Lactobacillus reuteri CCFM8631 Alleviates Hypercholesterolaemia Caused by the Paigen Atherogenic Diet by Regulating the Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Probiotic Strains and Culture
2.2. Mice and Dietary Interventions
2.3. Sample Collection
2.4. Biochemical Analyses
2.5. Histopathological Analysis
2.6. Faecal Short-Chain Fatty Acid Analysis
2.7. Analysis of the Gut Microbiota in Faecal Samples
2.8. Statistical Analysis
3. Results
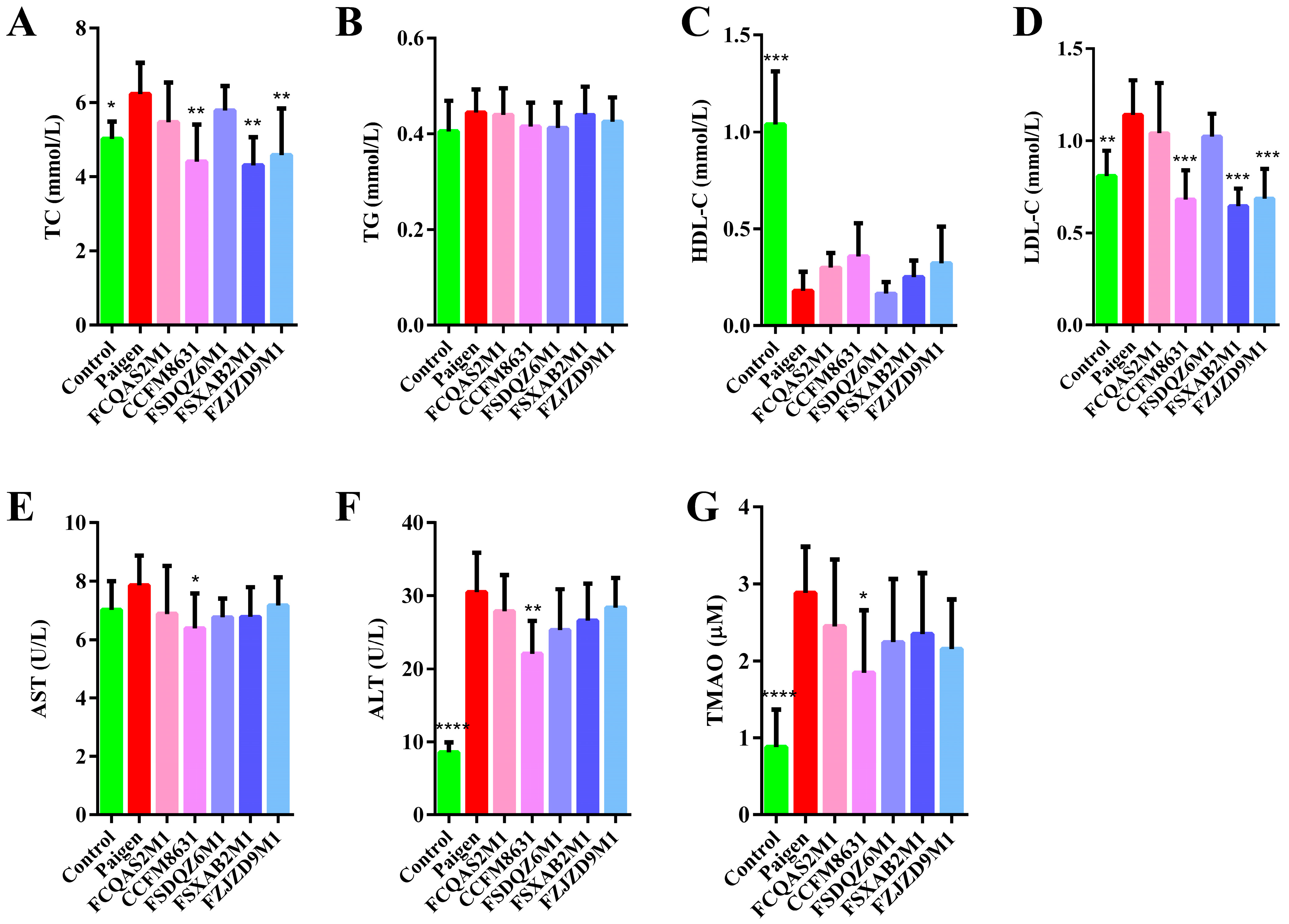
3.1. Lactobacillus Reuteri CCFM8631 Has the Potential to Reduce Blood Lipid Concentrations and Alleviate Liver Injury after Paigen Atherogenic Diet Treatment
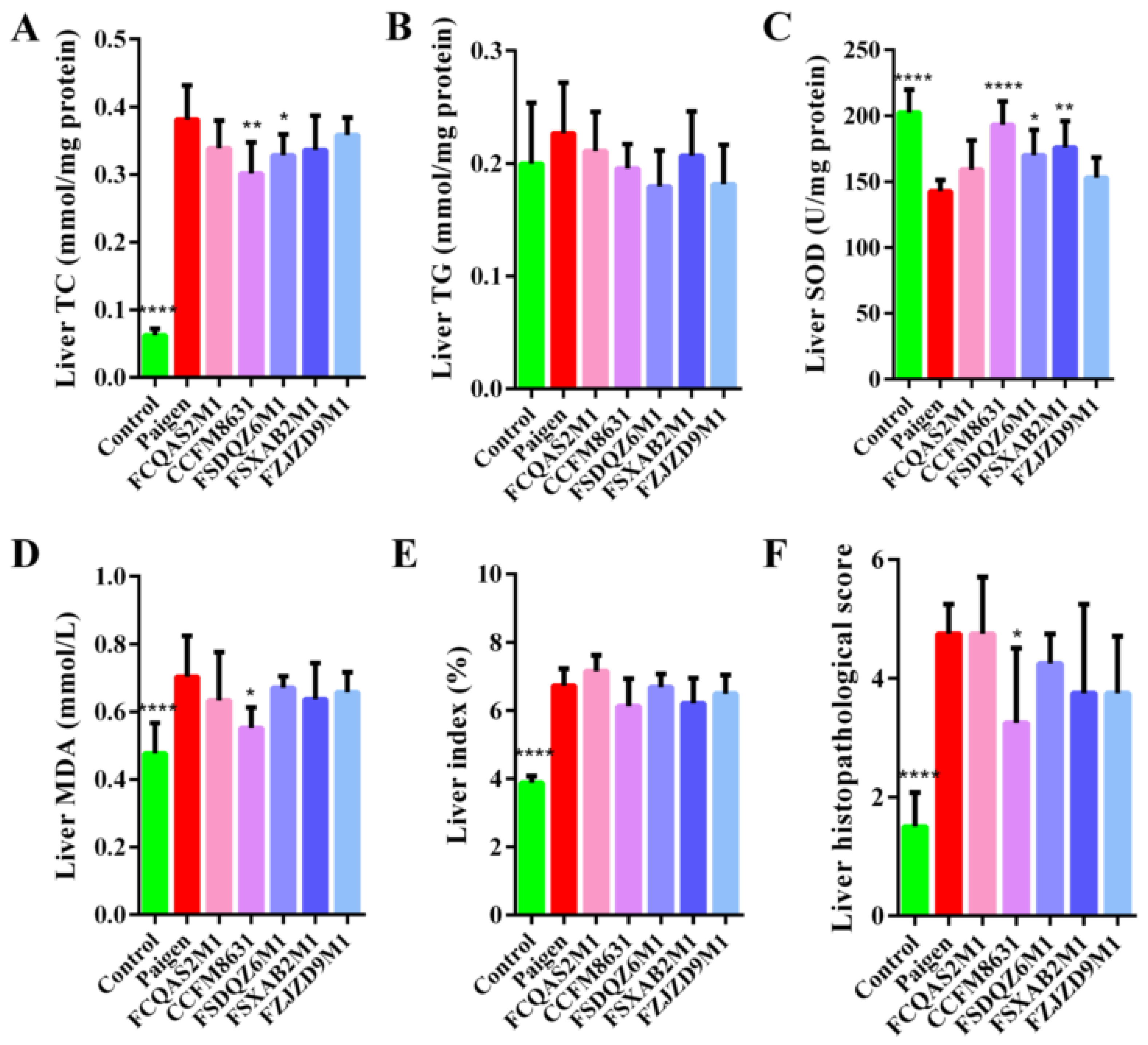
3.2. Lactobacillus Reuteri CCFM8631 Reduced the Lipid Cholesterol Concentration and Oxidative Liver Damage Caused by a Paigen Atherogenic Diet
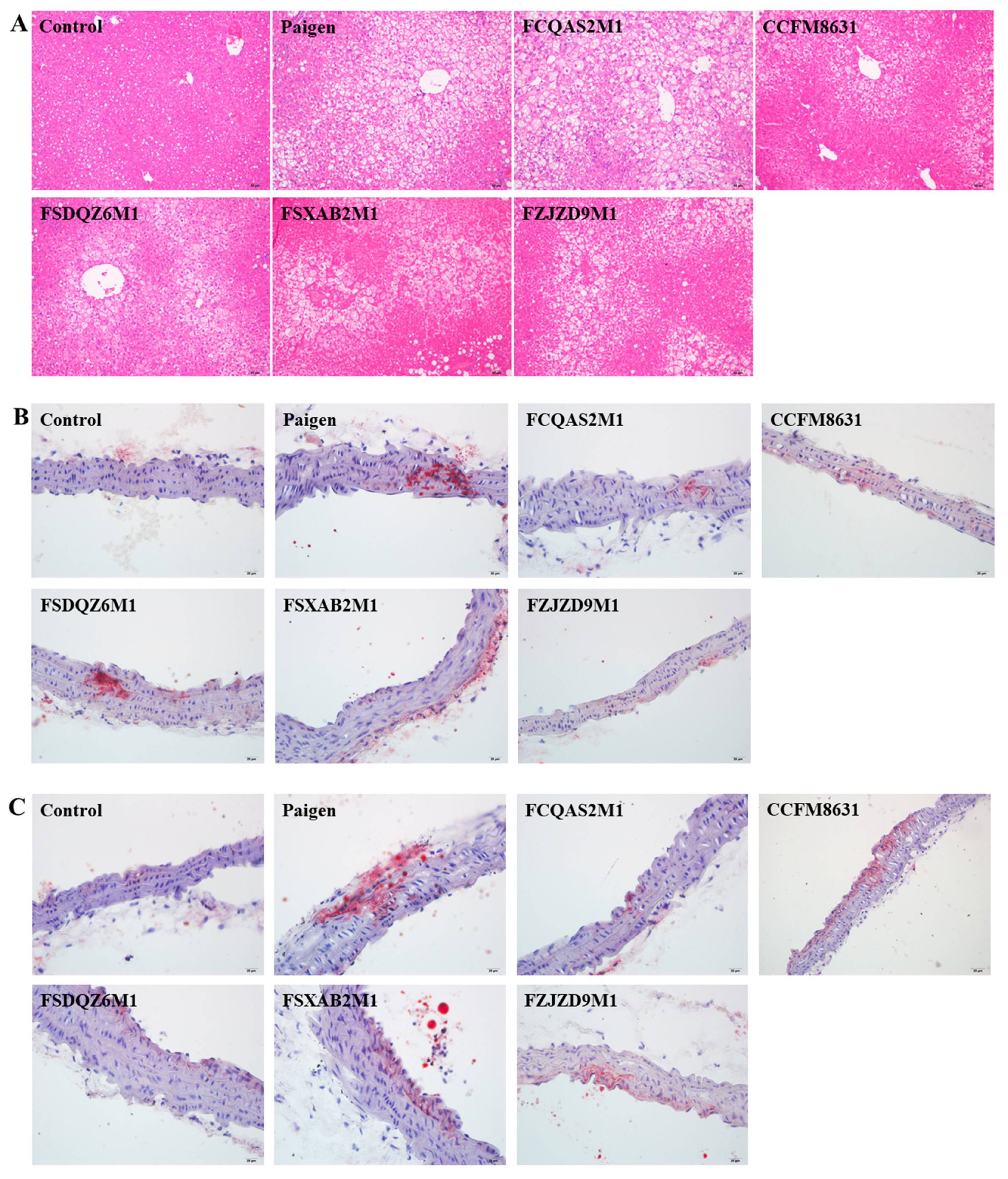
3.3. Lactobacillus Reuteri CCFM8631 Significantly Alleviated Liver Injury, but Only Slightly Decreased the Production of Foam Cells in the Abdominal Aorta and Aortic Arch after Paigen Atherogenic Diet Treatment
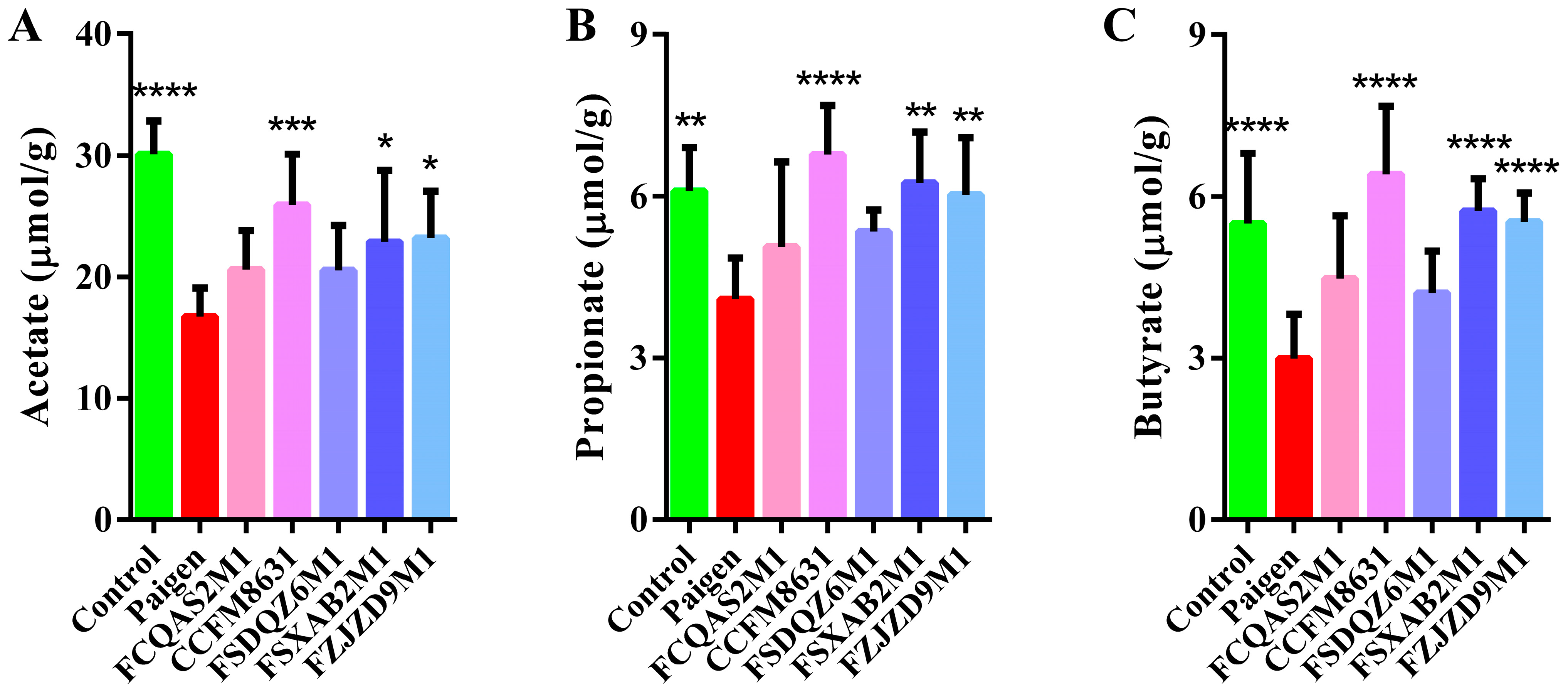
3.4. Lactobacillus Reuteri CCFM8631 Significantly Affected the Composition of Short-Chain Fatty Acids in Mice Fed a Paigen Atherogenic Diet
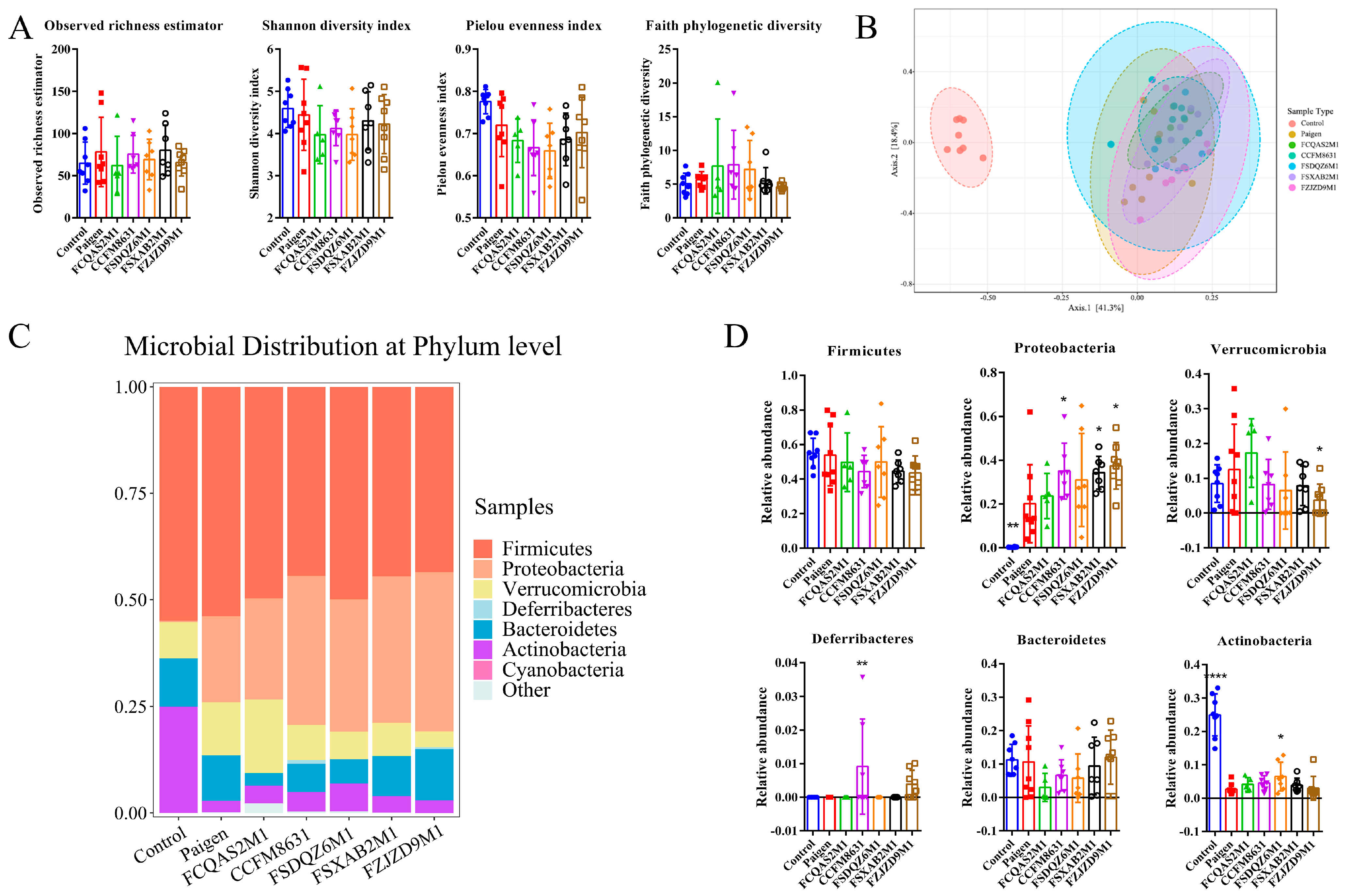
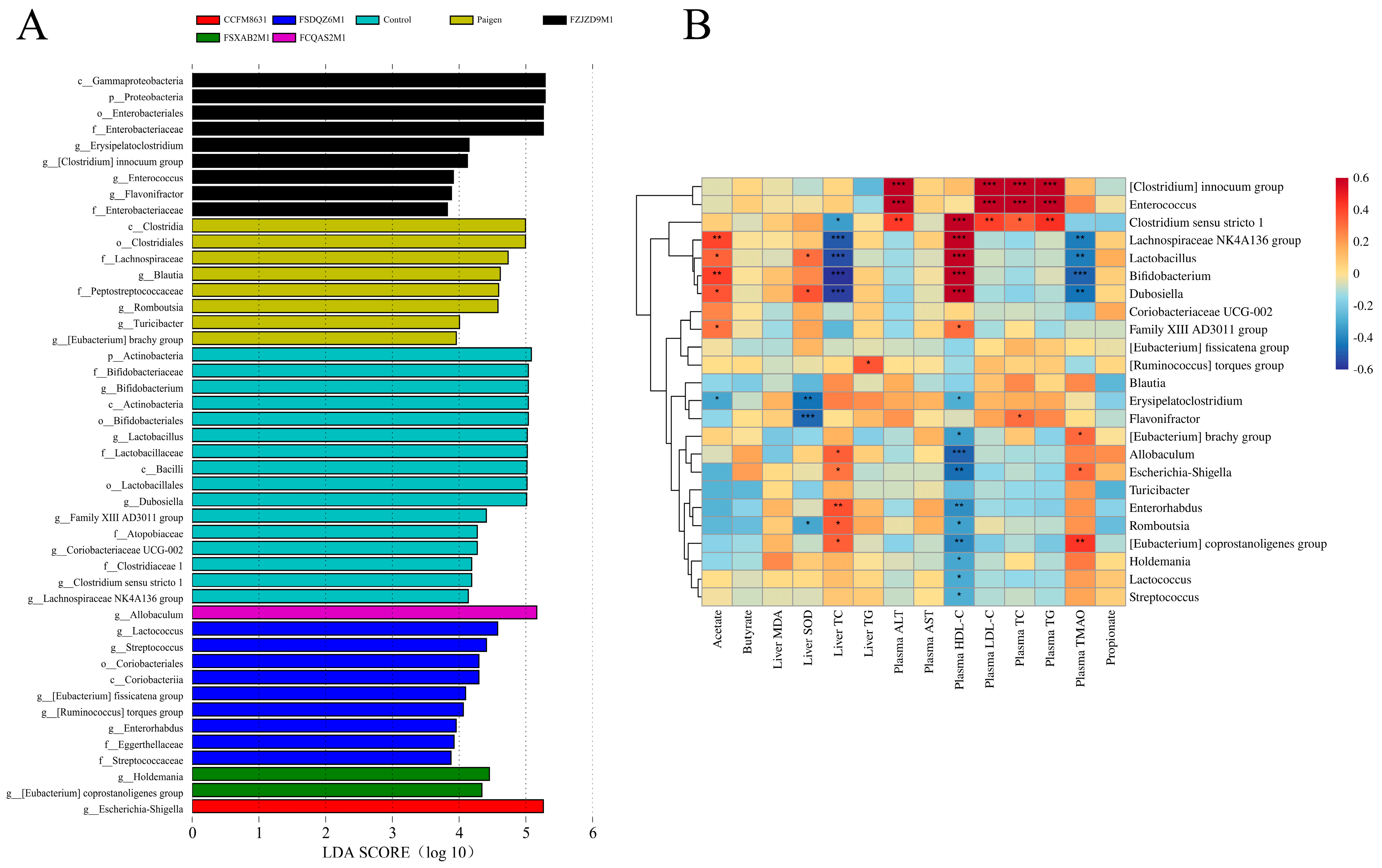
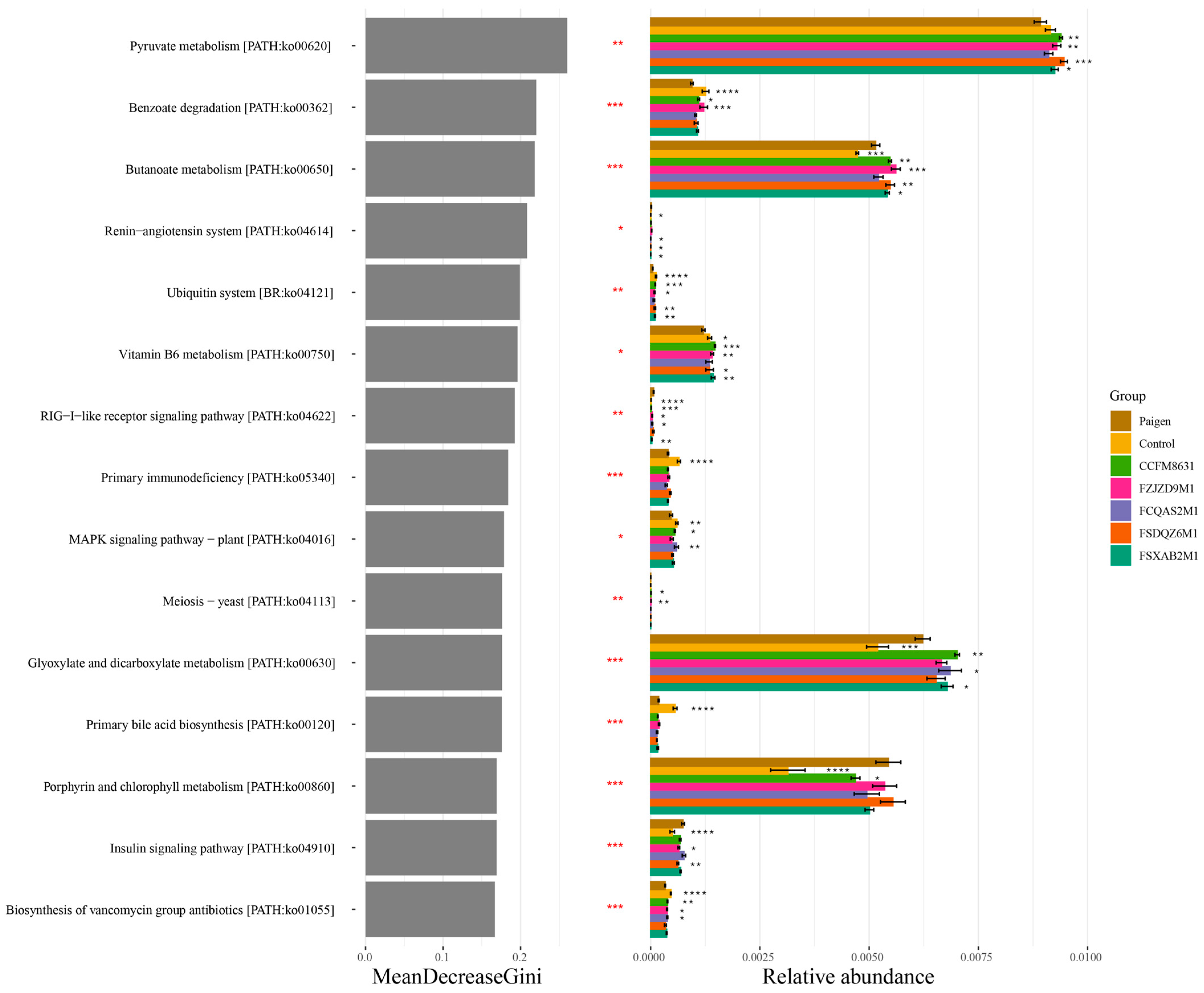
