Effect of Copper and Selenium Supplementation on the Level of Elements in Rats’ Femurs under Neoplastic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval Statement
2.2. Dietary Ingredients
2.3. Animal Experiments and Experimental Procedure
- -
- Copper—0.639 mg/mL (0.256 mg Cu(II)/day/rat, as CuSO4·5H2O in aqueous suspension);
- -
- Selenium—0.018 mg/mL (0.0072 mg Se(VI)/day/rat, as Na2SeO4 in aqueous suspension).
- (1)
- 5 fold for determination of Co, Cu, Mn, Mo, Ni, Se and Zn;
- (2)
- 500 fold for determination of Ca, K, Sr and Fe.
2.3.1. Chemicals and Reagents
2.3.2. Analytical Procedure
2.3.3. Instrumentation
2.4. Statistics
3. Results
3.1. Experimental to Control Group Comparison
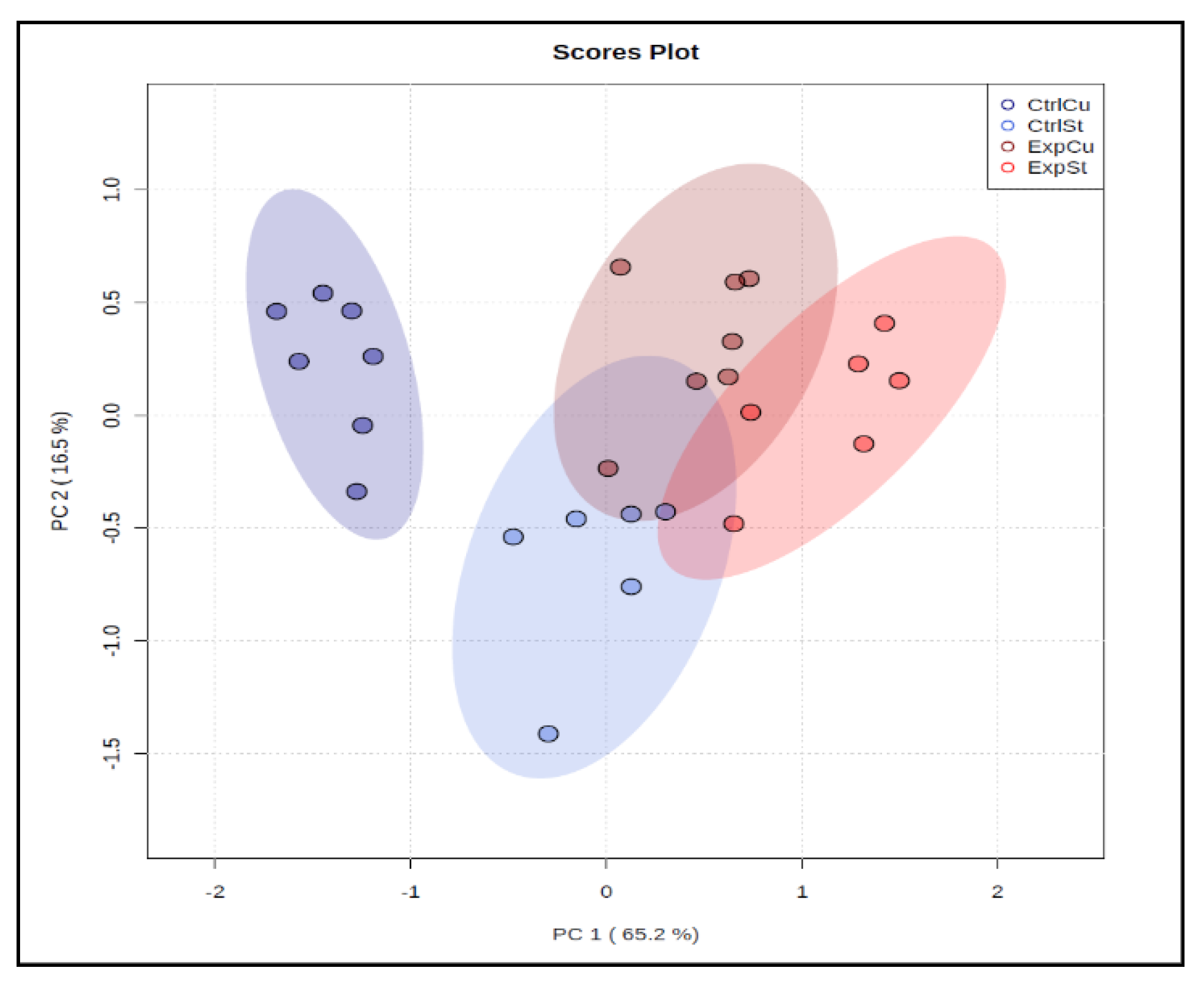
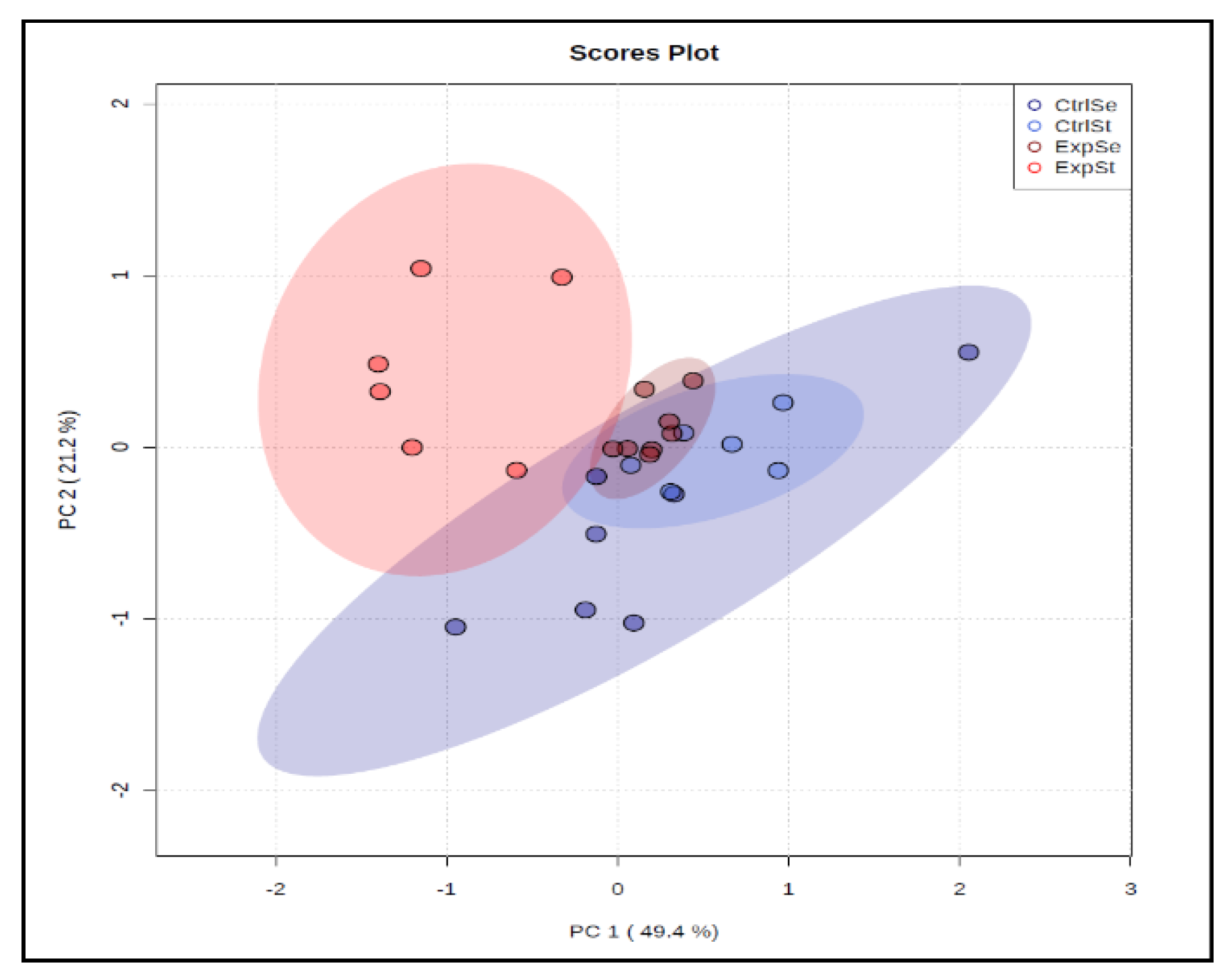
3.2. Comparison of Diet Groups
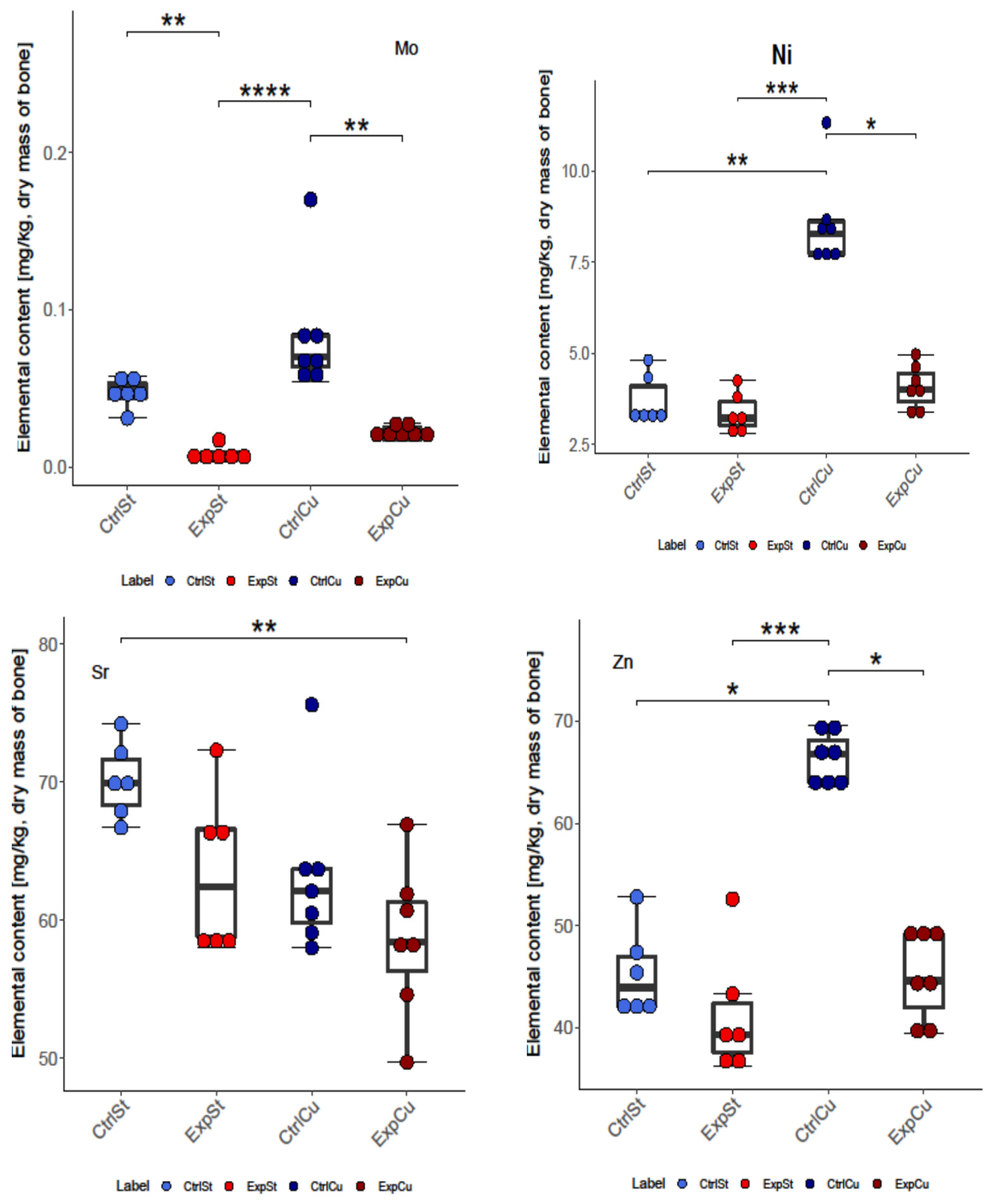
3.2.1. Dietary Supplementation with Copper Relative to Other Experimental Groups
- -
- The greatest number of statistically significant differences between nearly all groups was shown for the levels of Fe, Co, Mo, Ni, and Zn. It is particularly worth noting the significant decrease in the content of these elements in the groups with implanted LNCaP cells (ExpCu) in comparison with the control group (CtrlCu) without implanted cancer cells.
