003, 107, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Lind, L.; Berglund, L.; Larsson, A.; Sundstrom, J. Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation 2011, 123, 1545–1551. [Google Scholar] [CrossRef] [Green Version]
- Yeboah, J.; Crouse, J.R.; Hsu, F.C.; Burke, G.L.; Herrington, D.M. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The Cardiovascular Health Study. Circulation 2007, 115, 2390–2397. [Google Scholar] [CrossRef]
- Yeboah, J.; Folsom, A.R.; Burke, G.L.; Johnson, C.; Polak, J.F.; Post, W.; Lima, J.A.; Crouse, J.R.; Herrington, D.M. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: The multi-ethnic study of atherosclerosis. Circulation 2009, 120, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef] [Green Version]
- Luukinen, H.; Koski, K.; Laippala, P.; Kivela, S.L. Factors predicting fractures during falling impacts among home-dwelling older adults. J. Am. Geriatr. Soc. 1997, 45, 1302–1309. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Laukkanen, P.; Heikkinen, E.; Kauppinen, M. Muscle strength and mobility as predictors of survival in 75–84-year-old people. Age Ageing 1995, 24, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Santos-Parker, J.R.; Strahler, T.R.; Bassett, C.J.; Bispham, N.Z.; Chonchol, M.B.; Seals, D.R. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging 2017, 9, 187–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitrou, P.; Petsiou, E.; Papakonstantinou, E.; Maratou, E.; Lambadiari, V.; Dimitriadis, P.; Spanoudi, F.; Raptis, S.A.; Dimitriadis, G. The role of acetic acid on glucose uptake and blood flow rates in the skeletal muscle in humans with impaired glucose tolerance. Eur. J. Clin. Nutr. 2015, 69, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.A.; Clerk, L.H.; Lindner, J.R.; Price, W.J.; Jahn, L.A.; Leong-Poi, H.; Barrett, E.J. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am. J. Physiol.-Endocrinol. Metab. 2006, 290, E1191–E1197. [Google Scholar] [CrossRef]
- Khan, M.S.; Muhammad, T.; Ikram, M.; Kim, M.O. Dietary Supplementation of the Antioxidant Curcumin Halts Systemic LPS-Induced Neuroinflammation-Associated Neurodegeneration and Memory/Synaptic Impairment via the JNK/NF-kappaB/Akt Signaling Pathway in Adult Rats. Oxidative Med. Cell. Longev. 2019, 2019, 7860650. [Google Scholar] [CrossRef] [Green Version]
- Kondamudi, P.K.; Kovelamudi, H.; Nayak, P.G.; Rao, M.C.; Shenoy, R.R. Curcumin half analog modulates interleukin-6 and tumor necrosis factor-alpha in inflammatory bowel disease. Pharmacogn. Mag. 2015, 11, S296–S302. [Google Scholar] [CrossRef] [Green Version]
- Suhett, L.G.; de Miranda Monteiro Santos, R.; Silveira, B.K.S.; Leal, A.C.G.; de Brito, A.D.M.; de Novaes, J.F.; Lucia, C.M.D. Effects of curcumin supplementation on sport and physical exercise: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 61, 946–958. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [Green Version]
- Tanabe, Y.; Chino, K.; Ohnishi, T.; Ozawa, H.; Sagayama, H.; Maeda, S.; Takahashi, H. Effects of oral curcumin ingested before or after eccentric exercise on markers of muscle damage and inflammation. Scand. J. Med. Sci. Sports 2019, 29, 524–534. [Google Scholar] [CrossRef]
- He, J.; Xie, H.; Wu, S. Dietary Supplementation of Curcumin Alleviates NF-kappaB-dependent Skeletal Muscle Wasting in Rat. Endocr. Metab. Immune Disord. Drug Targets 2016, 16, 140–147. [Google Scholar] [CrossRef]
- Huang, W.C.; Chiu, W.C.; Chuang, H.L.; Tang, D.W.; Lee, Z.M.; Wei, L.; Chen, F.A.; Huang, C.C. Effect of curcumin supplementation on physiological fatigue and physical performance in mice. Nutrients 2015, 7, 905–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Tang, J.; Li, Y.; Xie, Y.; Shan, H.; Chen, M.; Zhang, J.; Yang, X.; Zhang, Q.; Yang, X. Curcumin attenuates skeletal muscle mitochondrial impairment in COPD rats: PGC-1alpha/SIRT3 pathway involved. Chem. Biol. Interact. 2017, 277, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Hallajzadeh, J.; Milajerdi, A.; Kolahdooz, F.; Amirani, E.; Mirzaei, H.; Asemi, Z. The effects of curcumin supplementation on endothelial function: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 2989–2995. [Google Scholar] [CrossRef] [PubMed]