3.5. Supplementation of Lactobacillus and Bifidobacterium Alleviated the Gut Microbiota Dysbiosis Caused by Paigen Atherogenic Diet Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Di Angelantonio, E.; Kaptoge, S.; Pennells, L.; De Bacquer, D.; Cooney, M.T.; Kavousi, M.; Stevens, G.; Riley, L.; Savin, S.; Altay, S.; et al. World Health Organization cardiovascular disease risk charts: Revised models to estimate risk in 21 global regions. Lancet Glob. Health 2019, 7, E1332–E1345. [Google Scholar] [CrossRef] [Green Version]
- Davis, C.E.; Rifkind, B.M.; Brenner, H.; Gordon, D.J. A single cholesterol measurement underestimates the risk of coronary heart disease. An empirical example from the Lipid Research Clinics Mortality Follow-up Study. JAMA 1990, 264, 3044–3046. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.J.; Hsu, W.H.; Huang, J.J.; Wu, S.C. Effect of pigeon pea (Cajanus cajan L.) on high-fat diet-induced hypercholesterolemia in hamsters. Food Chem. Toxicol. 2013, 53, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Tunstall-Pedoe, H.; Vanuzzo, D.; Hobbs, M.; Mahonen, M.; Cepaitis, Z.; Kuulasmaa, K.; Keil, U.; Project, W.M. Estimation of contribution of changes in coronary care to improving survival, event rates, and coronary heart disease mortality across the WHO MONICA Project populations. Lancet 2000, 355, 688–700. [Google Scholar] [CrossRef]