- -
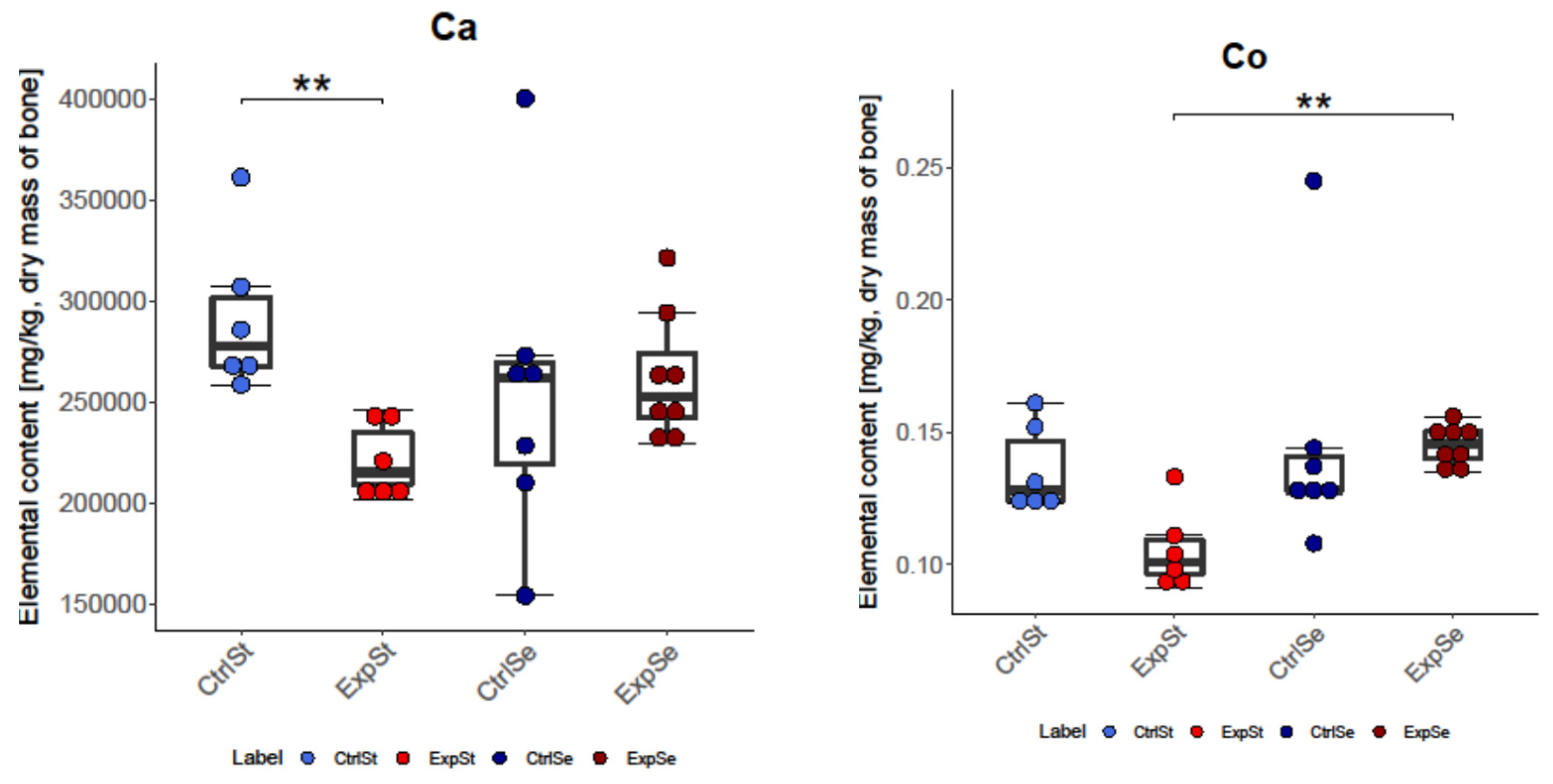
- In the case of Ca and Mo, a decrease was noted in the experimental groups whose diet was not supplemented (ExpSt) relative to the corresponding control group (CtrlSt). Comparison of the control groups with and without copper supplementation (CtrlCu vs. CtrlSt) also showed a decrease in Ca levels, but an increase in the content of Co in the control group receiving additional copper (CtrlCu).
- -
- Comparison of groups ExpSt and CtrlCu showed an increase in Co, Mn, Mo, Ni, and Zn levels in the control group. Comparison of ExpCu vs. CtrlSt revealed a decrease in K, Sr, and Fe in group ExpCu.
- -
- Single changes were observed for K and Sr.
3.2.2. Dietary Supplementation with Selenium Relative to Other Experimental Groups
- -
- The greatest number of changes was noted for Mo. There was a significant decrease in its content in group ExpSt relative to groups CtrlSt, CtrlSe, and ExpSe.
- -
- For iron, there was a decrease in the bones of rats in group CtrlSe relative to groups CtrlSt and ExpSe.
- -
- Potassium content increased in the bones of rats with implanted LNCaP (ExpSe) in comparison to the control group on the same diet (CtrlSe) and ExpSt.
- -
- Single changes were observed for Ca (CtrlSt vs. ExpSt), Co (ExpSt vs. ExpSe), and Ni (ExpSt vs. CtrlSe).
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Weatherholt, A.M.; Fuchs, R.K.; Warden, S.J. Specialized Connective Tissue: Bone, the Structural Framework of the Upper Extremity. J. Hand Ther. 2012, 25, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muselin, F.; Gârban, Z.; Cristina, R.T.; Doma, A.O.; Dumitrescu, E.; Vițălaru, A.B.; Bănățean-Dunea, I. Homeostatic Changes of Some Trace Elements in Geriatric Rats in the Condition of Oxidative Stress Induced by Aluminum and the Beneficial Role of Resveratrol. J. Trace Elem. Med. Biol. 2019, 55, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.; Pleshko Camacho, N. FT-IR Imaging of Native and Tissue-Engineered Bone and Cartilage. Biomaterials 2007, 28, 2465–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, N.H.; Nimphius, S.; Rantalainen, T.; Ireland, A.; Siafarikas, A.; Newton, R.U. Mechanical Basis of Bone Strength: Influence of Bone Material, Bone Structure and Muscle Action. J. Musculoskelet. Neuronal Interact. 2017, 17, 114–139. [Google Scholar] [PubMed]
- Boskey, A.L.; Coleman, R. Aging and Bone. J. Dent. Res. 2010, 89, 1333–1348. [Google Scholar] [CrossRef] [PubMed]
- Robey, P.G.; Boskey, A.L.; Leikin, S. Chapter 8—The regulatory role of matrix proteins in mineralization of bone. In Marcus and Feldman’s Osteoporosis, 5th ed.; Dempster, D.W., Cauley, J.A., Bouxsein, M.L., Cosman, F., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 165–187. ISBN 978-0-12-813073-5. [Google Scholar]
- Hernandez, R.K.; Wade, S.W.; Reich, A.; Pirolli, M.; Liede, A.; Lyman, G.H. Incidence of Bone Metastases in Patients with Solid Tumors: Analysis of Oncology Electronic Medical Records in the United States. BMC Cancer 2018, 18, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanocha, N.; Kalisinska, E.; Kosik-Bogacka, D.I.; Budis, H.; Sokolowski, S.; Bohatyrewicz, A.; Lanocha, A. The Effect of Environmental Factors on Concentration of Trace Elements in Hip Joint Bones of Patients after Hip Replacement Surgery. Ann. Agric. Environ. Med. 2013, 20, 487–493. [Google Scholar] [PubMed]
- Brodziak-Dopierala, B.; Kwapulinski, J.; Kusz, D.; Gajda, Z.; Sobczyk, K. Interactions between Concentrations of Chemical Elements in Human Femoral Heads. Arch. Environ. Contam. Toxicol. 2009, 57, 203–210. [Google Scholar] [CrossRef]
- Kubaszewski, Ł.; Zioła-Frankowska, A.; Gasik, Z.; Frankowski, M.; Dąbrowski, M.; Molisak, B.; Kaczmarczyk, J.; Gasik, R. Chemometric Evaluation of Concentrations of Trace Elements in Intervertebral Disc Tissue in Patient with Degenerative Disc Disease. Ann. Agric. Environ. Med. 2017, 24, 610–617. [Google Scholar] [CrossRef]
- Bi, X.; Sterling, J.A.; Merkel, A.R.; Perrien, D.S.; Nyman, J.S.; Mahadevan-Jansen, A. Prostate Cancer Metastases Alter Bone Mineral and Matrix Composition Independent of Effects on Bone Architecture in Mice—A Quantitative Study Using MicroCT and Raman Spectroscopy. Bone 2013, 56, 454–460. [Google Scholar] [CrossRef] [Green Version]
- Syniachenko, O.V.; Dumanckyi, Y.V.; Yehudina, Y.D.; Bevzenko, T.B.; Yarmola, T.I. Bone Tissue Lesion in Oncological Disease (Literature Review and Own Research Data). Wiadomości Lek. 2018, 71, 1262–1266. [Google Scholar]
- Pepa, G.D.; Brandi, M.L. Microelements for Bone Boost: The Last but Not the Least. Clin. Cases Miner. Bone Metab. 2016, 13, 181–185. [Google Scholar] [CrossRef]
- Dermience, M.; Lognay, G.; Mathieu, F.; Goyens, P. Effects of Thirty Elements on Bone Metabolism. J. Trace Elem. Med. Biol. 2015, 32, 86–106. [Google Scholar] [CrossRef] [PubMed]
- Denoyer, D.; Clatworthy, S.A.S.; Masaldan, S.; Meggyesy, P.M.; Cater, M.A. Heterogeneous Copper Concentrations in Cancerous Human Prostate Tissues. Prostate 2015, 75, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Cao, J.J.; Combs, G.F. Selenium in Bone Health: Roles in Antioxidant Protection and Cell Proliferation. Nutrients 2013, 5, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Hoeg, A.; Gogakos, A.; Murphy, E.; Mueller, S.; Köhrle, J.; Reid, D.M.; Glüer, C.C.; Felsenberg, D.; Roux, C.; Eastell, R.; et al. Bone Turnover and Bone Mineral Density Are Independently Related to Selenium Status in Healthy Euthyroid Postmenopausal Women. J. Clin. Endocrinol. Metab. 2012, 97, 4061–4070. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Wang, C.; Yu, W.; Fan, W.; Wang, S.; Shen, N.; Wu, P.; Li, X.; Wang, F. Selenium Exposure and Cancer Risk: An Updated Meta-Analysis and Meta-Regression. Sci. Rep. 2016, 6, 19213. [Google Scholar] [CrossRef] [Green Version]