- Fleenor, B.S.; Sindler, A.L.; Marvi, N.K.; Howell, K.L.; Zigler, M.L.; Yoshizawa, M.; Seals, D.R. Curcumin ameliorates arterial dysfunction and oxidative stress with aging. Exp. Gerontol. 2013, 48, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Changal, K.H.; Khan, M.S.; Bashir, R.; Sheikh, M.A. Curcumin Preparations Can Improve Flow-Mediated Dilation and Endothelial Function: A Meta-Analysis. Complementary Med. Res. 2020, 27, 272–281. [Google Scholar] [CrossRef]
- Nakayama, H.; Tsuge, N.; Sawada, H.; Masamura, N.; Yamada, S.; Satomi, S.; Higashi, Y. A single consumption of curry improved postprandial endothelial function in healthy male subjects: A randomized, controlled crossover trial. Nutr. J. 2014, 13, 67. [Google Scholar] [CrossRef]
- Kuszewski, J.C.; Wong, R.H.X.; Wood, L.G.; Howe, P.R.C. Effects of fish oil and curcumin supplementation on cerebrovascular function in older adults: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 625–633. [Google Scholar] [CrossRef]
- Bangen, K.J.; Werhane, M.L.; Weigand, A.J.; Edmonds, E.C.; Delano-Wood, L.; Thomas, K.R.; Nation, D.A.; Evangelista, N.D.; Clark, A.L.; Liu, T.T.; et al. Reduced Regional Cerebral Blood Flow Relates to Poorer Cognition in Older Adults With Type 2 Diabetes. Front. Aging Neurosci. 2018, 10, 270. [Google Scholar] [CrossRef] [Green Version]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef] [Green Version]
- Cleasby, M.E.; Jamieson, P.M.; Atherton, P.J. Insulin resistance and sarcopenia: Mechanistic links between common co-morbidities. J. Endocrinol. 2016, 229, R67–R81. [Google Scholar] [CrossRef]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of Non-alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-controlled Trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Na, L.X.; Li, Y.; Pan, H.Z.; Zhou, X.L.; Sun, D.J.; Meng, M.; Li, X.X.; Sun, C.H. Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: A double-blind, placebo-controlled trial. Mol. Nutr. Food Res. 2013, 57, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Hodaei, H.; Adibian, M.; Nikpayam, O.; Hedayati, M.; Sohrab, G. The effect of curcumin supplementation on anthropometric indices, insulin resistance and oxidative stress in patients with type 2 diabetes: A randomized, double-blind clinical trial. Diabetol. Metab. Syndr. 2019, 11, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Park, J.M.; Kim, E.-K.; Lee, J.O.; Lee, S.K.; Jung, J.H.; You, G.Y.; Park, S.H.; Suh, P.-G.; Kim, H.S. Curcumin stimulates glucose uptake through AMPK-p38 MAPK pathways in L6 myotube cells. J. Cell. Physiol. 2010, 223, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Mohiti-Ardekani, J.; Asadi, S.; Ardakani, A.M.; Rahimifard, M.; Baeeri, M.; Momtaz, S. Curcumin increases insulin sensitivity in C2C12 muscle cells via AKT and AMPK signaling pathways. Cogent Food Agric. 2019, 5, 1577532. [Google Scholar] [CrossRef]
- Hellmann, F.; Verdi, M.; Schlemper, B.R., Jr.; Caponi, S. 50th anniversary of the Declaration of Helsinki: The double standard was introduced. Arch. Med. Res. 2014, 45, 600–601. [Google Scholar] [CrossRef] [PubMed]
- Gota, V.S.; Maru, G.B.; Soni, T.G.; Gandhi, T.R.; Kochar, N.; Agarwal, M.G. Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J. Agric. Food Chem. 2010, 58, 2095–2099. [Google Scholar] [CrossRef]
- Sian, T.S.; Din, U.S.U.; Deane, C.S.; Smith, K.; Gates, A.; Lund, J.N.; Williams, J.P.; Rueda, R.; Pereira, S.L.; Phillips, B.E.; et al. Cocoa Flavanols Adjuvant to an Oral Nutritional Supplement Acutely Enhances Nutritive Flow in Skeletal Muscle without Altering Leg Glucose Uptake Kinetics in Older Adults. Nutrients 2021, 13, 1646. [Google Scholar] [CrossRef]