- Browning, J.D.; Horton, J.D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Investig. 2004, 114, 147–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.M.; Zhao, L.; Xiong, X.L.; He, Y.; Huang, W.; Liu, Z.H.; Ji, L.; Pan, B.; Guo, X.F.; Wang, L.B.; et al. TMAVA, a Metabolite of Intestinal Microbes, Is Increased in Plasma from Patients with Liver Steatosis, Inhibits gamma-Butyrobetaine Hydroxylase, and Exacerbates Fatty Liver in Mice. Gastroenterology 2020, 158, 2266–2281.e27. [Google Scholar] [CrossRef] [PubMed]
- Bellosta, S.; Paoletti, R.; Corsini, A. Safety of statins—Focus on clinical pharmacokinetics and drug interactions. Circulation 2004, 109, 50–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, G. Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; Report of a Joint FAO/WHO Expert Consultation; Scientific Research Publishing: Wuhan, China, 2001; p. 30. [Google Scholar]
- Begley, M.; Hill, C.; Gahan, C.G.M. Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 2006, 72, 1729–1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.H.; Kim, J.G.; Shin, Y.W.; Kim, S.H.; Whang, K.Y. Effect of dietary inclusion of Lactobacillus acidophilus ATCC 43121 on cholesterol metabolism in rats. J. Microbiol. Biotechnol. 2007, 17, 655–662. [Google Scholar] [PubMed]
- Liong, M.T.; Shah, N.P. Acid and bile tolerance and cholesterol removal ability of lactobacilli strains. J. Dairy Sci. 2005, 88, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Paigen, B.; Morrow, A.; Brandon, C.; Mitchell, D.; Holmes, P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis 1985, 57, 65–73. [Google Scholar] [CrossRef]