- Beukhof, C.M.; Medici, M.; van den Beld, A.W.; Hollenbach, B.; Hoeg, A.; Visser, W.E.; de Herder, W.W.; Visser, T.J.; Schomburg, L.; Peeters, R.P. Selenium Status Is Positively Associated with Bone Mineral Density in Healthy Aging European Men. PLoS ONE 2016, 11, e0152748. [Google Scholar] [CrossRef] [Green Version]
- Kubiak, K.; Klimczak, A.; Dziki, Ł.; Modranka, R.; Malinowska, K. Influence of copper (II) complex on the activity of selected oxidative enzymes. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2010, 28, 22–25. [Google Scholar]
- Li, B.; Yu, S. In vitro study of the effects of copper ion on osteoclastic resorption in various dental mineralized tissues. Zhonghua Kou Qiang Yi Xue Za Zhi Zhonghua Kouqiang Yixue Zazhi Chin. J. Stomatol. 2007, 42, 110–113. [Google Scholar]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological Relevance and Mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Burkhead, J.L.; Gray, L.W.; Lutsenko, S. Systems Biology Approach to Wilson’s Disease. Biometals 2011, 24, 455–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Romaña, D.L.; Olivares, M.; Uauy, R.; Araya, M. Risks and Benefits of Copper in Light of New Insights of Copper Homeostasis. J. Trace Elem. Med. Biol. 2011, 25, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Golabek, T.; Darewicz, B.; Borawska, M.; Socha, K.; Markiewicz, R.; Kudelski, J. Copper, Zinc, and Cu/Zn Ratio in Transitional Cell Carcinoma of the Bladder. Urol. Int. 2012, 89, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Malavolta, M.; Piacenza, F.; Basso, A.; Giacconi, R.; Costarelli, L.; Mocchegiani, E. Serum Copper to Zinc Ratio: Relationship with Aging and Health Status. Mech. Ageing Dev. 2015, 151, 93–100. [Google Scholar] [CrossRef]
- Mandair, D.; Rossi, R.E.; Pericleous, M.; Whyand, T.; Caplin, M.E. Prostate Cancer and the Influence of Dietary Factors and Supplements: A Systematic Review. Nutr. Metab. 2014, 11, 30. [Google Scholar] [CrossRef] [Green Version]
- Goodman, V.L.; Brewer, G.J.; Merajver, S.D. Copper Deficiency as an Anti-Cancer Strategy. Endocr. Relat. Cancer 2004, 11, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Bian, W.; Liu, S.; Huang, K. Selenium Protects Bone Marrow Stromal Cells against Hydrogen Peroxide-Induced Inhibition of Osteoblastic Differentiation by Suppressing Oxidative Stress and ERK Signaling Pathway. Biol. Trace Elem. Res. 2012, 150, 441–450. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenoproteins and Human Health: Insights from Epidemiological Data. Biochim. Biophys. Acta 2009, 1790, 1533–1540. [Google Scholar] [CrossRef] [Green Version]
- Mody, N.; Parhami, F.; Sarafian, T.A.; Demer, L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 2001, 31, 509–519. [Google Scholar] [CrossRef]
- Hiraoka, K.; Komiya, S.; Hamada, T.; Zenmyo, M.; Inoue, A. Osteosarcoma cell apoptosis induced by selenium. J. Orthop. Res. 2001, 19, 809–814. [Google Scholar] [CrossRef]
- Yang, T.; Lee, S.J.; Park, K.C.; Park, S.H.; Chung, J.; Lee, S. The Effects of Selenium on Bone Health: From Element to Therapeutics. Molecules 2022, 27, 392. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.; Eastell, R. Circulating osteoprotegerin and receptor activator for nuclear factor (B ligand: Clinical utility in metabolic bone disease assessment. J. Clin. Endocrinol. Metab. 2005, 90, 6323–6331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofbauer, L.C.; Schoppet, M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA 2004, 292, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Keightley, A.; Guthrie, J.; Veno, P.A.; Harris, S.E.; Bonewald, L.F. Identification of osteocyte-selective proteins. Proteomics 2010, 10, 3688–3698. [Google Scholar] [CrossRef] [Green Version]
- Moon, H.J.; Ko, W.K.; Han, S.W.; Kim, D.S.; Hwang, Y.S.; Park, H.K.; Kwon, I.K. Antioxidants, like coenzyme Q10, selenite, and curcumin, inhibited osteoclast differentiation by suppressing reactive oxygen species generation. Biochem. Biophys. Res. Commun. 2012, 418, 247–253. [Google Scholar] [CrossRef]
- Ebert, R.; Ulmer, M.; Zeck, S.; Meissner-Weigl, J.; Schneider, D.; Stopper, H.; Schupp, N.; Kassem, M.; Jakob, F. Selenium supplementation restores the antioxidative capacity and prevents cell damage in bone marrow stromal cells in vitro. Stem Cells 2006, 24, 1226–1235. [Google Scholar] [CrossRef]
- Cao, J.J.; Gregoire, B.R.; Zeng, H. Selenium Deficiency Decreases Antioxidative Capacity and Is Detrimental to Bone Microarchitecture in Mice. J. Nutr. 2012, 142, 1526–1531. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Munger, R.G.; West, N.A.; Cutler, D.R.; Wengreen, H.J.; Corcoran, C.D. Antioxidant Intake and Risk of Osteoporotic Hip Fracture in Utah: An Effect Modified by Smoking Status. Am. J. Epidemiol. 2006, 163, 9–17. [Google Scholar] [CrossRef]
- Wang, X.; Ning, Y.; Yang, L.; Yu, F.; Guo, X. Zinc: The Other Suspected Environmental Factor in Kashin-Beck Disease in Addition to Selenium. Biol. Trace Elem. Res. 2017, 179, 178–184. [Google Scholar] [CrossRef]
- Mlakar, S.J.; Osredkar, J.; Prezelj, J.; Marc, J. The Antioxidant Enzyme GPX1 Gene Polymorphisms Are Associated with Low BMD and Increased Bone Turnover Markers. Dis. Markers 2010, 29, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sun, Q.; Lu, S. From Selenoprotein to Endochondral Ossification: A Novel Mechanism with MicroRNAs Potential in Bone Related Diseases? Med. Hypotheses 2011, 77, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Bos, S.D.; Kloppenburg, M.; Suchiman, E.; van Beelen, E.; Slagboom, P.E.; Meulenbelt, I. The Role of Plasma Cytokine Levels, CRP and Selenoprotein S Gene Variation in OA. Osteoarthr. Cartil. 2009, 17, 621–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Xie, D.; Wu, J.; Wu, Z.; He, H.; Yang, Z.; Yang, T.; Wang, Y. Selenium and bone health: A protocol for a systematic review and metaanalysis. BMJ 2020, 10, e036612. [Google Scholar] [CrossRef]