- Din, U.S.U.; Sian, T.S.; Deane, C.S.; Smith, K.; Gates, A.; Lund, J.N.; Williams, J.P.; Rueda, R.; Pereira, S.L.; Atherton, P.J.; et al. Green Tea Extract Concurrent with an Oral Nutritional Supplement Acutely Enhances Muscle Microvascular Blood Flow without Altering Leg Glucose Uptake in Healthy Older Adults. Nutrients 2021, 13, 3895. [Google Scholar] [CrossRef]
- Sjoberg, K.A.; Rattigan, S.; Hiscock, N.; Richter, E.A.; Kiens, B. A new method to study changes in microvascular blood volume in muscle and adipose tissue: Real-time imaging in humans and rat. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, H450–H458. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Bukhari, S.S.I.; Phillips, B.E.; Limb, M.C.; Cegielski, J.; Brook, M.S.; Rankin, D.; Mitchell, W.K.; Kobayashi, H.; Williams, J.P.; et al. Effects of leucine-enriched essential amino acid and whey protein bolus dosing upon skeletal muscle protein synthesis at rest and after exercise in older women. Clin. Nutr. 2018, 37, 2011–2021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinenno, F.A.; Jones, P.P.; Seals, D.R.; Tanaka, H. Limb blood flow and vascular conductance are reduced with age in healthy humans: Relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 1999, 100, 164–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, B.; Williams, J.; Atherton, P.; Smith, K.; Hildebrandt, W.; Rankin, D.; Greenhaff, P.; Macdonald, I.; Rennie, M.J. Resistance exercise training improves age-related declines in leg vascular conductance and rejuvenates acute leg blood flow responses to feeding and exercise. J. Appl. Physiol. 2012, 112, 347–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Sorond, F.A.; Lipsitz, L.A.; Hollenberg, N.K.; Fisher, N.D. Cerebral blood flow response to flavanol-rich cocoa in healthy elderly humans. Neuropsychiatr. Dis. Treat. 2008, 4, 433–440. [Google Scholar] [PubMed]
- Sorond, F.A.; Schnyer, D.M.; Serrador, J.M.; Milberg, W.P.; Lipsitz, L.A. Cerebral blood flow regulation during cognitive tasks: Effects of healthy aging. Cortex 2008, 44, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Purkayastha, S.; Sorond, F. Transcranial Doppler ultrasound: Technique and application. Semin. Neurol. 2012, 32, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Englund, E.K.; Rodgers, Z.B.; Langham, M.C.; Mohler, E.R., 3rd; Floyd, T.F.; Wehrli, F.W. Simultaneous measurement of macro- and microvascular blood flow and oxygen saturation for quantification of muscle oxygen consumption. Magn. Reson. Med. 2018, 79, 846–855. [Google Scholar] [CrossRef]
- Abumrad, N.N.; Rabin, D.; Diamond, M.P.; Lacy, W.W. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism 1981, 30, 936–940. [Google Scholar] [CrossRef]
- Jakobsson, F.; Borg, K.; Edstrom, L. Fibre-type composition, structure and cytoskeletal protein location of fibres in anterior tibial muscle. Comparison between young adults and physically active aged humans. Acta Neuropathol. 1990, 80, 459–468. [Google Scholar] [CrossRef]
- Porter, M.M.; Stuart, S.; Boij, M.; Lexell, J. Capillary supply of the tibialis anterior muscle in young, healthy, and moderately active men and women. J. Appl. Physiol. 2002, 92, 1451–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, L.; Morrison, D.J.; Wadley, G.D.; Shaw, C.S.; Betik, A.C.; Roberts-Thomson, K.; Kaur, G.; Keske, M.A. Prior exercise enhances skeletal muscle microvascular blood flow and mitigates microvascular flow impairments induced by a high-glucose mixed meal in healthy young men. J. Physiol. 