- Ocque, A.J.; Stubbs, J.R.; Nolin, T.D. Development and validation of a simple UHPLC-MS/MS method for the simultaneous determination of trimethylamine N-oxide, choline, and betaine in human plasma and urine. J. Pharm. Biomed. Anal. 2015, 109, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Ramirezzacarias, J.L.; Castromunozledo, F.; Kuriharcuch, W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with oil red-O. Histochemistry 1992, 97, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Bedossa, P.; Consortium, F.P. Utility and Appropriateness of the Fatty Liver Inhibition of Progression (FLIP) Algorithm and Steatosis, Activity, and Fibrosis (SAF) Score in the Evaluation of Biopsies of Nonalcoholic Fatty Liver Disease. Hepatology 2014, 60, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Samuel, B.S.; Gordon, J.I. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc. Natl. Acad. Sci. USA 2006, 103, 10011–10016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, B.Y.; Li, D.Y.; Zhao, J.X.; Liu, X.M.; Gu, Z.N.; Chen, Y.Q.; Zhang, H.; Chen, W. Metagenomic Insights into the Effects of Fructo-oligosaccharides (FOS) on the Composition of Fecal Microbiota in Mice. J. Agric. Food Chem. 2015, 63, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cui, Y.M.; Li, X.Z.; Yao, M.J. microeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.N.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.M.