- Rivas, A.; Romero, A.; Mariscal-Arcas, M.; Monteagudo, C.; López, G.; Lorenzo, M.L.; Ocaña-Peinado, F.M.; Olea-Serrano, F. Association between dietary antioxidant quality score (DAQs) and bone mineral density in Spanish women. Nutr. Hosp. 2012, 27, 1886–1893. [Google Scholar]
- Sun, L.L.; Li, B.L.; Xie, H.L.; Fan, F.; Yu, W.Z.; Wu, B.H.; Xue, W.Q.; Chen, Y.M. Associations between the dietary intake of antioxidant nutrients and the risk of hip fracture in elderly Chinese: A case-control study. Br. J. Nutr. 2014, 112, 1706–1714. [Google Scholar] [CrossRef] [Green Version]
- Melhus, H.; Michaëlsson, K.; Holmberg, L.; Wolk, A.; Ljunghall, S. Smoking, antioxidant vitamins, and the risk of hip fracture. J. Bone Miner. Res. 1999, 14, 129–135. [Google Scholar] [CrossRef]
- Wolf, R.L.; Cauley, J.A.; Pettinger, M.; Jackson, R.; Lacroix, A.; Leboff, M.S.; Lewis, C.E.; Nevitt, M.C.; Simon, J.A.; Stone, K.L.; et al. Lack of a relation between vitamin and mineral antioxidants and bone mineral density: Results from the women’s health Initiative. Am. J. Clin. Nutr. 2005, 82, 581–588. [Google Scholar] [CrossRef]
- Arikan, D.C.; Coskun, A.; Ozer, A.; Kilinc, M.; Atalay, F.; Arikan, T. Plasma selenium, zinc, copper and lipid levels in postmenopausal Turkish women and their relation with osteoporosis. Biol. Trace Elem. Res. 2011, 144, 407–417. [Google Scholar] [CrossRef]
- Ilich, J.Z.; Cvijetic, S.; Baric, I.C.; Cecic, I.; Saric, M.; Crncevic-Orlic, Z.; Blanusa, M.; Korsic, M. Nutrition and lifestyle in relation to bone health and body weight in Croatian postmenopausal women. Int. J. Food Sci. Nutr. 2009, 60, 319–332. [Google Scholar] [CrossRef]
- Wang, L.; Yu, H.; Yang, G.; Zhang, Y.; Wang, W.; Su, T.; Ma, W.; Yang, F.; Chen, L.; He, L.; et al. Correlation between bone mineral density and serum trace element contents of elderly males in Beijing urban area. Int. J. Clin. Exp. Med. 2015, 8, 19250–19257. [Google Scholar] [PubMed]
- Liu, S.-Z.; Yan, H.; Xu, P.; Li, J.-P.; Zhuang, G.-H.; Zhu, B.-F.; Lu, S.-M. Correlation analysis between bone mineral density and serum element contents of postmenopausal women in Xi’an urban area. Biol. Trace Elem. Res. 2009, 131, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.S.; Jacques, R.M.; Schomburg, L.; Hill, T.R.; Mathers, J.C.; Williams, G.R.; Eastell, R. Effect of selenium supplementation on musculoskeletal health in older women: A randomised, double-blind, placebo-controlled trial. Lancet Healthy Longev. 2021, 2, e212–e221. [Google Scholar] [CrossRef]
- Perri, G.; Hill, T.; Mathers, J.C.; Walsh, J.; Gossiel, F.; Winther, K.; Frölich, J.; Folkestad, L.; Cold, S.; Eastell, R. Effect of selenium supplementation on biomarkers of bone turnover. Proc. Nutr. Soc. 2021, 80, E110. [Google Scholar] [CrossRef]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of Selenium and Vitamin E on Risk of Prostate Cancer and Other Cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA J. Am. Med. Assoc. 2009, 301, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Stranges, S.; Marshall, J.R.; Natarajan, R.; Donahue, R.P.; Trevisan, M.; Combs, G.F.; Cappuccio, F.P.; Ceriello, A.; Reid, M.E. Effects of Long-Term Selenium Supplementation on the Incidence of Type 2 Diabetes: A Randomized Trial. Ann. Intern. Med. 2007, 147, 217–223. [Google Scholar] [CrossRef]
- Fulda, S. Targeting apoptosis signaling pathways for anticancer therapy. Front. Oncol. 2011, 1, 23. [Google Scholar] [CrossRef] [Green Version]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria dependent and mitochondria independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- Aki, T.; Funakoshi, T.; Uemura, K. Regulated necrosis and its implications in toxicology. Toxicology 2015, 333, 118–126. [Google Scholar] [CrossRef]
- Ju-Kun, S.; Yuan, D.-B.; Rao, H.-F.; Chen, T.-F.; Luan, B.-S.; Xu, X.-M.; Jiang, F.-N.; Zhong, W.-D.; Zhu, J.-G. Association between Cd Exposure and Risk of Prostate Cancer. Medicine 2016, 95, e2708. [Google Scholar] [CrossRef]
- Banas, A.; Kwiatek, W.M.; Banas, K.; Gajda, M.; Pawlicki, B.; Cichocki, T. Correlation of Concentrations of Selected Trace Elements with Gleason Grade of Prostate Tissues. J. Biol. Inorg. Chem. 2010, 15, 1147–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lener, M.; Jaworska, K.; Muszyńska, M.; Sukiennicki, G.; Durda, K.; Gupta, S.; Złowocka-Perłowska, E.; Kładny, J.; Wiechowska-Kozłowska, A.; Grodzki, T.; et al. Selenium as Marker for Cancer Risk and Prevention. Pol. Przegl. Chir. 2012, 84, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.P.; Banerjee, S.; Brown, T.R.; Zirkin, B.R. Androgen Action in Prostate Function and Disease. Am. J. Clin. Exp. Urol. 2018, 6, 62–77. [Google Scholar] [PubMed]
- Menter, D.G.; Sabichi, A.L.; Lippman, S.M. Selenium Effects on Prostate Cell Growth. Cancer Epidemiol. Biomark. Prev. 2000, 9, 1171–1182. [Google Scholar]
- Brozmanová, J.; Mániková, D.; Vlčková, V.; Chovanec, M. Selenium: A Double-Edged Sword for Defense and Offence in Cancer. Arch. Toxicol. 2010, 84, 919–938. [Google Scholar] [CrossRef]
- Uysal, H.; Agar, G. Selenium Protective Activity against Aflatoxin B1 Adverse Affects on Drosophila Melanogaster. Braz. Arch. Biol. Technol. 2005, 48, 227–233. [Google Scholar] [CrossRef]