2021, 599, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Thota, R.N.; Acharya, S.H.; Garg, M.L. Curcumin and/or omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance and blood lipids in individuals with high risk of type 2 diabetes: A randomised controlled trial. Lipids Health Dis. 2019, 18, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Melo, I.S.V.; Dos Santos, A.F.; Bueno, N.B. Curcumin or combined curcuminoids are effective in lowering the fasting blood glucose concentrations of individuals with dysglycemia: Systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2018, 128, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Babraj, J.A.; Mustard, K.; Sutherland, C.; Towler, M.C.; Chen, S.; Smith, K.; Green, K.; Leese, G.; Hardie, D.G.; Rennie, M.J.; et al. Blunting of AICAR-induced human skeletal muscle glucose uptake in type 2 diabetes is dependent on age rather than diabetic status. Am. J. Physiol.-Endocrinol. Metab. 2009, 296, E1042–E1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, B.E.; Atherton, P.J.; Varadhan, K.; Wilkinson, D.J.; Limb, M.; Selby, A.L.; Rennie, M.J.; Smith, K.; Williams, J.P. Pharmacological enhancement of leg and muscle microvascular blood flow does not augment anabolic responses in skeletal muscle of young men under fed conditions. Am. J. Physiol.-Endocrinol. Metab. 2014, 306, E168–E176. [Google Scholar] [CrossRef]
- Oliver, J.M.; Stoner, L.; Rowlands, D.S.; Caldwell, A.R.; Sanders, E.; Kreutzer, A.; Mitchell, J.B.; Purpura, M.; Jager, R. Novel Form of Curcumin Improves Endothelial Function in Young, Healthy Individuals: A Double-Blind Placebo Controlled Study. J. Nutr. Metab. 2016, 2016, 1089653. [Google Scholar] [CrossRef] [Green Version]
- Barber-Chamoux, N.; Milenkovic, D.; Verny, M.A.; Habauzit, V.; Pereira, B.; Lambert, C.; Richard, D.; Boby, C.; Mazur, A.; Lusson, J.R.; et al. Substantial Variability Across Individuals in the Vascular and Nutrigenomic Response to an Acute Intake of Curcumin: A Randomized Controlled Trial. Mol. Nutr. Food Res. 2018, 62, 1700418. [Google Scholar] [CrossRef]
- Jamwal, R. Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers. J. Integr. Med. 2018, 16, 367–374. [Google Scholar] [CrossRef]
- Moghaddam, N.S.A.; Oskouie, M.N.; Butler, A.E.; Petit, P.X.; Barreto, G.E.; Sahebkar, A. Hormetic effects of curcumin: What is the evidence? J. Cell. Physiol. 2019, 234, 10060–10071. [Google Scholar] [CrossRef]
| Parameter | Volunteers (n = 12) |
|---|---|
| Gender (% M) | 50 |
| Age (years) | 73 ± 1 |
| Height (cm) | 171.5 ± 2.8 |
| Body mass (kg) | 79.4 ± 4.4 |
| BMI (kg/m2) | 26.7 ± 0.8 |
| Lean mass (kg) | 50.0 ± 3.5 |
| Resting heart rate (bpm) | 62 ± 2 |
| Resting systolic blood pressure (mmHg) | 137 ± 3 |
| Resting diastolic blood pressure (mmHg) | 79 ± 3 |
| Grip strength (kg) | 29.7 ± 2 |
| SPPB | 11 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deane, C.S.; Din, U.S.U.; Sian, T.S.; Smith, K.; Gates, A.; Lund, J.N.; Williams, J.P.; Rueda, R.; Pereira, S.L.; Atherton, P.J.; et al. Curcumin Enhances Fed-State Muscle Microvascular Perfusion but Not Leg Glucose Uptake in Older Adults. Nutrients 2022, 14, 1313. https://doi.org/10.3390/nu14061313
Deane CS, Din USU, Sian TS, Smith K, Gates A, Lund JN, Williams JP, Rueda R, Pereira SL, Atherton PJ, et al. Curcumin Enhances Fed-State Muscle Microvascular Perfusion but Not Leg Glucose Uptake in Older Adults. Nutrients. 2022; 14(6):1313. https://doi.org/10.3390/nu14061313
Chicago/Turabian StyleDeane, Colleen S., Ushnah S. U. Din, Tanvir S. Sian, Ken Smith, Amanda Gates, Jonathan N. Lund, John P. Williams, Ricardo Rueda, Suzette L. Pereira, Philip J. Atherton, and et al. 2022. "Curcumin Enhances Fed-State Muscle Microvascular Perfusion but Not Leg Glucose Uptake in Older Adults" Nutrients 14, no. 6: 1313. https://doi.org/10.3390/nu14061313
APA StyleDeane, C. S., Din, U. S. U., Sian, T. S., Smith, K., Gates, A., Lund, J. N., Williams, J. P., Rueda, R., Pereira, S. L., Atherton, P. J., & Phillips, B. E. (2022). Curcumin Enhances Fed-State Muscle Microvascular Perfusion but Not Leg Glucose Uptake in Older Adults. Nutrients, 14(6), 1313. https://doi.org/10.3390/nu14061313