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Zhu, G.; Chen, C.; Zheng, Y.; Ma, F.; Zhao, J.; Lee, Y.K.; Zhang, H.; Chen, W. Lactobacillus strains derived from human gut ameliorate metabolic disorders via modulation of gut microbiota composition and short-chain fatty acids metabolism. Benef. Microbes 2021, 12, 267–281. [Google Scholar] [CrossRef]
- Wang, G.; Si, Q.; Yang, S.R.; Jiao, T.; Zhu, H.Y.; Tian, P.J.; Wang, L.L.; Li, X.; Gong, L.; Zhao, J.X.; et al. Lactic acid bacteria reduce diabetes symptoms in mice by alleviating gut microbiota dysbiosis and inflammation in different manners. Food Funct. 2020, 11, 5898–5914. [Google Scholar] [CrossRef] [PubMed]
- Gang, W.; Ting, J.; Yue, X.; Daozheng, L.; Qian, S.; Jianfeng, H.; Jianxin, Z.; Hao, Z.; Wei, C. Bifidobacterium adolescentis and Lactobacillus rhamnosus alleviate non-alcoholic fatty liver disease induced by a high-fat, high-cholesterol diet through modulation of different gut microbiota-dependent pathways. Food Funct. 2020, 11, 6115–6127. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, B.; Ross, R.P.; Jin, Y.; Stanton, C.; Zhao, J.X.; Zhang, H.; Chen, W. Orally Administered CLA Ameliorates DSS-Induced Colitis in Mice via Intestinal Barrier Improvement, Oxidative Stress Reduction, and Inflammatory Cytokine and Gut Microbiota Modulation. J. Agric. Food Chem. 2019, 67, 13282–13298. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.; McCoy, K.D.; Macpherson, A.J. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin. Immunol. 2007, 19, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [Green Version]