- Rejali, L.; Jaafar, M.H.; Ismail, N.H. Serum Selenium Level and Other Risk Factors for Breast Cancer among Patients in a Malaysian Hospital. Environ. Health Prev. Med. 2007, 12, 105–110. [Google Scholar] [CrossRef]
- Sandsveden, M.; Manjer, J. Selenium and Breast Cancer Risk: A Prospective Nested Case–Control Study on Serum Selenium Levels, Smoking Habits and Overweight. Int. J. Cancer 2017, 141, 1741–1750. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Kuai, Y.; Zhu, R.; Zhou, C.; Tao, Y.; Han, W.; Chen, Q. Prognosis of Prostate Cancer and Bone Metastasis Pattern of Patients: A SEER-Based Study and a Local Hospital Based Study from China. Sci. Rep. 2020, 10, 9104. [Google Scholar] [CrossRef]
- Zhang, X.H.-F.; Jin, X.; Malladi, S.; Zou, Y.; Wen, Y.H.; Brogi, E.; Smid, M.; Foekens, J.; Massagué, J. Selection of Bone Metastasis Seeds by Mesenchymal Signals in the Primary Tumor Stroma. Cell 2013, 154, 1060–1073. [Google Scholar] [CrossRef] [Green Version]
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to Bone: A Fatal Attraction. Nat. Rev. Cancer 2011, 11, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Dushyanthen, S.; Cossigny, D.A.F.; Quan, G.M.Y. The Osteoblastic and Osteoclastic Interactions in Spinal Metastases Secondary to Prostate Cancer. Cancer Growth Metastasis 2013, 6, 61–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.-C.; Zhang, J.-L.; Ge, C.-T.; Yu, Y.-Y.; Wang, P.; Yuan, T.-F.; Fu, C.-Y. Advances in Cancer Pain from Bone Metastasis. Drug Des. Dev. Ther. 2015, 9, 4239–4245. [Google Scholar] [CrossRef] [Green Version]
- Ando, T.; Watanabe, K.; Mizusawa, T.; Katagiri, A. Hypercalcemia Due to Parathyroid Hormone-Related Peptide Secreted by Neuroendocrine Dedifferentiated Prostate Cancer. Urol. Case Rep. 2018, 22, 67–69. [Google Scholar] [CrossRef]
- Cannarella, R.; Condorelli, R.A.; Barbagallo, F.; La Vignera, S.; Calogero, A.E. Endocrinology of the Aging Prostate: Current Concepts. Front. Endocrinol. 2021, 12, 554078. [Google Scholar] [CrossRef]
- Vuichoud, C.; Loughlin, K.R. Benign Prostatic Hyperplasia: Epidemiology, Economics and Evaluation. Can. J. Urol. 2015, 22 (Suppl. 1), 1–6. [Google Scholar] [PubMed]
- Daniyal, M.; Siddiqui, Z.A.; Akram, M.; Asif, H.M.; Sultana, S.; Khan, A. Epidemiology, Etiology, Diagnosis and Treatment of Prostate Cancer. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 9575–9578. [Google Scholar] [CrossRef] [Green Version]
- Cancer Today. Available online: http://gco.iarc.fr/today/home (accessed on 2 September 2021).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [Green Version]
- Keller, E.T.; Zhang, J.; Cooper, C.R.; Smith, P.C.; McCauley, L.K.; Pienta, K.J.; Taichman, R.S. Prostate Carcinoma Skeletal Metastases: Cross-Talk between Tumor and Bone. Cancer Metastasis Rev. 2001, 20, 333–349. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.K.; Mohamad, N.-V.; Giaze, T.R.; Chin, K.-Y.; Mohamed, N.; Ima-Nirwana, S. Prostate Cancer and Bone Metastases: The Underlying Mechanisms. Int. J. Mol. Sci. 2019, 20, 2587. [Google Scholar] [CrossRef] [Green Version]
- Gaffney-Stomberg, E. The Impact of Trace Minerals on Bone Metabolism. Biol. Trace Elem. Res. 2019, 188, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Noor, Z.; Sumitro, S.B.; Hidayat, M.; Rahim, A.H.; Sabarudin, A.; Umemura, T. Atomic Mineral Characteristics of Indonesian Osteoporosis by High-Resolution Inductively Coupled Plasma Mass Spectrometry. Sci. World J. 2012, 2012, e372972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonas, J.; Burns, J.; Abel, E.W.; Cresswell, M.J.; Strain, J.J.; Paterson, C.R. Impaired Mechanical Strength of Bone in Experimental Copper Deficiency. Ann. Nutr. Metab. 1993, 37, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Faliva, M.A.; Infantino, V.; Gasparri, C.; Iannello, G.; Perna, S.; Riva, A.; Petrangolini, G.; Tartara, A.; Peroni, G. Copper as Dietary Supplement for Bone Metabolism: A Review. Nutrients 2021, 13, 2246. [Google Scholar] [CrossRef] [PubMed]
- Milkovic, L.; Hoppe, A.; Detsch, R.; Boccaccini, A.R.; Zarkovic, N. Effects of Cu-Doped 45S5 Bioactive Glass on the Lipid Peroxidation-Associated Growth of Human Osteoblast-like Cells in Vitro. J. Biomed. Mater. Res. A 2014, 102, 3556–3561. [Google Scholar] [CrossRef]
- National Research Council (US). Subcommittee on Laboratory Animal Nutrition. In Nutrient Requirements of Laboratory Animals: Fourth Revised Edition; National Academies Press (US): Washington, DC, USA, 1995; ISBN 978-0-309-05126-2. [Google Scholar]
- Ott, G.; Havemeyer, A.; Clement, B. The Mammalian Molybdenum Enzymes of MARC. J. Biol. Inorg. Chem. JBIC 2015, 20, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.C.; Johns, L.E.; Meeker, J.D. Exploratory Analysis of the Potential Relationship between Urinary Molybdenum and Bone Mineral Density among Adult Men and Women from NHANES 2007–2010. Chemosphere 2016, 164, 677–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyskocil, A.; Viau, C. Assessment of Molybdenum Toxicity in Humans. J. Appl. Toxicol. JAT 1999, 19, 185–192. [Google Scholar] [CrossRef]
- Bhattacharya, P.T.; Misra, S.R.; Hussain, M. Nutritional Aspects of Essential Trace Elements in Oral Health and Disease: An Extensive Review. Scientifica 2016, 2016, e5464373. [Google Scholar] [CrossRef] [Green Version]
- Parry, N.M.; Phillippo, M.; Reid, M.D.; McGaw, B.A.; Flint, D.J.; Loveridge, N. Molybdenum-Induced Changes in the Epiphyseal Growth Plate. Calcif. Tissue Int. 1993, 53, 180–186. [Google Scholar] [CrossRef]
- Picco, S.; Ponzzinibio, M.V.; Mattioli, G.; Rosa, D.; Minatel, L.; Fazzio, L.; Seoane, A. Physiological and Genotoxic Effects of Molybdenum-Induced Copper Deficiency in Cattle. Agrociencia 2012, 46, 107–117. [Google Scholar]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The Importance of Mineral Elements for Humans, Domestic Animals and Plants—A Review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar] [CrossRef]
- Aschner, J.L.; Aschner, M. Nutritional Aspects of Manganese Homeostasis. Mol. Asp. Med. 2005, 26, 353–362. [Google Scholar] [CrossRef]
- Sansone, V.; Pagani, D.; Melato, M. The Effects on Bone Cells of Metal Ions Released from Orthopaedic Implants. A Review. Clin. Cases Miner. Bone Metab. 2013, 10, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Devitt, B.M.; Queally, J.M.; Vioreanu, M.; Butler, J.S.; Murray, D.; Doran, P.P.; O’Byrne, J.M. Cobalt Ions Induce Chemokine Secretion in a Variety of Systemic Cell Lines. Acta Orthop. 2010, 81, 756–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibon, E.; Amanatullah, D.F.; Loi, F.; Pajarinen, J.; Nabeshima, A.; Yao, Z.; Hamadouche, M.; Goodman, S.B. The Biological Response to Orthopaedic Implants for Joint Replacement: Part I: Metals. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2162–2173. [Google Scholar] [CrossRef] [PubMed]
- Queally, J.M.; Devitt, B.M.; Butler, J.S.; Malizia, A.P.; Murray, D.; Doran, P.P.; O’Byrne, J.M. Cobalt Ions Induce Chemokine Secretion in Primary Human Osteoblasts. J. Orthop. Res. 2009, 27, 855–864. [Google Scholar] [CrossRef]
- Tkaczyk, C.; Petit, A.; Antoniou, J.; Zukor, D.J.; Tabrizian, M.; Huk, O.L. Significance of Elevated Blood Metal Ion Levels in Patients with Metal-on-Metal Prostheses: An Evaluation of Oxidative Stress Markers. Open Orthop. J. 2010, 4, 221–227. [Google Scholar] [CrossRef]
- Tkaczyk, C.; Huk, O.L.; Mwale, F.; Antoniou, J.; Zukor, D.J.; Petit, A.; Tabrizian, M. Effect of Chromium and Cobalt Ions on the Expression of Antioxidant Enzymes in Human U937 Macrophage-like Cells. J. Biomed. Mater. Res. A 2010, 94A, 419–425. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. 2-Oxoglutarate-Dependent Dioxygenases Are Sensors of Energy Metabolism, Oxygen Availability, and Iron Homeostasis: Potential Role in the Regulation of Aging Process. Cell. Mol. Life Sci. 2015, 72, 3897–3914. [Google Scholar] [CrossRef]
- Díaz-Castro, J.; López-Frías, M.R.; Campos, M.S.; López-Frías, M.; Alférez, M.J.M.; Nestares, T.; Ojeda, M.L.; López-Aliaga, I. Severe Nutritional Iron-Deficiency Anaemia Has a Negative Effect on Some Bone Turnover Biomarkers in Rats. Eur. J. Nutr. 2012, 51, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Jeney, V. Clinical Impact and Cellular Mechanisms of Iron Overload-Associated Bone Loss. Front. Pharmacol. 2017, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Strause, L.; Saltman, P.; Smith, K.T.; Bracker, M.; Andon, M.B. Spinal Bone Loss in Postmenopausal Women Supplemented with Calcium and Trace Minerals. J. Nutr. 1994, 124, 1060–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eaton-Evans, J.; Mcllrath, E.M.; Jackson, W.E.; McCartney, H.; Strain, J.J. Copper Supplementation and the Maintenance of Bone Mineral Density in Middle-Aged Women. J. Trace Elem. Exp. Med. 1996, 9, 87–94. [Google Scholar] [CrossRef]
- Baker, A.; Harvey, L.; Majask-Newman, G.; Fairweather-Tait, S.; Flynn, A.; Cashman, K. Effect of Dietary Copper Intakes on Biochemical Markers of Bone Metabolism in Healthy Adult Males. Eur. J. Clin. Nutr. 1999, 53, 408–412. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, F.H.; Lukaski, H.C.; Johnson, L.K.; Roughead, Z.K.F. Reported Zinc, but Not Copper, Intakes Influence Whole-Body Bone Density, Mineral Content and T Score Responses to Zinc and Copper Supplementation in Healthy Postmenopausal Women. Br. J. Nutr. 2011, 106, 1872–1879. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Advances in Metal-Induced Oxidative Stress and Human Disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Yossepowitch, O.; Pinchuk, I.; Gur, U.; Neumann, A.; Lichtenberg, D.; Baniel, J. Advanced but Not Localized Prostate Cancer Is Associated with Increased Oxidative Stress. J. Urol. 2007, 178, 1238–1243. [Google Scholar] [CrossRef]
- Paschos, A.; Pandya, R.; Duivenvoorden, W.C.M.; Pinthus, J.H. Oxidative Stress in Prostate Cancer: Changing Research Concepts towards a Novel Paradigm for Prevention and Therapeutics. Prostate Cancer Prostatic Dis. 2013, 16, 217–225. [Google Scholar] [CrossRef]
- Ayala, G.E.; Dai, H.; Ittmann, M.; Li, R.; Powell, M.; Frolov, A.; Wheeler, T.M.; Thompson, T.C.; Rowley, D. Growth and Survival Mechanisms Associated with Perineural Invasion in Prostate Cancer. Cancer Res. 2004, 64, 6082–6090. [Google Scholar] [CrossRef] [Green Version]
- Vogt, T.M.; Ziegler, R.G.; Graubard, B.I.; Swanson, C.A.; Greenberg, R.S.; Schoenberg, J.B.; Swanson, G.M.; Hayes, R.B.; Mayne, S.T. Serum Selenium and Risk of Prostate Cancer in U.S. Blacks and Whites. Int. J. Cancer 2003, 103, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Liu, D.; Liu, C.; Liu, G. Serum Selenium Levels and Prostate Cancer Risk: A MOOSE-Compliant Meta-Analysis. Medicine 2017, 96, e5944. [Google Scholar] [CrossRef] [PubMed]
- Sayehmiri, K.; Azami, M.; Mohammadi, Y.; Soleymani, A.; Tardeh, Z. The Association between Selenium and Prostate Cancer: A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. APJCP 2018, 19, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Algotar, A.M.; Stratton, M.S.; Ahmann, F.R.; Ranger-Moore, J.; Nagle, R.B.; Thompson, P.A.; Slate, E.; Hsu, C.H.; Dalkin, B.L.; Sindhwani, P.; et al. Phase 3 Clinical Trial Investigating the Effect of Selenium Supplementation in Men at High-Risk for Prostate Cancer. Prostate 2013, 73, 328–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, E.A.; Thompson, I.M.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the Risk of Prostate Cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef]