- Jiao, X.Y.; Wang, Y.H.; Lin, Y.; Lang, Y.X.; Li, E.H.; Zhang, X.Y.; Zhang, Q.; Feng, Y.; Meng, X.J.; Li, B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef]
- Li, Y.M.; Luan, Y.P.; Yue, X.G.; Xiang, F.; Mao, D.C.; Cao, Y.; Xiong, Z. Effects of Codonopis bulleynana forest ex diels on Deferribacteres in constipation predominant intestine tumor: Differential analysis. Saudi J. Biol. Sci. 2019, 26, 395–401. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, J.Y.; Bonder, M.J.; Cenit, M.C.; Tigchelaar, E.F.; Maatman, A.; Dekens, J.A.M.; Brandsma, E.; Marczynska, J.; Imhann, F.; Weersma, R.K.; et al. The Gut Microbiome Contributes to a Substantial Proportion of the Variation in Blood Lipids. Circ. Res. 2015, 117, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.J.; Jiang, C.T.; Patterson, A.D. An Intestinal Microbiota-Farnesoid X Receptor Axis Modulates Metabolic Disease. Gastroenterology 2016, 151, 845–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ubbink, J.B.; Vermaak, W.J.H.; Vandermerwe, A.; Becker, P.J. Vitamin-B12, vitamin-B6, and folate nutritional-status in men with hyperhomocysteinemia. Am. J. Clin. Nutr. 1993, 57, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Chengfeng, Y.; Guopeng, L.; Yuqing, C.; Song, M.; Bin, L.; Chao, Z. Antidiabetic potential of green seaweed Enteromorpha prolifera flavonoids regulating insulin signaling pathway and gut microbiota in type 2 diabetic mice. J. Food Sci. 2019, 84, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Galie, S.; Garcia-Gavilan, J.; Camacho-Barcia, L.; Atzeni, A.; Muralidharan, J.; Papandreou, C.; Arcelin, P.; Palau-Galindo, A.; Garcia, D.; Basora, J.; et al. Effects of the Mediterranean Diet or Nut Consumption on Gut Microbiota Composition and Fecal Metabolites and their Relationship with Cardiometabolic Risk Factors. Mol. Nutr. Food Res. 2021, 65, 2000982. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.W.; de Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, Colonic Fermentation, and Gastrointestinal Health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Midtvedt, T.; Gordon, J.I. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu. Rev. Nutr. 2002, 22, 283–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.W.; Wang, J.H.; Xu, Y.L.; Yang, H.C.; Wang, J.F.; Xue, C.H.; Yan, X.J.; Su, L.J. Anti-inflammation effects of fucosylated chondroitin sulphate from Acaudina molpadioides by altering gut microbiota in obese mice. Food Funct. 2019, 10, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, J.; Hornung, B.; Renckens, B.; van Hijum, S.; dos Santos, V.; Rijkers, G.T.; Schaap, P.J.; de Vos, W.M.; Smidt, H. Genomic and functional analysis of Romboutsia ilealis CRIBT reveals adaptation to the small intestine. PeerJ 2017, 5, e3698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Serial Number | Species | Original Number | Sample |
|---|---|---|---|
| 1 | Lactobacillus plantarum | FCQAS2M1 | Human faeces |