- Nicastro, H.L.; Dunn, B.K. Selenium and Prostate Cancer Prevention: Insights from the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Nutrients 2013, 5, 1122–1148. [Google Scholar] [CrossRef] [Green Version]
- Bhaskaram, P. Micronutrient Malnutrition, Infection, and Immunity: An Overview. Nutr. Rev. 2002, 60, S40–S45. [Google Scholar] [CrossRef]
- Zeng, H. Selenite and Selenomethionine Promote HL-60 Cell Cycle Progression. J. Nutr. 2002, 132, 674–679. [Google Scholar] [CrossRef] [Green Version]
- Saito, Y.; Yoshida, Y.; Akazawa, T.; Takahashi, K.; Niki, E. Cell Death Caused by Selenium Deficiency and Protective Effect of Antioxidants. J. Biol. Chem. 2003, 278, 39428–39434. [Google Scholar] [CrossRef] [Green Version]
- Karp, H.J.; Ketola, M.E.; Lamberg-Allardt, C.J.E. Acute Effects of Calcium Carbonate, Calcium Citrate and Potassium Citrate on Markers of Calcium and Bone Metabolism in Young Women. Br. J. Nutr. 2009, 102, 1341–1347. [Google Scholar] [CrossRef] [Green Version]
- Sakhaee, K.; Maalouf, N.M.; Abrams, S.A.; Pak, C.Y.C. Effects of Potassium Alkali and Calcium Supplementation on Bone Turnover in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2005, 90, 3528–3533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbal, A.; Yılmaz, H.; Tutkun, E. Arsenic Exposure Associated with Decreased Bone Mineralization in Male. Aging Male 2014, 17, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Richette, P.; Ottaviani, S.; Vicaut, E.; Bardin, T. Musculoskeletal Complications of Hereditary Hemochromatosis: A Case-Control Study. J. Rheumatol. 2010, 37, 2145–2150. [Google Scholar] [CrossRef] [PubMed]
- Brissot, P.; Cavey, T.; Ropert, M.; Guggenbuhl, P.; Loréal, O. Genetic Hemochromatosis: Pathophysiology, Diagnostic and Therapeutic Management. Presse Med. 2017, 46, e288–e295. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-J.; Ahn, S.H.; Bae, S.J.; Kim, E.H.; Lee, S.-H.; Kim, H.-K.; Choe, J.W.; Koh, J.-M.; Kim, G.S. Iron Overload Accelerates Bone Loss in Healthy Postmenopausal Women and Middle-Aged Men: A 3-Year Retrospective Longitudinal Study. J. Bone Miner. Res. 2012, 27, 2279–2290. [Google Scholar] [CrossRef] [PubMed]


| ICP-MS Nexion 300 D | |
|---|---|
| plasma power, W | 1350 |
| nebulizer gas flow (AR), L min−1 | 0.9 |
| dwell time, ms | 50 |
| readings | 5 |
| sweeps | 1 |
| replicates | 3 |
| monitored isotopes | 27Al, 24Mg, 39K, 43Ca, 51V, 55Mn, 57Fe, 59Co, 63Cu, 60Ni, 66Zn, 78Se, 88Sr, 95Mo, 111Cd, 208Pb |
| Group/ Diet n = 6–8 | Ca (g/kg Dry Mass) | Zn (mg/kg Dry Mass) | K (mg/kg Dry Mass) | Fe (mg/kg Dry Mass) | Sr (mg/kg Dry Mass) | Ni (mg/kg Dry Mass) | Cu (mg/kg Dry Mass) | Mn (mg/kg Dry Mass) | Co (mg/kg Dry Mass) | Mo (mg/kg Dry Mass) |
|---|---|---|---|---|---|---|---|---|---|---|
| CtrlSt ExpSt | 291 ± 38 | 45.3 ± 4.3 | 2018 ± 206 | 1411 ± 158 | 70.1 ± 2.7 | 3.71 ± 0.69 | 0.494 ± 0.261 | 0.160 ± 0.014 | 0.136 ± 0.016 | 0.047 ± 0.010 |
| 221 ± 18 * | 41.3 ± 6.0 | 1561 ± 125 | 1281 ± 184 | 63.5 ± 5.8 * | 3.36 ± 0.55 | 0.272 ± 0.084 | 0.140 ± 0.010 * | 0.106 ± 0.015 | 0.008 ± 0.005 | |
| ↓ 24% | ↓ 9% | ↓ 23% | ↓ 9% | ↓ 9% | ↓ 9% | ↓ 45% | ↓ 13% | ↓ 22% | ↓ 83% | |
| (p = 0.002) | (ns) | (ns) | (ns) | (p = 0.029) | (ns) | (ns) | (p = 0.017) | (p = 0.007) | p = 4.67 × 10−6 | |
| CtrlCu ExpCu | 222 ± 17 | 66.3 ± 2.6 | 1827 ± 262 | 1289 ± 180 | 61.2 ± 2.4 | 8.1 ± 0.46 | 0.488 ± 0.045 | 0.327 ± 0.048 | 0.314 ± 0.009 | 0.070 ± 0.012 |
| 243 ± 23 | 45.1 ± 4.3 * | 1562 ± 191 | 866 ± 155 * | 58.6 ± 5.5 | 4.07 ± 0.59 * | 0.298 ± 0.039 * | 0.149 ± 0.013 * | 0.129 ± 0.017 * | 0.022 ± 0.005 * | |
| ↑ 10% | ↓ 32% | ↓ 15% | ↓ 33% | ↓ 4% | ↓ 50% | ↓ 61% | ↓ 54% | ↓ 59% | ↓ 69% | |
| (ns) | (p = 9.93 × 10−8) | (ns) | (p = 0.001) | (ns) | p = 2.23 × 10−6 | p = 2.23 × 10−6 | p = 2.23 × 10−6 | p = 2.23 × 10−8 | (p = 0.001) | |
| CtrlSe ExpSe | 256 ± 76 | 43.5 ± 4.3 | 1692 ± 463 | 887 ± 262 | 59.6 ± 18.0 | 4.08 ± 0.4 | 0.308 ± 0.051 | 0.179 ± 0.059 | 0.129 ± 0.012 | 0.040 ± 0.026 |
| 254 ± 22 | 45.0 ± 2.1 | 2402 ± 424 * | 1272 ± 106 * | 66.7 ± 4.3 | 3.76 ± 0.21 | 0.283 ± 0.027 | 0.157 ± 0.003 | 0.147 ± 0.007 | 0.031 ± 0.003 | |
| ↓ 1% | ↑ 3% | ↑ 42% | ↑ 43% | ↑ 12% | ↓ 8% | ↓ 8% | ↓ 12% | ↑ 14% | ↓ 23% | |
| (ns) | (ns) | (p = 0.008) | (p = 0.002) | (ns) | (ns) | (ns) | (ns) | (ns) | (ns) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skrajnowska, D.; Jagielska, A.; Ruszczyńska, A.; Idkowiak, J.; Bobrowska-Korczak, B. Effect of Copper and Selenium Supplementation on the Level of Elements in Rats’ Femurs under Neoplastic Conditions. Nutrients 2022, 14, 1285. https://doi.org/10.3390/nu14061285
Skrajnowska D, Jagielska A, Ruszczyńska A, Idkowiak J, Bobrowska-Korczak B. Effect of Copper and Selenium Supplementation on the Level of Elements in Rats’ Femurs under Neoplastic Conditions. Nutrients. 2022; 14(6):1285. https://doi.org/10.3390/nu14061285
Chicago/Turabian StyleSkrajnowska, Dorota, Agata Jagielska, Anna Ruszczyńska, Jakub Idkowiak, and Barbara Bobrowska-Korczak. 2022. "Effect of Copper and Selenium Supplementation on the Level of Elements in Rats’ Femurs under Neoplastic Conditions" Nutrients 14, no. 6: 1285. https://doi.org/10.3390/nu14061285
APA StyleSkrajnowska, D., Jagielska, A., Ruszczyńska, A., Idkowiak, J., & Bobrowska-Korczak, B. (2022). Effect of Copper and Selenium Supplementation on the Level of Elements in Rats’ Femurs under Neoplastic Conditions. Nutrients, 14(6), 1285. https://doi.org/10.3390/nu14061285