| 2 | Lactobacillus reuteri | CCFM8631 | Human faeces |
| 3 | Lactobacillus casei | FSDQZ6M1 | Human faeces |
| 4 | Bifidobacterium breve | FSXAB2M1 | Human faeces |
| 5 | Bifidobacterium adolescentis | FZJZD9M1 | Human faeces |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; He, Y.; Li, X.; Zhang, T.; Liang, M.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus reuteri CCFM8631 Alleviates Hypercholesterolaemia Caused by the Paigen Atherogenic Diet by Regulating the Gut Microbiota. Nutrients 2022, 14, 1272. https://doi.org/10.3390/nu14061272
Wang Q, He Y, Li X, Zhang T, Liang M, Wang G, Zhao J, Zhang H, Chen W. Lactobacillus reuteri CCFM8631 Alleviates Hypercholesterolaemia Caused by the Paigen Atherogenic Diet by Regulating the Gut Microbiota. Nutrients. 2022; 14(6):1272. https://doi.org/10.3390/nu14061272
Chicago/Turabian StyleWang, Qianqian, Yufeng He, Xiu Li, Ting Zhang, Ming Liang, Gang Wang, Jianxin Zhao, Hao Zhang, and Wei Chen. 2022. "Lactobacillus reuteri CCFM8631 Alleviates Hypercholesterolaemia Caused by the Paigen Atherogenic Diet by Regulating the Gut Microbiota" Nutrients 14, no. 6: 1272. https://doi.org/10.3390/nu14061272
APA StyleWang, Q., He, Y., Li, X., Zhang, T., Liang, M., Wang, G., Zhao, J., Zhang, H., & Chen, W. (2022). Lactobacillus reuteri CCFM8631 Alleviates Hypercholesterolaemia Caused by the Paigen Atherogenic Diet by Regulating the Gut Microbiota. Nutrients, 14(6), 1272. https://doi.org/10.3390/nu14061272






